Graphical abstract
Keywords: Unani medicine, Urolithiasis, Piper cubeba, Cystone, CaOx crystals
Abstract
Background
To investigate the antilithiatic effect of hydroalcoholic extract of Kabab Chini (Piper cubeba L.) fruit in male Sprague Dawley rats.
Methods
Rats were divided into six groups of six each. Group I received regular rat food and drinking water ad libitum. Groups II to VI were administered with ethylene glycol (EG) 0.75% (V/V) and ammonium chloride (AC) 1% (W/V) in drinking water for 7 days to induce urolithiasis. From 8th day Group I received 1 mL of 5% gum acacia. Group IV was treated with Cystone; V and VI groups with the hydro-alcoholic extract of Piper cubeba L. Treatment was continued for further 14 days, thereafter animals sacrificed. While Group II animals were sacrificed just after 7 days treatment with EG and AC. Group III was left untreated until 14 days and sacrificed on 22nd day. Crystalluria was analyzed on 8th and 22nd day while, urinary calcium, phosphorus, creatinine, sodium and magnesium on 22nd day. Biochemistry and histopathological studies of kidney were also carried out.
Results
Test groups showed significant reduction (p < 0.001) of crystals in urine. Serum creatinine and urea (p < 0.01) were also decreased significantly. Urine analysis showed significant increase in magnesium while calcium, sodium, chloride and phosphorus significantly decreased along with histopathological improvement in kidney tissue in treated groups.
Conclusion
From the above results it can be concluded that hydroalcoholic extract of P. cubeba L. fruit has significant inhibitory effect in calcium oxalate urolithiasis.
1. Introduction
Stone formation takes place in approximately 12% of the population with a recurrence rate of 70–80% in males and 47–60% in females.1 Northern India lies in the high occurrence of kidney stone area in the world.2 Its global increasing incidence and wide prevalence is a concern to the medical world which usually starts with obstruction and if left untreated results in severe complications like multiple infections and hemorrhage which requires the best medical care.3 Non steroidal anti-inflammatory drugs, calcium channel blockers, corticosteroids, and alpha adrenergic antagonists are used for the easy passage of stone but most of them lack scientific evidence to prove their efficacy. Endoscopic stone removal and extracorporeal shock wave lithotripsy (ESWL) have modernized the treatment of urolithiasis but do not check the possibility of new stone formation. These current treatment procedures are prohibitively expensive for the common man as well as with these procedures recurrence is quite common, and the patient has to be subjected to careful follow up for many years.4 Hence, the search for antilithiatic drugs from natural sources that are both effective and devoid of side effects has gained immense potential. Chronic use of synthetic drugs, which are not free from side effects, has motivated humans for alternative, safe, effective and potent remedies from natural sources.5
In Unani system of medicine several single and compound drugs have been used for the management of urolithiasis. Some of the drugs have been evaluated experimentally for their antiurolithiatic activity viz., Adiantum capillus veneris L.6, Peucedanum grande C.B. Clarke.7 In Unani medicine Piper cubeba L. is used for the treatment of renal disorders and other associated pathological conditions. P. cubeba is also used as an important ingredient in many formulations viz., Jawarish Zarooni, Majoon-e-Antaki, Laboob-e-Sagheer, Sufoof-e-Shora Murakkab which in turn are used in the treatment of cystitis, retention of urine, gonorrhea, and wounds in urinary tract.8 These plant derived drugs exert their antilithogenic properties by changing the ionic composition of urine for example decreasing the calcium ion concentration or increasing magnesium and citrate excretion. Many of them showed diuretic activity or contained saponin that act on mucoproteins (promoter of the crystallization process) and disaggregate them.4
P. cubeba L. fruit (Piperaceae) is widely prescribed for the treatment of gravel and stones in kidney and urinary bladder.9 Fruits and its oil are used therapeutically in various forms viz., decoction, powder, paste etc., to relieve a number of ailments. It is used in retention of urine, incontinence of urine, gonorrhea, and in healing of ulcer and wounds in the urinary tract.10,11 Some of the studies carried out on P. cubeba L. fruit include nephroprotective,12 antiulcer13 and antimicrobial14 activities. Phytochemical screening of P. cubeba L.15 showed the presence of alkaloid, glycosides, steroid, flavonoids, tannins, anthraquinones and phenols. HPLC analysis of P. cubeba L. showed presence of 0.0024% cubebin.16 The HPTLC for analysis of piperine in fruits of Piper species17 found that it contains piperine 11.19% and alkaloid cubebin which possess anti-inflammatory, antimicrobial, analgesic and antioxidant activities.16,18 There seems to be no report on its antilithiatic activity, therefore the present study was undertaken to evaluate the antilithiatic activity of P. cubeba L. fruit on calcium oxalate urolithiasis, induced by ethylene glycol and ammonium chloride in male Sprague Dawley (SD) rats.
2. Methods
2.1. Animals
The study was conducted on healthy male SD rats weighing between 200 and 250 g. The animals were obtained from registered breeders and were allowed to acclimatize for a week before starting the experiment. They were maintained under standard laboratory conditions and were provided with standard rat diet and water ad libitum. They were housed in clean polypropylene cages at room temperature 25 ± 2°C, humidity at 45–55% with 12 h light and 12 h dark cycle. The animal husbandry procedures and experimental protocol were in accord with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Before beginning the experiment ethical clearance was taken (reg. no. IAEC/IX/05/IA) from Institutional Animal Ethics Committee (IAEC).
2.2. Plant material and preparation of extract
The fruits of P. cubeba L. were collected from pharmacy of National Institute of Unani Medicine (NIUM), Bangalore, Karnataka. The drug was identified by a Botanist and the voucher specimen was deposited in the herbarium of NIUM, Bangalore (ref. no. 15/IA/Res/2014). The fruits were kept in drying chamber at 40°C for about 30 minutes to dry moisture on its surface and coarsely powdered in an electrical grinder, 100 g of which was extracted (in 50% distilled water and 50% ethanol) for 6 hours in Soxhlet's apparatus at 80°C. Extract was filtered by filter paper (Whatman no. 40) and then evaporated on water bath at 60°C till it dried completely.6,7,19 The yield of hydroalcoholic extract was found to be 10.05% w/w.
2.3. Dosage and drug administration
The human therapeutic dose of P. cubeba L. is 3 g20 as mentioned in the Unani classical literature. The dose for the rat was calculated by conversion factor of 7,21 and was found to be 350 mg/kg. The dose of the extract was determined with reference to yield % of extract with the dose of crude drug and was found to be 35 mg/kg. Further the second dose was calculated by the method of Miller and Tainter (1944)22 and was found to be 60 mg/kg. The doses of the extract calculated for rats were found to be extremely less than the safe dose i.e.1000 mg/kg.23 The extract was used orally, dissolved in 1 mL of 5% gum acacia. Each dose was prepared freshly before administration.
2.4. Ethylene glycol and ammonium chloride induced urolithiasis
The study was carried out by the method of Ahmed et al (2013) 6 and Kumar et al (2016)7 with some modification in the treatment schedule. All the animals were weighed and divided into six groups of six animals each. Group I served as plain control and received 1 mL of 5% gum acacia orally throughout the study period. While the animals of groups II, III, IV, V and VI were treated with ethylene glycol 0.75% (V/V) and ammonium chloride 1% (W/V) by adding in their drinking water for 7 days to induce urolithiasis. The animals of group II served as disease control A and were sacrificed just after 7 days of administration of ethylene glycol and ammonium chloride while the animals in group III served as Disease control B and were left untreated after 7 days administration of ethylene glycol and ammonium chloride. However, from 8th to 21st day, the animals of group IV were treated with Cystone® (750 mg/kg)24 (suspended in 1 mL of 5% gum acacia) and served as Standard control. While the animals of V and VI groups were treated with the hydro-alcoholic extract of P. cubeba L. in the dose of 35 mg/kg and 60 mg/kg and served as test groups A and B respectively. All the animals were sacrificed on the 22nd day.
2.5. Urine analysis
Urine analysis was done on the 8th day after administration of ethylene glycol and ammonium chloride and on the 22nd day after treatment with the test drug, by placing the animals individually in metabolic cages with water ad libitum for 3 hours to collect fresh urine samples. Urine was analyzed for crystalluria; for this, 10 µL of urine was placed in the slide and covered with a cover slip. The slide was kept undisturbed for about 30 minutes to get fixed and dried, and then it was visualized under light microscope (400×) for counting the number of calcium oxalate crystals.6,7,25 On the 22nd day, in addition to crystalluria examination, urine was also analyzed for calcium, phosphorus, creatinine, sodium, chloride and magnesium. For analysis, 1 mL of urine was taken in centrifugal tube and centrifuged at 2500 rpm for 5 minutes; the supernatant was estimated for the above mentioned constituents by Star 21 plus Auto analyzer.26
2.6. Serum analysis
After collection of urine, rats were sacrificed under thiopentone anesthesia (50 mg/kg IP). Blood samples were collected by cardiac puncture. The serum was separated (REM R8C Laboratory Centrifuge), centrifuged at 10,000 rpm for 10 minutes. The supernatant was analyzed for calcium, phosphorus, urea and creatinine levels by using auto analyzer and specific kits.6
2.7. Kidney homogenate analysis
After collection of blood both the kidneys were identified and carefully dissected. Isolated right kidneys trimmed off from extraneous tissue and preserved in specimen container containing 10% formalin and sent for histopathological examination. While left kidneys of all rats of each group were used for homogenate analysis. The kidney was dried at 80°C in a hot air oven. A sample of 100 mg of the dried kidney was boiled in 10 mL of 1N hydrochloric acid for 30 minutes and homogenised (IKA T10 basic Ultra-Turrax tissue homogenizer). The homogenate was centrifuged at 2000 rpm for 10 minutes. The supernatant was separated. Calcium and phosphorus content in the kidney homogenate were determined.27
2.8. Statistical analysis
The data were analyzed using Graph Pad software. The results among the groups were analyzed by one-way ANOVA post-test with Tukey's Kramer Multiple Comparisons test. Results were expressed as mean ± SEM. Statistical difference was considered significant at p < 0.05.
3. Results
3.1. Urine analysis
After administration of ethylene glycol (0.75%) and ammonium chloride (1%) for 7 days, significantly increased (p < 0.001) calcium oxalate crystals in urine of each group of animals was observed when compared to Plain control. On administration of hydroalcoholic extract of P. cubeba L. at 35 mg/kg and 60 mg/kg for 14 days, significant (p < 0.001) reduction of calcium oxalate crystals in urine were noted when compared to disease control A and B. Similar results were showed by cystone also. The animals in disease control B left untreated after 7 days administration of lithogenic agents and sacrificed on the 22nd day to see any auto healing effect. The result showed no significant reduction in urinary crystals when compared to disease control A (Table 1). In ethylene glycol (EG) and ammonium chloride (AC) treated groups, calcium (p < 0.001), phosphorus (p < 0.001) and sodium (p < 0.01) were found to be increased significantly in urine. Cystone reduced the levels of phosphorus (p < 0.05) and sodium (p < 0.01) while the levels of magnesium (p < 0.01) and creatinine (p < 0.05) increased significantly in urine when compared to disease controls. The test extract at both the doses significantly (p < 0.001) reduced calcium, when compared to disease control A and B, but no effect was observed on phosphorous level. On the contrary the level of sodium was found to be reduced significantly (p < 0.01, p < 0.05) at both low and high doses respectively when compared to disease control A. While magnesium level increased significantly (p < 0.01, p < 0.05) when compared to disease control A and B respectively in rats treated with higher dose. No significant effect was observed in chloride excretion in urine however it was slightly reduced on treatment with P. cubeba extract at both the doses. Creatinine level also reached up to the normal level in urine but was statistically not significant. Again in disease control B animals on 22nd day, no significant alteration in any of the parameters was found when compared to disease control A (Table 2).
Table 1.
Effect of Piper cubeba L. on urine calcium oxalate crystals
| Groups | Treatment | No. of CaOx crystals | |
|---|---|---|---|
| 8th day 22nd day | |||
| Plain control | 1 mL of 5% GA | 7.5 ± 0.88 | 6.66 ± 0.66 |
| Disease control A | EG 0.75% and AC1% | 131.7 ± 10.93a, *** | – |
| Disease control B | EG 0.75% and AC1% | 130.8 ± 10.83a, *** | 92.50 ± 8.24a, *** |
| Standard control | EG 0.75% and AC1% + cystone 750 mg/kg | 132.7 ± 16.98a, *** | 8.33 ± 1.87b, ***, c, *** |
| Test group A | EG 0.75% and AC1% + Piper cubeba L.35 mg/kg | 122.5 ± 12.83a, *** | 7.83 ± 0.83b, ***, c, *** |
| Test group B | EG 0.75% and AC1% + Piper cubeba L.60 mg/kg | 113.3 ± 13.82a, *** | 7.33 ± 0.95b, ***, c, *** |
EG, ethylene glycol; AC, ammonium chloride; GA, gum acacia.
Values expressed as mean ± SEM.
n = 6 animals in each group.
Test used one-way ANOVA followed by Tukey–Kramer Multiple Comparisons test.
Disease control A, B; standard control; test group A, B vs plain control on 7th day.
Standard control; test group A, B vs disease control A on 22nd day.
Standard control; test group A, B vs disease control B on 22nd day.
p < 0.001.
Table 2.
Effect of Piper cubeba L. on urinary parameters in rats
| Groups | Treatment | Ca (mg/dl) | Creatinine (mg/dl) | P (mg/dl) | Na (mEq/dl) | Cl (mg/dl) | Mg (mg/dl) |
|---|---|---|---|---|---|---|---|
| Plain control | 1 mL of 5% GA | 1.57 ± 0.17 | 0.88 ± 0.221 | 63.28 ± 1.53 | 141.9 ± 10.19 | 131.6 ± 14.84 | 1.50 ± 0.28 |
| Disease control A | EG 0.75% and AC1% | 5.81 ± 0.97a, *** | 0.10 ± 0.138a, * | 73.47 ± 0.86a, *** | 210.2 ± 21.05a, ** | 146.1 ± 6.66 | 0.37 ± 0.12a, ** |
| Disease control B | EG 0.75% and AC1% | 8.07 ± 0.21a, *** | 0.17 ± 0.15a, * | 70.88 ± 0.32a, *** | 182.8 ± 10.89 | 147.7 ± 17.17 | 0.45 ± 0.10a, ** |
| Standard control | EG 0.75% and AC1% + cystone 750 mg/kg | 1.82 ± 0.25b, ***, c, *** | 0.82 ± 0.03b, *, c, * | 69.71 ± 0.43b, * | 142.4 ± 9.36b, ** | 133.8 ± 6.73 | 1.42 ± 0.12b, **, c, * |
| Test group A | EG 0.75% and AC1% + Piper cubebaL. 35 mg/kg | 2.09 ± 0.23b, ***, c, *** | 0.71 ± 0.11 | 70.31 ± 0.03 | 146.1 ± 4.00b, * | 144.6 ± 5.10 | 0.9587 ± 0.11 |
| Test group B | EG 0.75% and AC1% + Piper cubebaL. 60 mg/kg | 1.83 ± 0.08b, ***, c, *** | 0.73 ± 0.07 | 70.80 ± 0.04 | 143.4 ± 2.11b, ** | 136.6 ± 11.20 | 1.37 ± 0.17b, **, c, * |
EG, ethylene glycol; AC, ammonium chloride; GA, gum acacia.
Values expressed as mean ± SEM.
n = 6 animals in each group.
Test used one-way ANOVA followed by Tukey–Kramer Multiple Comparisons test.
Disease control A, B vs plain control.
Standard control; test group A, B vs disease control A.
Standard control; test group A, B vs disease control B.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Serum analysis
On administration of EG and AC for 7 days it was observed that serum urea and calcium (p < 0.01) in some groups while creatinine (p < 0.01) in all the groups significantly increased when compared to plain control. On treatment with P. cubeba extract for 14 days at both the doses (35 mg/kg and 60 mg/kg bw); serum creatinine was found to be significantly (p < 0.01) reduced when compared to disease control ‘A’, same effect was observed by cystone also. Low dose of test extract significantly (p < 0.01) reduced serum urea level, but slightly lesser reduction (p < 0.5) was observed at higher dose of test extract when compared to disease control ‘B’. Serum calcium increased significantly (p < 0.01) when compared to plain control but after treatment with the test extract at both lower and higher dose it decreased though it was not statistically significant. While cystone showed significant reduction (p < 0.05) when compared to disease control ‘A’. No significant elevation was observed in serum phosphorus level after 7 days administration of lithogenic agent, but the values were found to be nearly same as in plain control after treatment with the test and standard drugs (Table 3).
Table 3.
Effect of Piper cubeba L. on Serum Parameters (mg/dl) in Rats
| Groups | Treatment | Calcium | Creatinine | Urea | Phosphorus |
|---|---|---|---|---|---|
| Plain control | 1 mL of 5% GA | 8.05 ± 0.42 | 1.31 ± 0.129 | 40.26 ± 3.51 | 2.14 ± 0.32 |
| Disease control A | EG 0.75% and AC1% | 10.40 ± 0.65a, ** | 3.05 ± 0.48a, ** | 53.65 ± 5.35 | 3.42 ± 0.45 |
| Disease control B | EG 0.75% and AC1% | 9.06 ± 0.89b, * | 3.02 ± 0.60a, ** | 72.15 ± 9.83a, ** | 3.18 ± 0.36 |
| Standard control | EG 0.75% and AC1% + cystone 750 mg/kg | 8.35 ± 0.49b, * | 1.39 ± 0.05b, **, c, ** | 42.80 ± 4.35c, ** | 2.41 ± 0.31 |
| Test group A | EG 0.75% and AC1% + Piper cubeba L. 35 mg/kg | 8.62 ± 0.33 | 1.41 ± 0.21b, **, c, ** | 45.99 ± 3.89c, * | 2.77 ± 0.49 |
| Test group B | EG 0.75% and AC1% + Piper cubeba L. 60 mg/kg | 8.51 ± 0.32 | 1.40 ± 0.06b, **, c, ** | 43.02 ± 2.92c, ** | 2.48 ± 0.19 |
EG, ethylene glycol; AC, ammonium chloride; GA, gum acacia.
Values expressed as mean ± SEM.
n = 6 animals in each group.
Test used one-way ANOVA followed by Tukey–Kramer Multiple Comparisons test.
Disease control A, B vs plain control.
Standard control; test group A, B vs disease control A.
Standard control; test group A, B vs disease control B.
p < 0.05.
p < 0.01.
3.3. Kidney homogenate analysis
In kidney homogenate analysis calcium and phosphorus were found to be significantly (p < 0.05) increased in the animals of disease control A. In test groups after treatment with hydroalcoholic extract of P. cubeba calcium level was significantly (p < 0.05) reduced when compared to disease control A. Phosphorus was also found to be reduced but it was not statistically significant. Cystone® did not show significant reduction in calcium but phosphorus significantly (p < 0.05) reduced when compared to disease control A. In disease control B which was left untreated after 7 days administration of lithogenic agent, slight reduction in calcium and phosphorus level was observed but it was not statistically significant (Table 4).
Table 4.
Effect of Piper cubeba L. on Kidney Homogenate Parameters (mg/100 g) in Rats
| Kidney homogenate analysis | |||
|---|---|---|---|
| Groups | Treatment | Calcium | Phosphorus |
| Plain control | 1 mL of 5% GA | 0.83 ± 0.39 | 2.22 ± 0.35 |
| Disease control A | EG 0.75% and AC1% | 3.33 ± 1.04a, * | 4.29 ± 0.29a, * |
| Disease control B | EG 0.75% and AC1% | 2.12 ± 0.58 | 3.69 ± 0.61 |
| Standard control | EG 0.75% and AC1% + cystone 750 mg/kg | 1.05 ± 0.19 | 2.33 ± 0.32b, * |
| Test group A | EG 0.75% and AC1% + Piper cubeba L. 35 mg/kg | 0.83 ± 0.23b, * | 2.66 ± 0.35 |
| Test group B | EG 0.75% and AC1% + Piper cubeba L. 60 mg/kg | 0.85 ± 0.17b, * | 2.52 ± 0.32 |
EG, ethylene glycol; AC, ammonium chloride; GA, gum acacia.
Values expressed as mean ± SEM.
n = 6 animals in each group.
Test used one-way ANOVA followed by Tukey–Kramer Multiple Comparisons test.
Disease control A vs plain control.
Standard; test group A, B vs disease control A.
p < 0.05.
3.4. Histopathology
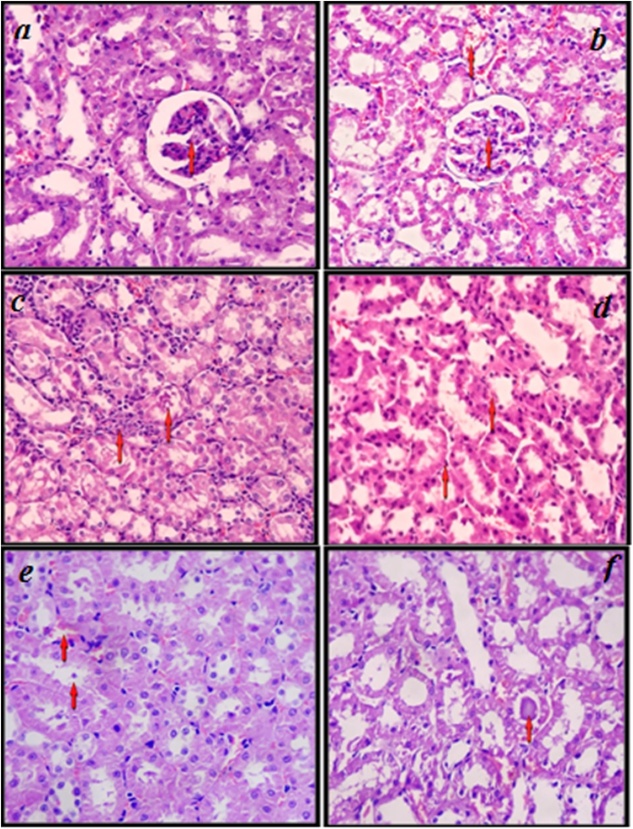
Histopathological reports revealed normal architecture of kidney tissue in plain control. In urolithiatic group the entire architecture of kidney was intact with mild increased hypercellularity in Bowman's space. Some tubules have shown irregular homogenous amorphous eosinophilic material. Dilated and congested blood vessels and mononuclear inflammatory infiltration were found in interstitium. On treatment with P. cubeba L. fruit extract much improvement in histopathological derangement was observed. Higher dose of extract showed remarkable effect as histopathological features f the kidney tissue reached up to normal level which was almost similar to plain control (Fig. 1a–f).
Fig. 1.
Microscopic images by light microscopy (400×) of kidney sections after Haematoxylin and Eosin staining from Plain control (a) showing intact architecture with glomerulus, tubules and blood vessels. Disease control (b) showing normal architecture with mild hyper cellularity in glomerulus while in disease control (c) intact architecture, mild hyper cellularity with increased mesengial cells, congested blood vessels. Standard control (d) showing normal cellularity, degenerative changes in tubular epithelial cells, dilated and congested blood vessels. Test group A (e) showing normal cellularity and mild congested blood vessels. Test group B (f) showing normal cellularity, eosinophilic material in tubules and normal blood vessels.
4. Discussion
Super saturation of urine with respect to stone forming substances is one of the essential factors in stone formation. Ethylene glycol (EG) ingestion to rats has been widely used as an experimental model for the study of nephrolithiasis. However, when EG is used alone, kidney crystal deposition can be quite inconsistent. To achieve uniformly high rate of kidney crystal deposition, ammonium chloride (AC) has been used in combination with ethylene glycol. When male Sprague Dawley (SD) rats were treated by 0.75% ethylene glycol with 1% or 2% ammonium chloride, almost all of them showed deposition of the calcium oxalate (CaOx) crystals in the kidney within seven days.28 Male rats are selected to induce urolithiasis because testosterone plays a significant role in oxalate production as increased serum testosterone level in male rats results in increased endogenous production of oxalate by liver and further ethylene glycol solutions of low concentration induces calcium oxalate nephrolithiasis in male rats does not produce similar results in females that is why male rats are more susceptible to develop CaOx stone than female rats.28,29 Therefore, in the present study male SD rats were treated with ethylene glycol (0.75% V/V) and ammonium chloride (1% SW/V) for 7 days. It has been reported that EG is oxidized to oxalic acid by non-specific dehydrogenase which leads to hyperoxalaurea key factor for urolithiasis. EG metabolizes into CaOx monohydrate and produces renal mitochondrial toxicity similar to clinical CaOx renal calculi.30
Crystalluria could occur in both healthy and stone forming individuals wherein stone formers tend to excrete larger and aggregated particles than healthy individuals.31 After 7 days of administration of lithogenic agent, large and plentiful crystals were observed in the urine of untreated animals. The similar finding was also found in various studies.28,32,33 But after treatment with P. cubeba L. fruit extract for 14 days urinary crystals were reduced significantly (p < 0.001) at both low and high dose. This effect could be helpful in preventing stone formation by excretion of small particles and reducing their chances of retention in the urinary tract.
The analysis of urine with respect to stone forming agents is an apt indicator for the risk of stone formation. Previous studies reported that EG causes hypercalciuria, hyperphosphaturia and hyperoxaluria. The increased urinary calcium is a reason for favoring the nucleation and precipitation of calcium oxalate from urine and subsequently crystal growth.34 In the present study, urinary calcium increased significantly (p < 0.001) in lithiatic animals. The test extract in both the doses and cystone showed significant reduction in urinary calcium. Reduction of calcium level in urine provides less calcium to bind with oxalate which results in reduction of calcium oxalate crystals, apparent in urinary microscopy observations. Further reduction in urinary calcium also reduces super saturation which is the major risk factor for stone formation.35 Though the serum calcium level was not significantly reduced in treated groups; it was much lesser than the disease control. On the contrary, phosphorus level remained unaffected. The reason behind this is that the phosphorus level in blood is reliant on the calcium level and activity of parathyroid hormone thus at times it is difficult to regulate the blood.
Urinary magnesium (Mg) is an important inhibitor in stone formation. Magnesium turns soluble after binding with oxalate thus decreasing oxalate availability to bind with calcium and the formation of calcium oxalate in turn decreasing super saturation.33 Low level of magnesium is observed in stone formers as well as in stone forming rats.36,37 After treatment with the test drug Mg level reached nearly upto normal. The animals treated with high dose of test extract showed significant increase (p < 0.01) in Mg level in the urine. The results evidently indicate the efficacy of test extract toward the elevation of magnesium level in urine and prevention of stone formation. On contrary, Urinary sodium was found to be significantly (p < 0.01) increased in untreated animals, after treatment with test extract and Cystone, significant reduction was noted. The result showed that P. cubeba L. fruit was comparable with standard drug in reducing super saturation by decreasing sodium level in urine.
Some inorganic substances such as sodium, calcium, magnesium and potassium38 are also found in P. cubeba L. Magnesium is the inhibitor of stone formation while sodium and calcium are promoters.35,39 Urinary magnesium increased significantly in all the groups, but no significant reductions were observed in sodium and calcium. These findings clearly demonstrate that there is a direct relationship in the ionic content of urine and phyto constituents present in the test drug.
Creatinine and urea are the indicators of kidney and tubular damage. Glomerular filtration rate (GFR) decreases in kidney tissue injury; may be due to the presence of stone in urinary tract, which obstructs urine flow and the waste products, particularly nitrogenous substances thus an increase in their level is noted.40 Urinary creatinine was found to be increased in disease control groups, may be due to decrease in GFR. Treatment with the test extract showed significant decrease in serum creatinine and urea in test groups. Improvement in GFR after the treatment with test drug may be due to reduction in inflammation and injury of kidney tissues. P. cubeba L. has been reported for anti-microbial, anti-inflammatory14 and nephroprotective activities 12 thus the efficacy of the test drug is justified by these properties.
The histological features also supported the above findings. In lithiatic animals, there were cellular derangement, hyper cellularity and injured glomerulus remarkably observed. However, on treatment with test extract, notable improvements were observed, particularly of the animals treated with high dose of test extract. Studies reported that exposure to high level of oxalate and calcium oxalate crystals causes cellular injury due to membrane lipid peroxidation through intracellular reactive oxygen species generation. Therefore, reduction in renal oxidative stress could be an effective approach in the treatment of urolithiasis. As reported in earlier studies that the antioxidant effect of flavonoid found in green tea 41 and Orthosiphon grandioxorum42 decreases oxidative injury in renal tubular cells and calcium oxalate deposition in rat kidney. The chemical constituents of P. cubeba might exert their effect through anti-oxidant activity to make expulsion of renal stone easy.32
Stones are formed due to deficit in inhibitory substances in urine and the presence of promoters. When the conditions are favorable for stone formation; the antiadherent layer of glycosaminoglycans (GAGs) acts as a protective barrier against urinary stone disease. If this layer is damaged due to consequence of bacterial attack, a stone nucleus develops leading to a full fledged stone in urinary tract. At this stage the drugs having antimicrobial property may be helpful in protecting the GAGs layer by covering the epithelium of collecting system.42 The antimicrobial property of test drug has already been reported,14,12 therefore it may be considered as one of the likely mechanism of the test drug action. Phytochemical constituents of P. cubeba L. such as cubebin, hinokinin, yatein, dihydrocubeib43 are reported to possess anti-inflammatory and analgesic activities. This further corroborates that the test drug produced its effect through diverse mechanism complementing each other.
The overall limitation of the study is that the drug extract has not been quantified for the constituents responsible for antiurolithiatic activity. Therefore, characterization and isolation of major active compound from the drug extract are required and could be analyzed for future studies.
In conclusion, based on above discussion it is concluded that the hydroalcoholic extract of P. cubeba L. fruit demonstrated significant lithotriptic effect on experimentally induced calcium oxalate urolithiasis. Present study is in consonance with the studies reporting that the presence of flavonoid and alkaloids are responsible for lithotriptic activity of herbal drugs. This investigation validates the therapeutic potential of Kabab Chini as claimed by Unani physicians. Further, studies are mandatory to know the exact mechanism of action of test extract for its litholytic effect.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
Authors are extremely thankful to Professor Abdul Wadud, HoD, Department of Ilmul Advia, NIUM for providing best possible facilities for smooth proceeding of the research and Dr. G. Sofi Reader, Dept. of Ilmul Advia, NIUM for his contribution in statistical analysis.
References
- 1.Smith CL, Guay DRP. Nephrolithiasis. In: Di Piero J.T., Talbert R.L., Hyes P.E., Yee G.C., Matzke G.R., Posey L.M., editors. Pharmacotherapy and pathophysiology. Approach. 2nd ed. Elsevier; New York: 1992. pp. 720–736. [Google Scholar]
- 2.Abbagani S, Gundimeda SD, Varre S, Ponnala D, Mundluru HP. Kidney stone disease: etiology and evaluation. Int J Appl Biol Pharmaceut Technol. 2010;1:175–182. [Google Scholar]
- 3.Baheti DG, Kadam SS. Antiurolithiatic activity of a polyherbal formulation against calcium oxalate induced urolithiasis in rats. J Adv Pharm Educ Res. 2013;3:31–41. [Google Scholar]
- 4.Chen YT. Urolithiasis update. Evaluation and management. Urol Sci. 2012;23:5–8. [Google Scholar]
- 5.Atodariya U, Barad R, Upadhyay S, Upadhyay U. Anti-urolithiatic activity of Dolichos biflorus seeds. J Pharmacogn Phytochem. 2013;2:209–213. [Google Scholar]
- 6.Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S. Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol. 2013;146:411–416. doi: 10.1016/j.jep.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Kumar BN, Wadud A, Jahan N, Sofi G, Bano H, Makbul SAA, Husain S. Antilithiatic effect of Peucedanum grande C.B. Clarke in chemically induced urolithiasis in rats. J Ethnopharmacol. 2016;194:1122–1129. doi: 10.1016/j.jep.2016.10.081. [DOI] [PubMed] [Google Scholar]
- 8.Kabeeruddin HM. 1938. Bayaze Kabeer, Vol. II. Hikmat book depot: Hyderabad Deccan; pp. 1–179. [Google Scholar]
- 9.Baitar I. vols. I and III. CCRUM; New Delhi: 2002. (Al Jami li Mufradat al Adviawal Aghzia (Urdu Translation)). p. 46–7,128. [Google Scholar]
- 10.Razi ABZ. vol. X. CRUM; New Delhi: 2001. pp. 108–125. (Al Hawi Fil Tib (Urdu translation)). [Google Scholar]
- 11.Qamri AMH. 1st ed. CCRUM; New Delhi: 2008. Ghina Mana (Urdu translation) pp. 288–295. [Google Scholar]
- 12.Ahmad QZ, Jahan N, Ahmad G, Tajuddin Nephroprotective effect of Kabab Chini (Piper cubeba) in Gentamycin induced nephrotoxicity. Saudi J Kidney Dis Transplant. 2012;23:773–781. doi: 10.4103/1319-2442.98159. [DOI] [PubMed] [Google Scholar]
- 13.Parvez M, Gayasuddin M, Basheer MK, Janakiraman Screening of Piper cubeba L. fruits for anti-ulcer activity. Int J Pharm Tech Res. 2010;2:1128–1132. [Google Scholar]
- 14.Aneja KR, Joshi R, Sharma C, Aneja A. Antimicrobial efficacy of fruit extracts of two Piper species against selected bacterial and oral fungal pathogens. Braz J Oral Sci. 2010;9:421–426. [Google Scholar]
- 15.Nanak G, Sahu RK. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J Appl Pharmaceut Sci. 2011;8:153–157. [Google Scholar]
- 16.Mulik MB, Laddha KS. Isolation, characterization and quantification of bioactive dibenzylbutyrolactone lignin cubebin from fruits of Piper cubeba L. f. Indian J Nat Prod Resour. 2015;6:189–193. [Google Scholar]
- 17.Rajupadhyaye AA, Upadhye AS, Mujumdar AM. HPTLC method for analysis of Piperine in fruits of Piper species. J Planar Chromatogr. 2011;24:57–59. [Google Scholar]
- 18.JK Pandey, Singh DK. Molluscicidal activity of Piper cubeba Linn, Piper longum Linn. and Tribulus terristeris Linn. and their combination against snail Indiplanorbis exustus Desh. Ind J Exp Biol. 2009;47:643–648. [PubMed] [Google Scholar]
- 19.Saiyed A, Jahan N, Makbul SAA, Ansari M, Bano H, Habib SH. Effect of combination of Withania somnifera Dunal and Tribulus terrestris Linn on laterazole induced polycystic ovarian syndrome in rats. Integr Med Res. 2016;5:293–300. doi: 10.1016/j.imr.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabiruddin M. IdarahKitabu-us-Shifa; New Delhi: 2007. Makhzanul Mufradat (Urdu) p. 316. [Google Scholar]
- 21.Freirich EJ, Gehan EA, Rall DP, Scunidt, Skipper HE. Qualitative comparison of toxicity of anti cancer agent in mouse, rat, dog, monkey and man. Cancer Chemother Rep. 1968;50:219–244. [PubMed] [Google Scholar]
- 22.Miller LC, Tainter ML. Estimation of ED50 and its error by means of Logprobit graph paper. Proc Soc Exp Bio Med. 1944;57:261–269. [Google Scholar]
- 23.Al-Said M, Mothana R, Raish M, Al-Sohaibani M, Al-Yahya M, Ahmad A, Al-Dosari M, Rafatullah S. Evaluation of the effectiveness of Piper cubeba extract in the amelioration of CCl4 induced liver injuries and oxidative damage in the rodent model. Biomed Res Int. 2015:1–11. doi: 10.1155/2015/359358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra SK, Gopumadhavan S, Venkataranganna MV, Sundaram R. Effect of Cystone, a herbal formulation, on glycolic acid-induced urolithiasis in rats. Phytother Res. 1998;12:372–374. [Google Scholar]
- 25.Kaur T, Bijarnia RK, Singla SK, Tandon C. Purification and characterization of an anticalcifying protein from the seeds of Trachyspermum ammi (L) Protein Pept Lett. 2009;16:173–181. doi: 10.2174/092986609787316252. [DOI] [PubMed] [Google Scholar]
- 26.Dodoala S, Diviti R, Koganti B, KVSRG Prasad. Effect of ethanolic extract of Phyla nodiflora L. Greene against calculi producing diet induced urolithiasis. Indian J Nat Prod Resour. 2010;1:314–321. [Google Scholar]
- 27.Dhaval PM, Arvind LN. Evaluation of the efficacy of methanolic extract of Foeniculum vulgare in urolithiasis on Wistar rats. Int J Pharm Res Technol. 2013;3:22–25. [Google Scholar]
- 28.Fan J, Glass MA, Chandhoke PS. Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Scan Microsc Int. 1999;13:299–306. [Google Scholar]
- 29.Khan SR. Animal models of kidney stone formation: an analysis. World J Urol. 1997;15:236–243. doi: 10.1007/BF01367661. [DOI] [PubMed] [Google Scholar]
- 30.Mc Martin KE, Wallace KB. Calcium oxalate monohydrate, a metabolite of ethylene glycol is toxic for rat renal mitochondrial function. Toxicol Sci. 1984:195–200. doi: 10.1093/toxsci/kfi062. [DOI] [PubMed] [Google Scholar]
- 31.Fleish H. Inhibitors and promoters of stone formation. Kidney Int. 1978;13:361–371. doi: 10.1038/ki.1978.54. [DOI] [PubMed] [Google Scholar]
- 32.Rathod NR, Biswas D, Chitme HR, Ratna S, Muchandia IS, Chandra R. Anti-urolithiatic effects of Punica granatum in male rats. J Ethnopharmacol. 2012;140:234–238. doi: 10.1016/j.jep.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48:1013–1018. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Lemann J, Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis. 1991;17:386–391. doi: 10.1016/s0272-6386(12)80628-7. [DOI] [PubMed] [Google Scholar]
- 35.Dadametirkee RB, Biyani CS, Browning AJ, Cartledge JJ. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. Eau-Ebu Upd Ser. 2007;5:126–136. [Google Scholar]
- 36.Soundarajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 37.Rushton HG, Spector M, Rodgers AL, Magura CE. Crystal deposition in the renal tubules of hyperoxaluric and hypomagnesemic rats. Scan Electron Microsc. 1980;3:387–394. [PubMed] [Google Scholar]
- 38.Anonymous . CCRUM; New Delhi: 1997. Standardization of single drugs of Unani medicine. Part II, III. p. 182–88, 223. [Google Scholar]
- 39.Gupta M, Bhayana S, Sikka SK. Role of urinary inhibitors and promoters in calcium oxalate crystallisation. Int J Res Pharm Chem. 2011;1:793–798. [Google Scholar]
- 40.Patel PK, Patel MA, Saralai MG, Gandhi TR. Antiurolithiatic effects of Solanum xanthocarpum fruit extract on ethylene-glycol-induced nephrolithiasis in rats. J Young Pharm. 2012;4:164–170. doi: 10.4103/0975-1483.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong BC, Kim BS, Kim JI, Kim HH. Effects of green tea on urinary stone formation: an in vivo and in vitro study. J Endourol. 2006;20:356–361. doi: 10.1089/end.2006.20.356. [DOI] [PubMed] [Google Scholar]
- 42.Akanae W, Tsujihata M, Yoshioka I, Nonomura N, Okuyama A. Orthosiphon grandioxorum has a protective effect in a calcium oxalate stone forming rat model. Urol Res. 2010;38:89–96. doi: 10.1007/s00240-010-0265-6. [DOI] [PubMed] [Google Scholar]
- 43.Parmar VS, Jain SC, Bisht KS, Jain R Taneja P, Jha A. Phytochemistry of the genus Piper. Phytochemistry. 1997;46:597–673. [Google Scholar]