Abstract
Eosinophilic esophagitis (EoE) is a chronic inflammatory disease of the esophagus, which requires short- and long-term treatment. In addition, patients under long-term treatment for any chronic condition should have a structured follow-up. The mainstays in EoE treatment are drugs (such as swallowed topical corticosteroids [STC] and proton pump inhibitors), dietary exclusions, and endoscopic dilations. STC are the most widely used treatment and have proven efficacy in inducing clinical, endoscopic and histological remission in active EoE. However, data regarding maintaining disease remission and long-term management are limited. Ongoing disease activity and relapses despite STC treatment are frequently observed. This sheds light on the urgent need for adequate maintenance strategies, which have not been well defined. In terms of follow-up concepts, to date neither guidelines nor consensus recommendations have been published. To summarize the current knowledge on long-term diagnostic and therapeutic STC management of EoE, we conducted a literature search using PubMed and Embase applying the following key search items: Eosinophilic esophagitis, eosinophils, esophagus, swallowed topical corticosteroids, fluticasone, budesonide, long-term, treatment, therapy, and follow-up. In addition, we present empirically developed long-term management concepts applied at two large EoE centers, with a special focus on STC treatments. Finally, we highlight areas of future research and perspectives regarding the long-term management of EoE.
Introduction
Eosinophilic esophagitis (EoE) is a chronic inflammatory disorder of the esophagus characterized clinically by symptoms reflecting esophageal dysfunction such as dysphagia or bolus impaction, and histologically by an eosinophil-predominant infiltration of the esophageal mucosa1. The disease course of EoE is chronic in most if not all patients with persistence of symptoms and esophageal eosinophilia if not treated properly. The current treatment options consist of the 3D’s: (1) drugs (swallowed topical corticosteroids [STC] and proton pump inhibitors [PPIs]), (2) dietary restrictions (allergen avoidance) and—if disease is advanced with stricture formation—(3) endoscopic dilation2. PPI-response has long been used as exclusion criteria for the diagnosis of EoE. However, in recent years “PPI-responsive EoE” (PPI-REE) has been recognized as a subgroup of EoE3. Despite therapeutic advances, ongoing disease activity and relapses are frequently observed, particularly after cessation of treatment. This sheds light on the urgent need for adequate maintenance strategies, which have not been defined so far. In addition, given the chronic nature of the disease, patients should have a structured follow-up, yet neither guidelines nor expert consensus on the interval or type of follow-up visits exist1,4.
In this review, we summarize existing knowledge on long-term diagnostic and therapeutic EoE management and currently applied empiric long-term treatment concepts at two large EoE centers, with a special focus on STC treatments. Finally, we highlight future perspectives on therapeutics, diagnostics, which may have their place in EoE long-term management. A literature review was conducted using PubMed and Embase. The following key search items were used: Eosinophilic esophagitis, eosinophils, esophagus, swallowed topical corticosteroids, fluticasone, budesonide, long-term, treatment, therapy, and follow-up.
Treatment of EoE with swallowed topical corticosteroids
While PPIs are efficacious in inducing remission in at least a subset of EoE patients, their role in the (long-term) treatment algorithm remains unclear. In terms of dietary exclusions, the need for multiple endoscopic evaluations and challenges regarding compliance limit their use in daily practice. Therefore, STC—either fluticasone or budesonide—are the most widely applied treatment modalities in EoE5. Until very recently, STC have been used off-label in EoE treatment. In late 2017, the European Medicines Agency (EMA) approved an orodispersable budesonide tablet (Jorveza®) for short-term EoE treatment. To date—however—there is still no FDA approval for STC.
STC inhibit several key pathogenic mechanisms of EoE: IL-13-induced pathways and genes—key regulators in allergy and EoE—are largely reversible by STC treatment6. On a cellular level, a two-week treatment course with STC significantly decreases esophageal eosinophilia, reduces epithelial cell apoptosis, and leads to a decrease in levels of mast cells with down regulation of mast cell genes, T-cells and the proinflammatory cytokine TNFα7,8. Also seen with STC are restoration of tight junction proteins and the epithelial barrier9. Tissue remodeling and fibrosis are also targeted7,10. However, these findings were not confirmed in the long-term11.
STC are either applied orally as nebulized liquid using a spray (fluticasone, from metered-dose inhaler used for asthma treatment), as powder (fluticasone, from a discus inhaler) or using a viscous preparation of liquid budesonide12–15. The mode of delivery of STC is one key to efficacy. Administration of viscous budesonide leads to a greater quantity and longer exposure to esophageal mucosa than does nebulized budesonide12. Optimal doses of STC treatment remain unclear: current induction treatment regimens consist of 880ug fluticasone or 1.0–2.0 mg budesonide twice daily for two to eight weeks. After induction of disease remission, there are three possible approaches: (1) continuation of induction dose, (2) reduction of induction dose (e.g., induction regimen once a day instead of b.i.d.), or (3) switch to a lower maintenance dose (0.25 mg b.i.d.). Dose-reduction strategies in case of adequately controlled disease are extrapolated from recommendations on long-term asthma treatment, although such dose-reductions may result in treatment failure16–19.
Safety of long-term STC treatment is a concern as short-term data cannot be effectively extrapolated. Recently published data suggest that rates of candida infections are considerably lower in the long-term (2.7 compared to up to 22%), at least with a low-dose regimen7,11,13,20–24. So far, there is no evidence demonstrating that prolonged use of STC leads to esophageal mucosal atrophy which could theoretically facilitate increased antigen presentation to the esophageal mucosa24. No robust studies are available yet regarding STC-induced osteoporosis, but inhaled steroids used for asthma have been associated with an increase in osteoporotic fractures25. Nevertheless, preliminary data in EoE patients demonstrate that in at least a 1–3 year follow-up study of 17 adults, no patient had a significant change in bone density with a daily dose of 1.5 mg budesonide26. A recent meta-analysis showed STC were associated with chemical but not clinical adrenal insufficiency in only a minority of patients, but long-term data are missing27.
Prospective trials of chronic STC in EoE
Given the chronic nature of EoE, long-term treatment appears needed. Two prospective trials have been performed (Table 1). One was a randomized-controlled trial conducted in adults, the other one was a non-controlled study in pediatric patients.
Table 1.
Overview of so far published maintenance treatment studies
| Study design | Treatment regimen | Treatment concept | Outcome | Study population | Follow-up | Comments | |
|---|---|---|---|---|---|---|---|
| Straumann 2011 | Randomized-controlled | 0.25 mg budesonide b.i.d | 50-week treatment, clinical follow-up every 3 months, telephone interview every 4 weeks | Reduction of symptoms, esophageal eosinophilia and mucosal wall thickness | Adults, n = 28, in clinical and histological remission after introduction treatment with 1 mg budesonide b.i.d (15 days) | 50 weeks | No validated PRO instruments, limited sample size |
| Andreae 2016 | Open-label | 2 puffs of fluticasone b.i.d (44, 110, or 220ug according to age) | If histological remission achieved, treatment continued with clinical follow-up every 4 months | Improvement of symptoms, endoscopy and histology | Children, n = 54 | Median 20.4 months | No PRO instruments, no control group |
| Kuchen 2014 | Observational | Fluticasone or budesonide, low-dose | Annual follow-up with assessment of clinical, endoscopic and histological activity | Higher frequency of STC use was associated with a lower risk for bolus impactions | Adults, n = 206 | Median 5 years | Bolus impaction as the only readout |
| Greuter 2017 | Observational | 1 mg STC b.i.d for 2–4 weeks, followed by 0.25 mg STC b.i.d | Annual follow-up with assessment of clinical, endoscopic and histological activity. Treatment stopped in case of long-lasting deep remission | Only 9.4% achieved deep remission. Relapse occurred in 81.8% after discontinuation of treatment | Adults, n = 33 | Median 6 years | Only patients in deep remission were analyzed in detail |
| Greuter 2018 | Observational | 1 mg STC b.i.d for 2–4 weeks, followed by 0.25 mg STC b.i.d | Annual follow-up with assessment of clinical, endoscopic and histological activity. | Higher rates of clinical, endoscopic and histological remission at visits under STC. Higher doses and longer treatment associated with complete remission. | Adults, n = 229 | Median 5 years | Low maintenance dose, possibly inadequate dosing |
| Eluri 2017 | Observational | STC (dose, type and dose adjustments at discretion of individual care provider) | Follow-up at discretion of health care provider | High rates of loss of response to steroids, which was associated with steroid dose reduction | Adults, n = 55 | Median 11.7 months | Short follow-up, no uniform concept |
The first trial evaluated the efficacy and safety of 0.25 mg budesonide b.i.d over 50 weeks in 28 patients in a double-blind randomized-controlled fashion11. Adolescent and adult patients (>14 years old) with histological remission defined by < 5 eos/hpf after STC induction treatment (1 mg b.i.d.) were included. Budesonide treated patients showed a significantly less pronounced increase in the peak eosinophil count over time. 35.7% of the patients in the interventional group maintained < 5 eos/hpf, while 14.3% remained at least under 20 eos/hpf compared to controls with remission rates of 0 and 28.6%, respectively. At 1 year, 64.3% remained in clinical remission. No cases of clinically relevant esophageal candida or other opportunistic infections were identified.
The second trial prospectively analyzed the long-term outcome of 54 children treated with oral fluticasone28. Patients were treated according to their age with 2 puffs of fluticasone b.i.d. (44 μg per puff for age 2–4, 110 μg per puff for age 5–11, and 220 μg per puff for age ≥ 12, no reduction of induction dose over time). At predefined time-points ( < 4 months, 4–12 months, 1–24 months, and > 24 months), histological disease activity was assessed. At all time-points, peak eosinophil count was significantly decreased compared to the baseline counts before initiation of STC treatment. However, peak eosinophil counts increased over time. In the second year of treatment, the number of patients in histological remission decreased from 83% to 59% using a cut-off of < 15 eos/hpf and from 63% to 48% (for a cut-off < 5 eos/hpf). Only three cases of esophageal candida infection were observed. All patients were asymptomatic and responded to anti-fungal treatment. There was no effect on growth (based on z-scores) over the total follow-up period.
While both trials suggest efficacy and safety of STC in the long-term, the small study sample size and the treatment duration of only one year in the Swiss trial, and the unblinded, uncontrolled design in the Mount Sinai study limit the generalizability of these findings.
Observational studies
Four observational studies on long-term STC treatment have been published (Table 1). Three of these studies had a predefined therapeutic strategy with a low-dose STC regimen (fluticasone or budesonide 0.25 mg b.i.d.)24,29,30 and one had a non-uniform concept with dose reduction in some patients, while unchanged doses in others31.
A long-term low-dose STC treatment (0.25 mg b.i.d.), initiated after a short-term induction treatment (2–4 weeks), was associated with lower rates of bolus impaction (9.1 vs. 4.3% if not treated, p = 0.007) over a 5-year follow-up period in 206 patients29. The longer the patients were treated and the higher their adherence to the treatment regimen was, the less likely they experienced a long-lasting food impaction. Nevertheless, complications such as bolus obstruction still occurred in some patients with high adherence rates to long duration (1.7%). Long-term low-dose STC treatment was also more effective than no treatment with regards to clinical, endoscopic, histological ( < 15 eos/hpf), and complete remission, assessed in 229 patients over a 5-year follow-up24. Esophageal candida infections occurred in only 2.7% of patients and no mucosal atrophy was detected. Higher cumulative doses ( > 600 × 0.25 mg STC) and longer steroid treatment ( ≥ 1 year) were associated with higher rates of clinical and complete remission. The low-dose treatment regimen was also able to induce a long-lasting deep remission in a small subset of EoE patients (33/351, 9.5%), defined as clinical ( = absence of any EoE-attributable symptoms), endoscopic ( = absence of any inflammatory signs on endoscopy), and histologic remission (peak eosinophil count of < 5 eos/hpf) for at least 6 months30. However, in the vast majority of STC-treated patients, at least one of these measures of disease activity remained uncontrolled. In a small subset of STC-treated patients even strictures occur (4.8%, unpublished data from the Swiss EoE cohort). Of note, treatment discontinuation in the subgroup with deep remission led to high rates of clinical, and in some cases histologically proven relapse ( >80%), occurring within a few months30. Longer diagnostic delay and delayed time until clinical remission were identified as possible predictors for experiencing an EoE relapse. These findings promote evidence for the need of indefinite STC treatment, particularly in the context of their good safety profile and that achievement of deep remission does not reduce the need for continued treatment.
Eluri and colleagues31 analyzed the outcome of adult EoE patients in histologic remission ( < 15 eos/hpf) who continued to take topical steroids (with or without dose reduction) after an eight-week induction treatment with STC. 20/33 patients (61%) showed a histological relapse despite continuation of treatment, particularly those patients with a subsequent dose reduction. Histological relapse increased over time with 50% after 18.5 months and 75% after 29.6 months. There was no uniform management regarding dose reduction and chosen regimen, and follow-up evaluations did not follow a rigorous protocol. Patients with loss of relapse may have been over-represented in the group returning for further care.
Taken together currently available data support efficacy of STC in the long-term, but remission rates are lower than those seen after induction treatment. Long-lasting remission can be achieved, but only in a subset of patients with the lower doses of STC used to date. Nevertheless, discontinuation of treatment results in rapid relapse in most patients. This is why—even in well-controlled patients—treatment discontinuation cannot be recommended. Higher cumulative doses and longer treatment are associated with higher remission rates and lower rates of EoE complications, while dose reduction more often leads to a loss of response. Low-dose regimens show an excellent safety profile, while data for high-dose STC plans are currently limited. Although it would be appealing that EoE patients benefit from co-medication, there are not much available data supporting the concomitant use of two EoE treatment strategies.
Current concepts in acute and chronic treatment with STC
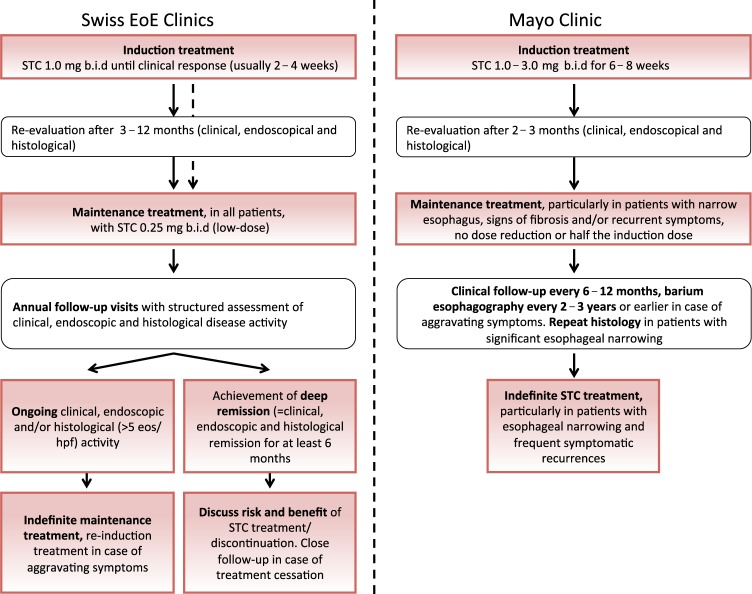
Despite its off-label use, there is wide consensus supporting the general concept of STC treatment for maintenance of EoE remission. However, no clear recommendations exist for the use, dose of STC and for adequate follow-up strategies. Given this lack of knowledge and consensus, a long-term therapeutic and follow-up concept was developed at the Swiss EoE Clinic in Olten, Switzerland, which was prospectively applied to the Swiss EoE cohort, one of largest cohorts worldwide. The concept was expanded based on the only available randomized-controlled maintenance trial in EoE and follows a paradigm similar to other chronic inflammatory disorders such as inflammatory bowel disease, where regular visits including assessment of endoscopic and histological disease activity are part of the work-up and help in guiding treatment decisions11,32. It consists of the following principles:
Patients with active EoE are treated with STC 1.0 mg b.i.d. until a clinical response is detected (two to four weeks). Induction treatment may be repeated in case of worsening symptoms;
After induction of a response, patients are treated with 0.25 mg STC b.i.d.;
Endoscopic and histological response to treatment is assessed within the first year after treatment initiation;
Patients are seen once per year with assessment of clinical (Straumann Dysphagia index7,11, EoE Activity Index [EEsAI])33, endoscopic (EoE Endoscopic Reference Score [EREFS] grading system)34 and histological disease activity (four biopsies are taken from each the proximal and distal esophagus). A cut-off of < 15 eos/hpf is chosen for dichotomizing activity into controlled and non-controlled. A cut-off of < 5 eos/hpf is used to classify histological activity as fully controlled;
The STC regimen is administered indefinitely except for patients achieving a clinical, endoscopic and histological remission ( < 5 eos/hpf) for the duration of at least six months ( = deep remission). In these patients, STC treatment is stopped and close clinical follow-up is maintained (assessment of clinical disease activity every three months);
In case of a relapse (defined as new-onset of EoE-attributed symptoms and/or esophageal eosinophilia ( ≥ 15 eos/hpf), treatment is re-initiated.
After thorough analysis of this concept and the high rates of clinical and histological relapses after discontinuation of treatment, the concept has been slightly modified; if long-lasting deep remission is achieved, the risks and benefits of treatment discontinuation are discussed with the patient in great detail. Treatment discontinuation is neither recommended nor rejected. The concept has been shown to be effective in maintaining disease remission in the long-term, although remission rates are considerable lower than in the short-term. One major limitation of the concept is that the chosen dose of 0.25 mg STC twice daily is low and the high rates of treatment failure may actually represent inadequate dosing. On the other side, this low-dose regimen imposes little if any risks or side-effects.
At the Mayo Clinic, a referral center treating a cohort of over 1500 EoE patients, the principles of long-term EoE management with STC are slightly different. A structure barium esophagogram is performed at baseline. An endoflip procedure to determine esophageal distensibility, similarly could be used at baseline if available. In general, patients with significant fibrotic disease and a narrowed esophagus will require dilation and in this group histologic remission is pursued on proven maintenance therapy. In patients with no significant esophageal narrowing, the goal is symptomatic remission with serial esophageal diameter assessments to exclude progressive esophageal narrowing. This group may go on maintenance therapy if required for recurrent or initially severe symptoms. Further principles of the concept are:
Active disease with an esophageal eosinophilia of ≥15 eos/hpf is an indication for treatment;
Patients are treated with an induction regimen (either 2 puffs 440 μg fluticasone twice daily, or 1.0 mg budesonide b.i.d) for eight weeks; for patients with severe symptoms accompanied by stricturing disease, 3.0 mg budesonide b.i.d. may be used;
Treatment response is assessed by endoscopy including biopsies at the end of induction phase as symptomatic responses may not correlate with histologic response, particularly in patients with significant esophageal narrowing;
Patients are alerted to contact the physician for any recurrent symptoms and clinical disease activity is assessed formally every six to twelve months by EEsAI and a detailed dysphagia history taken at each visit;
Patients with frequent symptomatic recurrences off steroid and those with significant esophageal narrowing are maintained on a reduced dose (budesonide 1.0–1.5 mg or fluticasone 880 mcg q day at bedtime) or on the induction dose without any further dose reduction. In patients with significant esophageal narrowing repeat histology is obtained after two months to confirm histologic remission on maintenance therapy and to eventually titrate STC maintenance doses;
A barium esophagogram is performed every two to three years for patients with stable symptoms or earlier for dysphagia recurrence. Esophageal dilation is performed for recurrent and/or worsening esophageal stricture formation.
This concept is based on the following rationale: (1) Regular assessment of histological disease activity in the absence of symptomatic or radiographic progression usually does not result in changes of the treatment plan in the presence of a maintenance STC dose previously shown to sustain histologic remission; (2) Optimal peak eosinophil count cut-off points for adequate disease maintenance are unknown, so there is no consensus about the exact target to treat even though < 15 eos/HPF is often cited as it has been shown to correlate with symptoms35 and changes in esophageal impedance36; (3) It is unclear if the presence of isolated esophageal eosinophilia in an asymptomatic patient with a low EREFS score predicts disease progression; and (4) It is unclear if a static measurement of esophageal eosinophilia represents absolute values on a month to month basis or the esophagus as a whole given the patchy distribution of esophageal eosinophilia. Esophagogram is used based on a recent study showing higher sensitivity for detecting strictures with barium esophagography compared to endoscopy. Indeed, sensitivity of endoscopy to detect a small caliber esophagus of less than 13 mm is less than 26% 37.
Differences in the long-term management of EoE at our two EoE referral centers highlight the lack of clear recommendations. Nonetheless, the two concepts applied at the Swiss EoE Clinic and Mayo Clinic have two important features in common: (1) indefinite treatment is recommended in most if not all EoE patients, and (2) disease activity should be monitored in the long-term by some objective measure which goes beyond assessment of symptoms alone (Fig. 1).
Fig. 1.
Current therapeutic concepts at the Swiss EoE Clinic and at the Mayo Clinic
Perspectives on long-term steroid treatment
Two areas of uncertainty have been identified: (1) optimal doses of STC in the maintenance phase and (2) method and frequency of diagnostic follow-up. Two currently ongoing phase III studies will close an important gap in terms of long-term EoE management (NCT02493335 and NCT03245840, clinicaltrials.gov) particularly in the context of very high histological response rates ( > 93%) with the budesonide regimen in the short-term38. However, the trials are not expected to be completed and published within the next two years. Until more data exist, one of the two or a blend of the two strategies might be followed after a successful induction treatment: (1) switch to a low maintenance dose, which is associated with significant better outcome than no treatment and has a well-documented safety profile or (2) treat patients with the same dose that brought them into remission. The fact that a dose reduction appears to be associated with a worse outcome and the findings of lower remission rates in the long-term than with high-dose induction treatment favor the second option. However, the high rates of loss of follow-up in the published observational studies appear to be an important confounder since non-compliant patients are more likely to show up for scheduled visits, which might result in falsely elevated rates of treatment failure. In addition, it is still unclear if dose reduction per se or rather a dose reduction below a specific level are responsible for an inferior outcome.
When it comes to the best follow-up strategies, there is no consensus without available data to better guide recommendations. However, close follow-up makes sense and is in accordance with other chronic inflammatory diseases. The type of diagnostic evaluation in the long-term phase has not yet been determined. In the future, newer diagnostic modalities may provide more detailed and comprehensive follow-up: the Cytosponge and the esophageal string, both minimally invasive tools, are able to detect active EoE with high sensitivity39–42. Such non-invasive approaches are urgently needed and might facilitate disease monitoring and evaluation of response to maintenance treatment regimens. Furthermore, functional lumen imaging probe (FLIP), an FDA-approved transoral intraesophageal balloon device that assesses pressure dynamics and diameter of the esophagus43 might be used to assess severity of EoE and to monitor EoE treatment similar to esophagography44,45. Proactive assessment of STC side-effects remains a matter of ongoing debate. Periodic laboratory testing for adrenal insufficiency may be considered in children with higher doses over a longer period of time46–48. Bone densitometry to assess for STC-induced osteoporosis cannot be universally recommended, but might be considered in high-risk situations.
Conclusions
Prospective and observational studies have shown efficacy for long-term treatment with swallowed topical corticosteroids in terms of disease activity and development of complications. Their safety profile is excellent. However, efficacy is considerably lower than that seen in the short-term. Based on the high rates of relapse after treatment cessation, currently applied therapeutic concepts advocate for indefinite long-term treatment, particularly in patients with severe symptomatic and/or endoscopic/radiographic manifestations of disease. Given EoE’s chronic nature, close follow-up with assessment of disease activity should be strived for in most if not all patients. Nonetheless, optimal doses for the maintenance phase have still to be defined and dose-finding trials are definitely needed. Current diagnostic and therapeutic concepts should be properly evaluated.
CONFLICTS OF INTEREST
Guarantor of article: Thomas Greuter, MD and David A. Katzka, MD.
Specific author contributions: All authors contributed to the conception and writing of the manuscript, reviewed it and approved the final version.
Financial support: This work was supported by a grant from the Swiss National Science Foundation to A.S. (grant no. 32003B_160115) and T.G. (grant no. P2ZHP3_168561), a young investigator award from the Swiss Society of Gastroenterology to T.G., a training grant from Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) to T.G. CEGIR (U54AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED and EFC.
Potential competing interests: A.S. has consultant contracts with Celgene-Receptos, Falk Pharma, Sanofi-Regeneron, and Tillots. T.G. received a travel grant from Falk Pharma GmbH and Vifor, has a consultant contract with Sanofi-Aventis and received an unrestricted research grant from Novartis. J.A.A. has a financial interest in Meritage Pharmacia and receives research funding from Shire. D.A.K. declares no conflicts of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J. Allergy Clin. Immunol. 2011;128:3–20.e26. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Straumann A, Katzka DA. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology. 2018;154:346–59. doi: 10.1053/j.gastro.2017.05.066. [DOI] [PubMed] [Google Scholar]
- 3.Molina-Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65:524–31. doi: 10.1136/gutjnl-2015-310991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucendo AJ, Molina-Infante J, Arias Aacute, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. U. Eur. Gastroenterol. J. 2017;5:335–58. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuta GT, Katzka DA. Eosinophilic esophagitis. N. Engl. J. Med. 2015;373:1640–8. doi: 10.1056/NEJMra1502863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 8.Hsu Blatman KS, Gonsalves N, Hirano I, Bryce PJ. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J. Allergy Clin. Immunol. 2011;127:1307–e1303. doi: 10.1016/j.jaci.2010.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzka DA, Tadi R, Smyrk TC, et al. Effects of topical steroids on tight junction proteins and spongiosis in esophageal epithelia of patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2014;12:1824–e1821. doi: 10.1016/j.cgh.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65:109–16. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2011;9:400–e401. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Sheikh A, Speck O, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143:321–e321. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miehlke S, Hruz P, Vieth M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65:390–9. doi: 10.1136/gutjnl-2014-308815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellon ES, Katzka DA, Collins MH, et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology. 2017;152:776–e775. doi: 10.1053/j.gastro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Hefner JN, Howard RS, Massey R, et al. A randomized controlled comparison of esophageal clearance times of oral budesonide preparations. Dig. Dis. Sci. 2016;61:1582–90. doi: 10.1007/s10620-015-3990-4. [DOI] [PubMed] [Google Scholar]
- 16.Koskela HO, Purokivi MK, Kokkarinen J. Stepping down from combination asthma therapy: the predictors of outcome. Respir. Med. 2016;117:109–15. doi: 10.1016/j.rmed.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Li AM, Tsang TW, Lam HS, Sung RY, Chang AB. Predictors for failed dose reduction of inhaled corticosteroids in childhood asthma. Respirology. 2008;13:400–7. doi: 10.1111/j.1440-1843.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 18.Rank MA, Johnson R, Branda M, et al. Long-term outcomes after stepping down asthma controller medications: a claims-based, time-to-event analysis. Chest. 2015;148:630–9. doi: 10.1378/chest.15-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crossingham I, Evans DJ, Halcovitch NR, Marsden PA. Stepping down the dose of inhaled corticosteroids for adults with asthma. Cochrane Database Syst. Rev. 2017;2:CD011802. doi: 10.1002/14651858.CD011802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig. Dis. Sci. 2010;55:1313–9. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin. Gastroenterol. Hepatol. 2008;6:165–73. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Greuter T, Safroneeva E, Bussmann C, et al. Maintenance Treatment of Eosinophilic Esophagitis With Swallowed Topical Steroids Alters Disease Course Over A 5-Year Follow-Up Period in Adult Patients. Clin Gastroenterol Hepatol. 2018 (epub ahead of print). [DOI] [PubMed]
- 25.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 26.Otaki F, Wacholz D, Geno DM, Tholen C, Alexander JA Interval bone density in patients with eosinophilic esophagitis on steroids. In ProcACG Congress 2017 P01090 (American College of Gastroenterology, Bethesda, MA, 2017).
- 27.Philpott H, Dougherty MK, Reed CC, et al. Systematic review: adrenal insufficiency secondary to swallowed topical corticosteroids in eosinophilic oesophagitis. Aliment Pharmacol. Ther. 2018;47:1071–8. doi: 10.1111/apt.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreae DA, Hanna MG, Magid MS, et al. Swallowed fluticasone propionate is an effective long-term maintenance therapy for children with eosinophilic esophagitis. Am. J. Gastroenterol. 2016;111:1187–97. doi: 10.1038/ajg.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchen T, Straumann A, Safroneeva E, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy. 2014;69:1248–54. doi: 10.1111/all.12455. [DOI] [PubMed] [Google Scholar]
- 30.Greuter T, Bussmann C, Safroneeva E, et al. Long-term treatment of eosinophilic esophagitis with swallowed topical corticosteroids: development and evaluation of a therapeutic concept. Am. J. Gastroenterol. 2017;112:1527–35. doi: 10.1038/ajg.2017.202. [DOI] [PubMed] [Google Scholar]
- 31.Eluri S, Runge TM, Hansen J, et al. Diminishing effectiveness of long-term maintenance topical steroid therapy in PPI non-responsive eosinophilic esophagitis. Clin. Transl. Gastroenterol. 2017;8:e97. doi: 10.1038/ctg.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina-Infante J, Schoepfer AM, Lucendo AJ, Dellon ES. Eosinophilic esophagitis: what can we learn from Crohn’s disease? U. Eur. Gastroenterol. J. 2017;5:762–72. doi: 10.1177/2050640616672953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–e1221. doi: 10.1053/j.gastro.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–95. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 35.Reed CC, Wolf WA, Cotton CC, et al. Optimal histologic cutpoints for treatment response in patients with eosinophilic esophagitis: analysis of data from a prospective cohort study. Clin. Gastroenterol. Hepatol. 2018;16:226–e222. doi: 10.1016/j.cgh.2017.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzka DA, Ravi K, Geno DM, et al. Endoscopic mucosal impedance measurements correlate with eosinophilia and dilation of intercellular spaces in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2015;13:1242–e1241. doi: 10.1016/j.cgh.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Podboy A, Katzka DA, Enders F, et al. Oesophageal narrowing on barium oesophagram is more common in adult patients with eosinophilic oesophagitis than PPI-responsive oesophageal eosinophilia. Aliment Pharmacol. Ther. 2016;43:1168–77. doi: 10.1111/apt.13601. [DOI] [PubMed] [Google Scholar]
- 38.Lucendo A, Miehlke, et al. Budesonide orodispersible tablets are highly effective for treatment of active eosinophilic esophagitis: results from a randomized, double-blind, placebo-controlled, pivotal multicentertrial (EOS-1) Gastroenterology. 2017;152:1–S207. doi: 10.1016/S0016-5085(17)30997-6. [DOI] [Google Scholar]
- 39.Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology. 2013;144:62–73.e66. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 40.Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2015;13:77–83.e72. doi: 10.1016/j.cgh.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Katzka DA, Smyrk TC, Alexander JA, et al. Accuracy and safety of the cytosponge for assessing histologic activity in eosinophilic esophagitis: a two-center study. Am. J. Gastroenterol. 2017;112:1538–44. doi: 10.1038/ajg.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395–405. doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano I, Pandolfino JE, Boeckxstaens GE. Functional lumen imaging probe for the management of esophageal disorders: expert review from the clinical practice updates committee of the AGA institute. Clin. Gastroenterol. Hepatol. 2017;15:325–34. doi: 10.1016/j.cgh.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicodème F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2013;11:1101–e1101. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy. 2016;48:794–801. doi: 10.1055/s-0042-107340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal insufficiency after chronic swallowed glucocorticoid therapy for eosinophilic esophagitis. J. Pediatr. 2016;170:240–5. doi: 10.1016/j.jpeds.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Hsu S, Wood C, Pan Z, et al. Adrenal insufficiency in pediatric eosinophilic esophagitis patients treated with swallowed topical steroids. Pediatr. Allergy Immunol. Pulmonol. 2017;30:135–40. doi: 10.1089/ped.2017.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoelwer MJ, Nebesio TD. Diagnosis of adrenal insufficiency in eosinophilic esophagitis: The importance of timing of cortisol measurements in interpreting low-dose adrenocorticotropic hormone stimulation testing. J. Pediatr. 2016;174:282. doi: 10.1016/j.jpeds.2016.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]