Abstract
Background
Two-dimensional (2D) speckle-tracking strain imaging is a novel method for assessment of regional myocardial deformation that uses tracking of acoustic speckles or kernels rather than Doppler myocardial velocities. It has been suggested that Left atrial (LA) strain as measured by 2D speckle tracking can be used to evaluate dynamic LA function.
Objective
To study the relation between left atrial deformation and the severity of coronary artery stenosis in patients with stable coronary artery disease (CAD).
Study design
30 patients with stable coronary artery disease (SCAD) with coronary artery stenosis (>50%) who were admitted for elective coronary angiography at Ain Shams University hospitals and AlAzhar University hospitals were included in the study. Measurements of conventional echocardiographic parameters as well as LA strain and strain rate parameters were obtained, Syntax (SX) score was calculated for all patients.
Results
Patients were categorized into 3 groups: low Syntax score of <23 (Group I), moderate syntax score 23–32 (Group II) and high syntax score of ≥33 (Group III). Peak atrial longitudinal strain (PALS) (Group I: 29.80 ± 4.48, Group II: 22.44 ± 1.42, Group III: 19.53 ± 4.46; p < 0.001) and Peak atrial contraction strain (PACS) (Group I: 13.43 ± 4.05, Group II: 10.84 ± 2.47, Group III: 7.19 ± 0.71; p < 0.022) were significantly lower in high syntax group. Significant negative correlation was found between SX score level and LA strain parameters (PALS and PACS) (r = 0.861; p < 0.001).
Conclusion
Left atrial deformation analysis by 2D Speckle tracking Doppler Echocardiography can predict the severity of coronary affection in patients with stable CAD.
Keywords: Left atrium, Deformation, Strain, Speckle tracking
1. Introduction
Coronary artery disease (CAD) is the leading cause of death all over the world. The World Health Organization estimates that approximately 17 million people die from CAD every year.1 LV diastolic function is abnormal in a high percentage of patients with CAD at rest independent of LV systolic function.2, 3 It has been suggested that left ventricular diastolic dysfunction may occur before left ventricular systolic dysfunction and therefore serve as an early and sensitive marker of ischemia.4
The principal role of the left atrium is to modulate left ventricular (LV) filling and cardiovascular performance by functioning as a reservoir for pulmonary venous return during ventricular systole, a conduit for pulmonary venous return during early ventricular diastole, and a booster pump that augments ventricular filling during late ventricular diastole.5 Two-dimensional (2D) speckle-tracking strain imaging is a novel method for assessment of regional myocardial deformation that uses tracking of acoustic speckles or kernels rather than Doppler myocardial velocities 6. It has been suggested that Left atrial strain as measured by 2D speckle tracking can be used to evaluate dynamic LA function.7
Coronary angiography is the gold standard for diagnosis of coronary artery disease.8 The Synergy between PCI with TAXUS and Cardiac Surgery score (SYNTAX score) is anatomically based risk calculation that characterize the severity of CAD.9, 10, 11
2. Aim of the work
The aim of this study is to evaluate the relation between LA strain and the severity of coronary artery stenosis in patients with CAD.
3. Patients and methods
3.1. Study population
Between March 2017 and February 2018, we included 30 patients with stable coronary artery disease with coronary artery stenosis (>50%) who were admitted for elective coronary angiography at Ain Shams University hospitals and AlAzhar University hospitals. Patients were categorized into 3 groups: low Syntax score of <23 (Group I), moderate syntax score 23–32(Group II) and high syntax score of ≥33 (Group III). Before coronary angiography, history taking, physical examinations and resting ECG were obtained. Venous samples for laboratory analysis were drawn after overnight fasting. We excluded patients with age more than 70 years, acute coronary syndrome, history of previous CABG, history of DM or chronic renal failure, hypertension with LVH and diastolic dysfunction > grade II, valvular heart disease, abnormal cardiac rhythm (especially atrial fibrillation), Heart failure with preserved or reduced ejection fraction, diastolic dysfunction >grade II, any types of cardiomyopathy, and/or systemic diseases that may affect the myocardium (metabolic storage disease).
3.2. Echocardiography
Transthoracic echocardiography was performed for enrolled patients at rest in the left lateral decubitus position by a professional echo-cardiographer (at least one year experience at Ain shams university hospital echo lab) who was blinded to the coronary angiography results, using General Electric Vivid 5 and 7 systems using an M4S transthoracic transducer on the same day of the angiographic studies. Complete two-dimensional, color, pulsed and continuous wave Doppler examinations were performed according to standard techniques.12, 13
3.3. LA deformation analysis by 2D speckle tracking
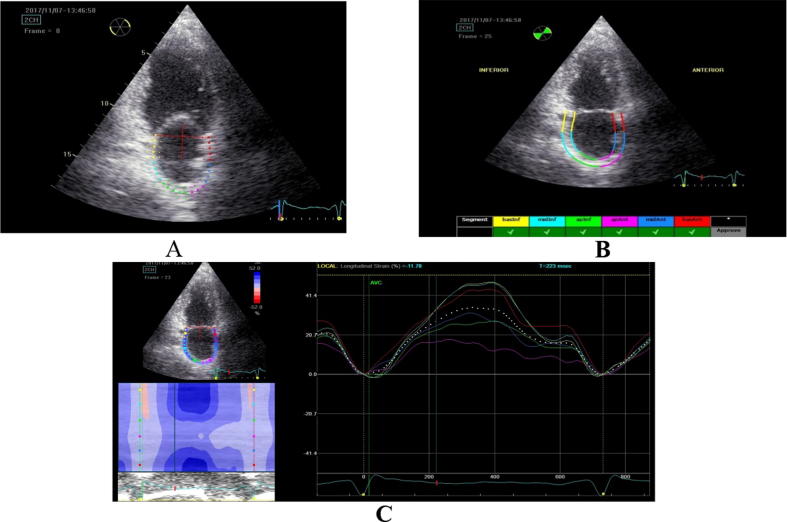
For the evaluation of the longitudinal deformation of the LA by 2D Speckle Tracking we stored three cardiac cycles of the apical two, three and four-chamber views at the end of expiration at a rate of 55–80 frames per second. The two-dimension strain analysis package available on the Echo PAC work station (GE Healthcare) was used to measure the LA strain and strain rate. At end-systole, LA endocardial surface is manually traced in four, three and two-chamber views by a point-and-click approach. An epicardial surface tracing is then automatically generated by the system, thus creating a region of interest (ROI). Confirmation was done by checking and adjusting the tracing process. The software then automatically divided the region of interest into six segments. After tracking myocardial motion frame by frame, the software marked these segments as acceptable or unacceptable one according whether their tracking quality were good enough. In segments with poor tracking, the observer readjusted the endocardial trace line until a better tracking quality was achieved. If this was not attainable, the segment was excluded. In the presence of three or more excluded segments, the subject was excluded from the study. The initiation of the QRS wave was set as 0 levels. Lastly the software generated the longitudinal strain and strain rate curves for each segment and a mean curve of all segments that reflects the pathophysiology of atrial function (Fig. 1).
Fig. 1.
Measurement of left atrial longitudinal strain by speckle tracking. (A) The atrial endocardial border was traced by a point-and-click method; (B) after automatic creation of a region of interest, the software then automatically divided the region of interest into six segments, (C) after approval by the user, segmental longitudinal strain curves were generated. The dashed curve represents the average strain.
3.4. LA strain curve
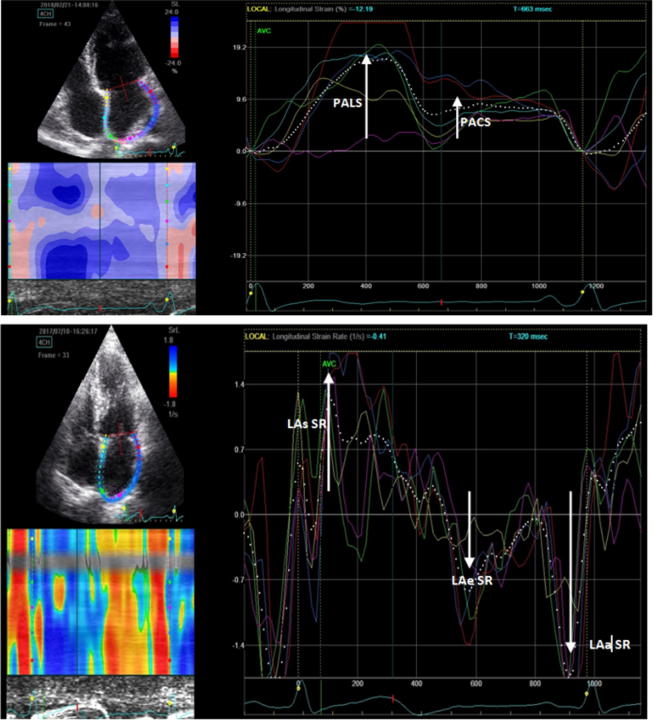
During the reservoir phase, the LA fills up, stretches itself, and for this reason, the atrial strain increases reaching a positive peak at the end of atrial filling before the opening of the mitral valve. After the opening of the mitral valve, LA empties quickly shortens itself so the strain decreases up to a plateau corresponding to the phase of diastasis followed by a second positive peak but less than the first which corresponds to the period preceding the atrial contraction, and finally a negative peak after the atrial contraction. This second positive deflection of the atrial strain curve, corresponding to atrial systole. Thus, as shown in Fig. 2 the following parameters were obtained: 1. Peak atrial longitudinal strain (PALS), measured at the end of the reservoir phase. 2. Peak atrial contraction strain (PACS), measured just before the start of the active atrial contractile phase.
Fig. 2.
Measurement of peak atrial longitudinal strain and strain rate using speckle tracking echocardiography from an apical 4-chamber view. An average atrial longitudinal strain along the cardiac cycle is depicted (dashed curve). AVC: aortic valve closure. Abbreviations: PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; LAs SR; Peak atrial longitudinal strain rate during ventricular systole, LAe SR; Peak atrial longitudinal strain rate during ventricular early diastole, LAa SR; Peak atrial longitudinal strain rate during ventricular late diastole.
3.5. LA strain rate curve
LA longitudinal strain rate profiles show one positive and two negative waves during LV systole and diastole, respectively. For this study, we measured the averaged values of peak global systolic strain rate (LAs SR), peak global early diastolic strain rate (LAe SR), and peak global late diastolic strain rate (LAa SR) from apical four, three and two-chamber images (Fig. 2).
LAs SR was positive during LV systole, which means that the LA wall was passively stretched during LV systole, so that this rate could be used as an index of LA reservoir function. LAe SR and LAa SR were negative during LV early and late diastole, which indicates shortening of the LA wall, so that these rates could be used as indices of LA conduit and LA booster functioning, respectively.
3.6. Coronary angiogram
Standard selective coronary angiography was done by a qualified interventional cardiologist (at least 3 years of experience at Ain shams university hospital cath lab) who was blinded to the study. All selective coronary angiographies were performed under local anesthesia via the right femoral artery according to the Judkin’s method with manual contrast media injections. Angiograms were obtained at various angles to allow more complete visualization of coronary arteries from several angles.14 CAD was defined as ≥50% angiographic lumen narrowing of at least one major coronary vessel. The name and number of the affected artery and segments were reported.
3.7. SYNTAX score
The coronary angiogram for each patient was reported, and an online calculator was used to determine the SYNTAX score (www.syntaxscore.com).
4. Results
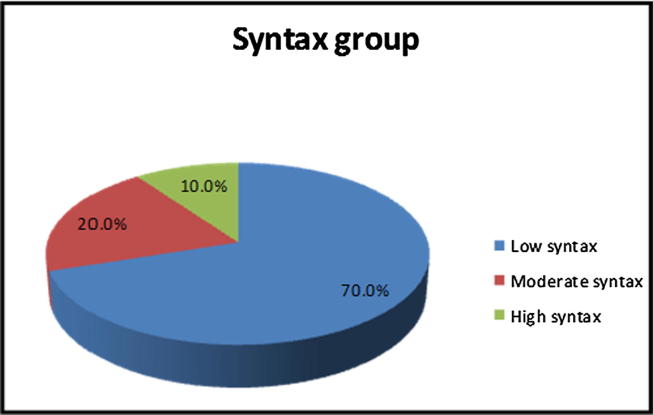
There was no statistically significant difference in age, gender distribution, smoking, and blood pressure between the three groups(Fig. 3, Table 1).
Fig. 3.
Pie chart syntax group distribution of the study group.
Table 1.
Comparison between syntax groups according to demographic data and co-morbidities.
| Demographic data and co-morbidities | Syntax group |
t/x2# | p-value | ||
|---|---|---|---|---|---|
| Low syntax (N = 21) | Moderate syntax (N = 6) | High syntax (N = 3) | |||
| Gender | 1.429# | 0.49 | |||
| Male | 17 (81.0%) | 4 (66.7%) | 3 (100.0%) | ||
| Female | 4 (19.0%) | 2 (33.3%) | 0 (0.0%) | ||
| Age (years) | 54.24 ± 9.20 | 58.33 ± 6.71 | 61.33 ± 9.07 | 1.177 | 0.323 |
| HTN | 4 (19.0%) | 3 (50.0%) | 1 (33.3%) | 2.362# | 0.307 |
| Smoking | 6 (28.6%) | 2 (33.3%) | 1 (33.3%) | 4.684# | 0.086 |
4.1. Echocardiographic features
Transthoracic echocardiographic parameters of the study population as well as Left atrial deformation parameters by 2D speckle tracking Doppler Echocardiography (PALS, PACS, LAs SR, LAe SR, and LAa SR) are presented in Table 2. All the subjects had normal LV systolic function. There were no significant differences between the three groups with respect to thickness of the inter-ventricular septum (IVS) and posterior wall (PW), LV end-diastolic dimensions and LV end-systolic dimensions.
Table 2.
Comparison between syntax score and echocardiographic parameters.
| Echo-cardiographic data | Syntax group |
p-value | ||
|---|---|---|---|---|
| Low syntax (N = 21) | Moderate syntax (N = 6) | High syntax (N = 3) | ||
| PALS (%) | 29.80 ± 4.48 | 22.44 ± 1.42 | 19.53 ± 4.46 | <0.001** |
| PACS (%) | 13.43 ± 4.05 | 10.84 ± 2.47 | 7.19 ± 0.71 | 0.022* |
| LAs SR (s−1) | 1.41 ± 0.17 | 1.15 ± 0.18 | 0.93 ± 0.21 | <0.001** |
| LAe SR (s−1) | −1.21 ± 0.22 | −1.06 ± 0.18 | −0.71 ± 0.22 | 0.003* |
| LAa SR (s−1) | −1.98 ± 0.30 | −1.79 ± 0.37 | −1.63 ± 0.47 | 0.031* |
| LVIDd (mm) | 49.58 ± 3.92 | 48.88 ± 5.38 | 48.00 ± 1.73 | 0.805 |
| LVIDd (mm) | 33.89 ± 4.58 | 31.38 ± 4.07 | 33.00 ± 3.61 | 0.407 |
| IVSd (mm) | 12.32 ± 2.29 | 13.75 ± 2.43 | 13.67 ± 2.89 | 0.308 |
| LVPWs (mm) | 13.21 ± 2.10 | 14.13 ± 2.36 | 13.33 ± 2.08 | 0.607 |
| EF% | 61.29 ± 5.26 | 65.67 ± 4.41 | 59.33 ± 7.57 | 0.157 |
| AP LA diameter (mm) | 33.71 ± 4.38 | 36.67 ± 8.19 | 39.00 ± 1.73 | 0.049* |
| LA volume (ml) | 43.90 ± 8.18 | 49.83 ± 15.66 | 55.00 ± 13.08 | 0.016* |
| E/A | 1.31 ± 1.50 | 1.03 ± 0.34 | 0.74 ± 0.14 | 0.728 |
| E/e′ | 5.68 ± 1.98 | 6.48 ± 1.70 | 6.41 ± 5.23 | 0.709 |
Data represented as Mean ± SD.
Abbreviations: PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; EF, ejection fraction, LAs SR; Peak atrial longitudinal strain rate during ventricular systole, LAe SR; Peak atrial longitudinal strain rate during ventricular early diastole, LAa SR; Peak atrial longitudinal strain rate during ventricular late diastole LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVPWd, Left ventricular posterior wall end diastole, IVSd, Interventricular septum end diastol.
p-value < 0.05 significant.
p-value < 0.001 highly significant.
The LA volumes were significantly higher in high syntax group (mild syntax, moderate syntax and high syntax groups were 43.90 ± 8.18 ml, 49.83 ± 15.66 ml and 55.00 ± 13.08 ml respectively (P Value 0.016)). Also LA diameters were higher in high syntax group. A positive correlation was reported between Syntax score and both LA volumes and diameters. The E/A ratio was non-significantly lower in the high syntax group (mild syntax, moderate syntax and high syntax groups were 1.31 ± 1.50, 1.03 ± 0.34 and 0.74 ± 0.14 respectively).
4.2. Left atrial deformation parameters by 2D speckle tracking Doppler Echocardiography
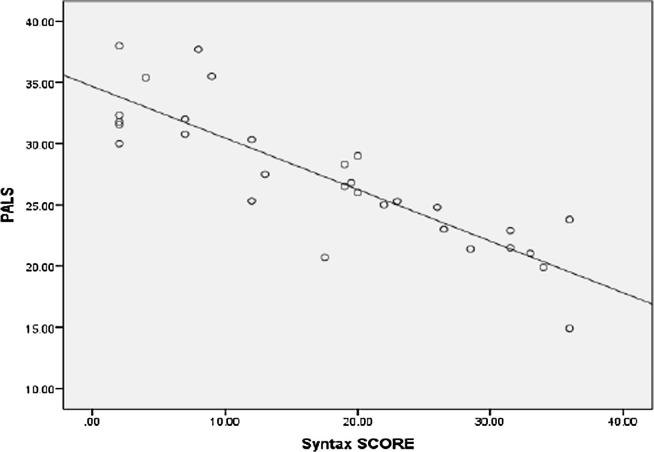
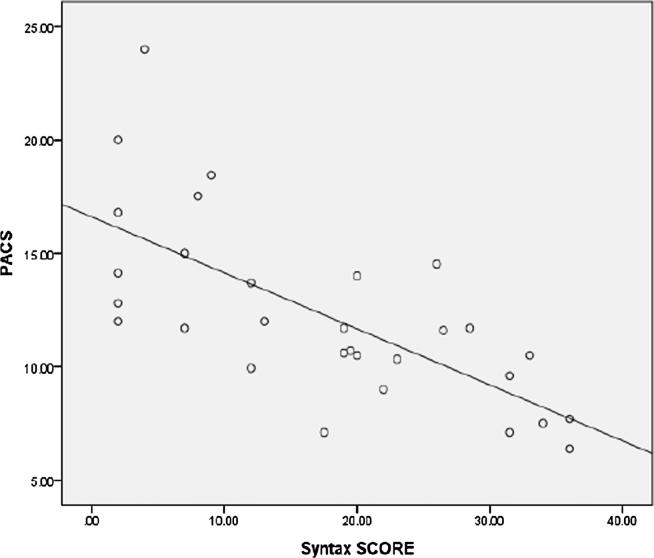
The left atrial deformation parameters (PALS, PACS, LAs SR, LAe SR, and LAa SR) were lower in patients with high syntax score. PALS was highly significantly lower in high syntax group (mild syntax, moderate syntax and high syntax groups were 29.80 ± 4.48%, 22.48 ± 2.10%, and 19.53 ± 4.46% respectively (P Value 0.001)). A highly significant negative correlation was found between Syntax score and PALS (r = −0.861, p < 0.001) (Fig. 7). PACS was significantly lower in high syntax group (mild syntax, moderate syntax and high syntax groups were 13.43 ± 4.05%, 10.84 ± 2.47% and 7.19 ± 0.71% respectively (P Value 0.022)). A highly significant negative correlation was found between Syntax score and PACS (r = −0.696, p < 0.001), (Fig. 8).
Fig. 7.
Scatter plot showing a Negative correlation between syntax score and LAa SR correlation between syntax score and PALS.
Fig. 8.
Scatter plot showing a negative correlation between syntax score and PACS.
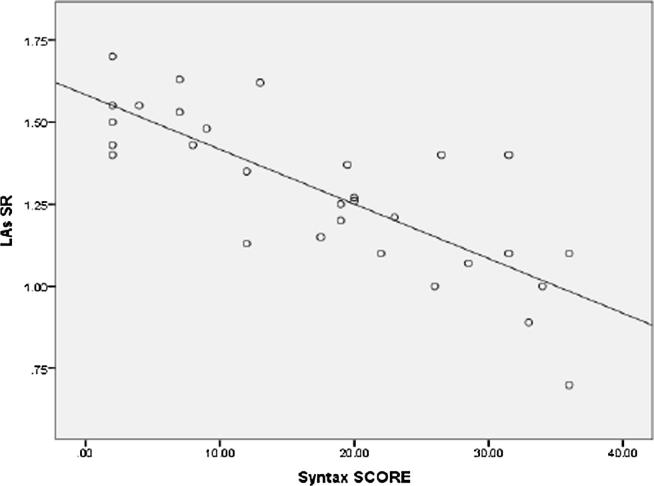
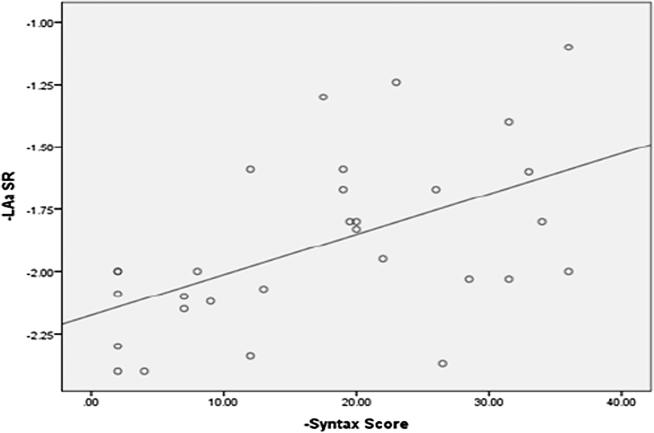
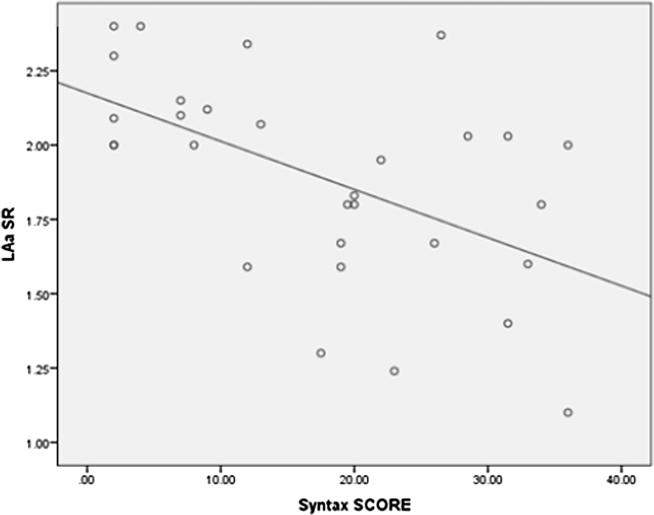
LAs SR was highly significantly lower in high syntax group (mild syntax, moderate syntax and high syntax groups were 1.41 ± 0.17 s−1, 1.15 ± 0.18 s−1 and 0.93 ± 0.21 s−1 respectively (P Value 0.001)). A highly significant negative correlation was found between Syntax score and LAs SR (r = −0.861, p < 0.001) (Fig. 4). LAe SR was significantly lower in high syntax group (mild syntax, moderate syntax and high syntax groups were – 1.21 ± 0.22 s−1, – 1.06 ± 0.18 s−1 and – 0.71 ± 0.22 s−1 respectively (P Value 0.003)). A significant negative correlation was found between Syntax score and LAs SR (P value 0.004) (Fig. 5). LAa SR was significantly lower in high syntax group (mild syntax, moderate syntax and high syntax groups were – 1.98 ± 0.30 s−1, – 1.79 ± 0.37 s−1 and – 1.63 ± 0.47 s−1 respectively (P Value 0.031)). A significant negative correlation was found between Syntax score and LAa SR (P value 0.002) (Fig. 6).
Fig. 4.
Scatter plot showing a Negative correlation between syntax score and LAs SR.
Fig. 5.
Scatter plot showing a Negative correlation between syntax score and LAs SR correlation between syntax score and LAe SR.
Fig. 6.
Scatter plot showing a Negative correlation between syntax score and LAa SR.
The results of LA deformation analysis in relation to the severity of CAD assessed by the number of vessels with significant lesions are shown in Table 3. The left atrial deformation parameters (PALS, PACS, LAs SR, and LAa SR) were significantly lower with increasing the number of coronary arteries affected. LAe SR was also lower in patients with three vessel disease but the differences didn’t reach statistical significance.
Table 3.
Comparison between Echocardiographic parameters and Number of vessels affected.
| Echocardiographic parameters | Number of vessels |
t-test | p-value | ||
|---|---|---|---|---|---|
| One (N = 13) | Two (N = 10) | Three (N = 7) | |||
| PALS (%) | 31.57 ± 4.70 | 26.08 ± 2.49 | 21.12 ± 3.29 | 18.151 | <0.001** |
| PACS (%) | 14.92 ± 4.43 | 11.35 ± 1.81 | 8.74 ± 2.05 | 8.673 | <0.001** |
| LAs SR (s−1) | 1.44 ± 0.16 | 1.27 ± 0.20 | 1.05 ± 0.22 | 9.929 | <0.001** |
| LAe SR (s−1) | −1.20 ± 0.20 | −1.13 ± 0.25 | −0.95 ± 0.31 | 2.452 | 0.105 |
| LAa SR (s−1) | −2.04 ± 0.31 | −1.90 ± 0.25 | −1.60 ± 0.37 | 4.828 | 0.016* |
| LVDd (mm) | 49.08 ± 2.90 | 49.10 ± 6.28 | 49.71 ± 2.43 | 0.058 | 0.944 |
| LVDs (mm) | 33.31 ± 5.17 | 33.20 ± 4.44 | 32.71 ± 3.04 | 0.041 | 0.960 |
| IVSd (mm) | 12.85 ± 2.23 | 12.00 ± 2.58 | 14.00 ± 2.24 | 1.486 | 0.244 |
| LVPWd (mm) | 13.62 ± 1.76 | 13.00 ± 2.58 | 13.86 ± 2.27 | 0.373 | 0.692 |
| EF% | 62.00 ± 6.28 | 63.10 ± 4.75 | 60.29 ± 5.31 | 0.521 | 0.600 |
| AP LA diameter (mm) | 32.69 ± 3.40 | 34.6 ± 7.04 | 39.14 ± 2.85 | 4.049 | 0.029* |
| LA volume (ml) | 43.92 ± 6.17 | 43.60 ± 12.10 | 54.14 ± 12.77 | 2.826 | 0.047* |
| E/A | 0.97 ± 0.18 | 1.70 ± 2.17 | 0.90 ± 0.23 | 1.178 | 0.323 |
| E/e' | 5.7 ± 1.84 | 5.96 ± 2.25 | 6.07 ± 3.29 | 0.033 | 0.968 |
Data represented as Mean ± SD; t-Independent Sample t-test.
Abbreviations: PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; EF, ejection fraction, LAs SR; Peak atrial longitudinal strain rate during ventricular systole, LAe SR; Peak atrial longitudinal strain rate during ventricular early diastole, LAa SR;Peak atrial longitudinal strain rate during ventricular late diastole LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVPWd, Left ventricular posterior wall end diastole, IVSd, Interventricular septal end diastole.
p-value < 0.05 significant.
p-value < 0.001 highly significant.
The results of LA deformation analysis in relation to the distribution pattern of involved coronary arteries are shown in Table 4. Among the patients with exclusively LAD disease and those with exclusively LCX or RCA disease, the left atrial longitudinal strain in LAD group (PALS), was significantly lower than LCX or RCA group. Also LAs SR, and LAe SR were lower in the LAD group but the differences didn’t reach statistical significance. By contrast, LAa SR was significantly higher in the LAD group. Also PACS was higher in the LAD group but the differences didn’t reach statistical significance. Among the patients with proximal LCX or RCA disease the Left ventricular posterior wall thickness was significantly decreased. (P value 0.045).
Table 4.
Comparison between Echocardiographic parameters and distribution pattern of CAD.
| LAD (N = 12) | Proximal LCX or RCA (N = 5) | p-value | |
|---|---|---|---|
| PALS (%) | 24.57 ± 2.84 | 30.27 ± 5.06 | 0.005 |
| PACS (%) | 13.27 ± 2.50 | 11.33 ± 1.98 | 0.091 |
| LAs SR (s−1) | 1.24 ± 0.20 | 1.33 ± 0.23 | 0.383 |
| LAe SR (s−1) | −1.12 ± 0.26 | −1.08 ± 0.07 | 0.713 |
| LAa SR (s−1) | −1.98 ± 0.25 | −1.71 ± 0.22 | 0.046 |
| LVDd (mm) | 49.60 ± 4.53 | 48.60 ± 3.58 | 0.661 |
| LVDs (mm) | 33.27 ± 5.18 | 33.60 ± 2.88 | 0.894 |
| IVSd (mm) | 12.93 ± 2.25 | 11.80 ± 1.92 | 0.328 |
| LVPWd (mm) | 13.87 ± 2.10 | 11.80 ± 1.79 | 0.045 |
| EF (%) | 61.13 ± 5.72 | 65.00 ± 5.57 | 0.204 |
| AP LA diameter (mm) | 36.40 ± 5.90 | 25.38 ± 13.00 | 0.016 |
| LA volume (ml) | 47.93 ± 12.06 | 42.00 ± 7.62 | 0.320 |
| E/A | 0.98 ± 0.27 | 1.03 ± 0.24 | 0.687 |
| E/e | 6.30 ± 1.32 | 5.59 ± 1.87 | 0.354 |
Data represented as Mean ± SD; t-Independent Sample t-test. *p-value < 0.05 significant; **p-value < 0.001 highly significant.
Abbreviations: PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; EF, ejection fraction, LAs SR; Peak atrial longitudinal strain rate during ventricular systole, LAe SR; Peak atrial longitudinal strain rate during ventricular early diastole, LAa SR; Peak atrial longitudinal strain rate during ventricular late diastole LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVPWd, Left ventricular posterior wall end diastole, IVSd, Interventricular septal end diastole.
5. Discussion
The understanding of cardiovascular system hemodynamics has traditionally focused on left ventricular structure and function with the left atrium being viewed simply as a passive transport chamber that empties into the left ventricle. Over the last decade there has been increasing recognition of the importance of left atrial (LA) structure and function as markers of adverse cardiovascular outcomes and cerebrovascular accidents such as AF, HTN, IHD, valvular heart disease, heart failure, and cardiomyopathy. So an early detection of LA dysfunction provides new insight into pathophysiology and management of those disorders.15
We evaluated left atrial functions by left atrial deformation analysis using 2D Speckle tracking Doppler Echocardiography among CAD patients with preserved LV function and investigated the value of left atrial strain and strain rate as a predictor of severity of coronary artery disease calculated with Syntax score following coronary angiography.
5.1. The main findings of this study were:
-
▪
Left atrial strain and strain rate parameters had a strong negative correlation with the severity of CAD and high syntax score.
-
▪
LA volume and diameter had a strong positive correlation with the severity of CAD and high syntax score.
Several studies cited in the literature had demonstrated that LA dysfunction appears early in coronary artery disease (CAD), irrespective of previous myocardial infarction, co-existing systolic dysfunction, severity of diastolic dysfunction or LA dimensions. Liu et al.16 studied left atrial function in 34 patients with CAD by two-dimensional strain echocardiographic imaging. They found that LA reservoir strain and strain rate (LAs S/SR) and passive conduit functions are impaired in patients with CAD even in the absence of LA enlargement. However they used 12 segments model for the analysis of LA strain using four and two chamber apical views which might overestimate the global S and SR parameters and is less accurate than the 15-segments model using 4, 2, and 3 chamber views used in our study.17 Another difference from our study is that they included patients with LV systolic dysfunction and DM which were strong risk factors for LA dysfunction.
Another study was performed by Yan et al. concerning the relationship between CAD, and left atrial deformation parameters, the authors detected a significant decrease in atrial deformation parameters measured by velocity vector imaging in patients with stable angina pectoris without significant further decrease with increasing severity of coronary stenosis.18
Our study showed a significant decrease in the LA strain and strain rate in CAD patients with normal LVEF. Also LA strain and strain rate had been decreased even in patients with normal LA dimensions and normal LV diastolic function. Moreover In our study the severity of the lesion on coronary angiography was determined by calculating the syntax Score and the patients were classified accordingly. We found significant further decrease in atrial deformation parameters with increasing severity of coronary artery stenosis and strong inverse correlation between Syntax score level and LA strain and strain rate parameters. These findings demonstrate that the evaluation of left atrial functions in patients with SCAD may be a potentially useful method, and in the future it may be considered as a predictor of severity of CAD.
Several studies have demonstrated an association between IHD and LA dilatation as a consequence to LV diastolic dysfunction. Tsang et al.19 analyzed a selected population of 1160 persons aged 65 years or older in sinus rhythm without significant valvular disease. They found that atrial volume was independent predictor of revascularization and MI. Similarly, in an even larger general population (45 years, n 2042) Pritchett et al. demonstrated that LA dimension and volume were associated with a history of CAD and MI.20 In our study, we found that LA volume and diameter had a strong positive correlation with the severity of CAD and high syntax score. These results are consistent with Nawathe et al.21 finding that LA Size correlates with the severity of the myocardial perfusion study defect.
Left ventricular diastolic dysfunction is one of the early findings of CAD. Deterioration of left ventricular diastolic function increases left ventricular filling pressure which may worsen left atrial deformation parameters. In our study although all patients had a significant decrease in LA atrial deformation parameters, none of them was found to have definitely elevated LV filling pressure (E/E′ ratio >15). These results are consistent with Yan et al.18 finding as they found that despite LA dysfunction, neither of their patients had E/E’ ratio >15 nor NT-pro BNP more than 200 pg/ml. These findings may minimize the effect of elevated LV filling pressure on atrial function and raise the possibility that the cause may be immune-inflammatory and neuro-hormonal responses due to myocardial ischemia according to Kosmala et al and his colleagues.22
The LA is perfused primarily by branches arising from the proximal left circumflex coronary artery with smaller contributions from the right coronary artery. LA contribution to left ventricular filling during ischemic insult in patients with LCX disease and in patients with LAD disease are different.23 Stefanadis et al.23 compared left atrial function in 32 patients with LAD and LCX coronary artery stenosis at rest and immediately after pacing induced tachycardia or during coronary occlusion. In patients with LAD stenosis, LA contraction was enhanced as a response to increased afterload imposed by the stiff left ventricle. In contrast in patients with proximal LCX disease left atrial ischemia may lead to LA dysfunction. Also Yan et al.18 measured left atrial longitudinal deformation using velocity vector imaging in 60 patients with CAD and 25 controls. They found that left atrial longitudinal strain rate during atrial contraction was increased in patients with LAD stenosis due to enhanced LA booster pump action compensated for the diminution of LV stroke work.
Similarly we compared left atrial deformation parameters between patients with exclusively LAD and exclusively proximal LCX or RCA coronary artery stenosis. We found that left atrial longitudinal strain rate during atrial contraction (LAs SR) which represents the LA booster pump function was increased in LAD patients despite decrease in the left atrial longitudinal strain (PALS) which represents the LA reservoir function. Also peak atrial contraction strain (PACS) was higher in LAD patients but did not reach significant difference making LAs SR is the most accurate index for evaluating LA booster pump function and these findings are consistent with Imanishi et al.24 findings.
The present study raises the possibility that LA reservoir dysfunction, which reflects LA diastolic dysfunction may occur before LA pump dysfunction and LV diastolic dysfunction in patients with SCAD.
6. Conclusion
Left atrial deformation analysis by 2D Speckle tracking Doppler Echocardiography may predict the severity of coronary artery disease as Left atrial strain and strain rate parameters had a strong negative correlation with the severity of CAD and high syntax score. In addition LA reservoir dysfunction, which reflects LA diastolic dysfunction may occur before LA pump dysfunction and LV diastolic dysfunction in patients with SCAD and evaluation of LA reservoir function by 2D Speckle tracking Doppler Echocardiography can be more sensitive than other atrial phasic functions for estimating severity of coronary stenosis in patients with SCAD.
7. Limitations
The current study has the following limitations; the small number of patients included in the study which may preclude the generalization of the results; The speckle tracking values for the left atrial function assessments are not well standardized like that of the LV (awaiting dedicated software for left atrial deformation analysis); And the lack of head to head comparison with other atrial phasic function assessment modalities.
Conflict of interest
No any COI with any of the Co-Authors (Ahmed Ibrahim Nassar, Ahmed Ahmed Fouad, Ali A. Ramzy, and Mustafa Fayed Fadle Abd Allah).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.World Health Organization: the atlas of heart disease and stroke. Available at <http://www.who.int/cardiovascular_diseases/resources/atlas/en/> [accessed June 10, 2014].
- 2.Bonow R.O., Bacharach S.L., Green M.V. Impaired left ventricular diastolic filling in patients with coronary artery disease: assessment with radionuclide angiography. Circulation. 1981;64:315–323. doi: 10.1161/01.cir.64.2.315. [DOI] [PubMed] [Google Scholar]
- 3.Reduto L.A., Wickemayer W.J., Young J.B. Left ventricular diastolic performance at rest and during exercise in patients with coronary artery disease. Circulation. 1981;63:1118–1137. doi: 10.1161/01.cir.63.6.1228. [DOI] [PubMed] [Google Scholar]
- 4.Labovitz A.L., Pearson A.C. Evaluation of left ventricular diastolic function: clinical relevance and recent Doppler echocardiographic insights. Am Heart J. 1987:1143836–1143851. doi: 10.1016/0002-8703(87)90795-2. [DOI] [PubMed] [Google Scholar]
- 5.Barbier P., Solomon S., Schiller N. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100(4):427–436. doi: 10.1161/01.cir.100.4.427. [DOI] [PubMed] [Google Scholar]
- 6.Leitman M., Lysyansky P., Sidenko S. Two-dimensional strain a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17(10):1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Vianna-Pinton R., Moreno C., Baxter C. Twodimensional speckle-tracking echocardiography of the left atrium. Feasibility andregional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Task Force Members. Montalescot G., Sechtem U. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of table coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 9.Sianos G., Morel M., Kappetein A. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227. [PubMed] [Google Scholar]
- 10.SHe J., Gao Y., Yu X. Syntax score predicts clinical outcome in patients with three-vessel coronary artery disease undergoing percutaneous coronary intervention. Chin Med J. 2011;124(5):704–709. [PubMed] [Google Scholar]
- 11.Brito J., Teles R., Almeida M. Predictive value of SYNTAX score in risk stratification of patients undergoing left main coronary artery angioplasty (abstr) Eurointervention. 2010;6:H69. [PubMed] [Google Scholar]
- 12.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. 1. [DOI] [PubMed] [Google Scholar]
- 13.Nagueh S.F., Smiseth O.A., Appleton C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Scanlon P.J., Faxon D.P., Audet A.M. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. Circulation. 1999;99:2345e57. doi: 10.1161/01.cir.99.17.2345. [DOI] [PubMed] [Google Scholar]
- 15.Hoit Brian D. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63(6):493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y.Y., Xie M.X., Xu J.F. Evaluation of left atrial function in patients with coronary artery disease by two-dimensional strain and strain rate imaging. Echocardiography. 2011;28(10):1095–1103. doi: 10.1111/j.1540-8175.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- 17.Rimbaş C., Roxana C., Raluca E.D. Methodological gaps in left atrial function assessment by 2D speckle tracking echocardiography. Arquivos brasileiros de Cardiologia. 2015;105(6):625–636. doi: 10.5935/abc.20150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan P., Sun B., Shi H. Left atrial and right atrial deformation in patients with coronary artery disease: a velocity vector imaging-based study. Plos One. 2012;7 doi: 10.1371/journal.pone.0051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang T.S., Barnes M.E., Gersh B.J. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–1205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 20.Pritchett Allison M. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45(1):87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 21.Nawathe A., Ariyarajah V., Apiyasawat S. Correlation of echocardiographic left atrial abnormality with myocardial ischemia during myocardial perfusion assessment in the presence of known left ventricular hypertrophy. Am J Cardiol. 2013;112(3):416–419. doi: 10.1016/j.amjcard.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Kosmala W., Derzhko R., Przewlocka-Kosmala M. Plasma levels of tnf-alpha, il-6, and il-10 and their relationship with left ventricular diastolic function in patients with stable angina pectoris and preserved left ventricular systolic performance. Coron Artery Dis. 2008;19:375–382. doi: 10.1097/MCA.0b013e3282fc617c. [DOI] [PubMed] [Google Scholar]
- 23.Stefanadis C., Dernellis J., Tsiamis E. Effects of pacing-induced and balloon coronary occlusion ischemia on left atrial function in patients with coronary artery disease. J Am Coll Cardiol. 1999;33(3):687–696. doi: 10.1016/s0735-1097(98)00623-8. [DOI] [PubMed] [Google Scholar]
- 24.Imanishi J., Tanaka H., Sawa T. Association of left atrial booster pump function with heart failure symptoms in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Echocardiography. 2015;32(5):758–767. doi: 10.1111/echo.12733. [DOI] [PubMed] [Google Scholar]