Abstract
Background
The prospective, observational XANTUS study demonstrated low rates of stroke and major bleeding in real-world rivaroxaban-treated patients with non-valvular atrial fibrillation (NVAF) from Western Europe, Canada and Israel. XANTUS-EL is a component of the overall XANTUS programme and enrolled patients with NVAF treated with rivaroxaban from Eastern Europe, the Middle East and Africa (EEMEA) and Latin America.
Methods
Patients with NVAF starting rivaroxaban for stroke prevention were consecutively recruited and followed for 1 year, at approximately 3-month intervals, or for ≥30 days after permanent rivaroxaban discontinuation. Primary outcomes were major bleeding, adverse events (AEs), serious AEs and all-cause mortality. Secondary outcomes included stroke, non-central nervous system systemic embolism (non-CNS SE), transient ischaemic attack (TIA), myocardial infarction (MI) and non-major bleeding. All major outcomes were centrally adjudicated.
Results
Overall, 2064 patients were enrolled; mean age ± standard deviation was 67.1 ± 11.32 years; 49.3% were male. Co-morbidities included heart failure (30.9%), hypertension (84.2%), diabetes mellitus (26.5%), prior stroke/non-CNS SE/TIA (16.2%) and prior MI (10.7%). Mean CHADS2, CHA2DS2-VASc and HAS-BLED scores were 2.0, 3.6 and 1.6, respectively. Treatment-emergent event rates were (events/100 patient-years, [95% confidence interval]): major bleeding 0.9 (0.5–1.4); all-cause mortality 1.7 (1.2–2.4); stroke/non-CNS SE 0.7 (0.4–1.2); any AE 18.1 (16.2–20.1) and any serious AE 8.3 (7.0–9.7). One-year treatment persistence was 81.9%.
Conclusions
XANTUS-EL confirmed low stroke and major bleeding rates in patients with NVAF from EEMEA and Latin America. The population was younger but with more heart failure and hypertension than XANTUS; stroke/SE rate was similar but major bleeding lower.
Keywords: EEMEA, Latin America, Rivaroxaban, Real-world, Stroke prevention
Abbreviations: AE, adverse event; AF, atrial fibrillation; CI, confidence interval; CNS, central nervous system; CrCl, creatinine clearance; EEMEA, Eastern Europe the Middle East and Africa; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; NOAC, non-vitamin K antagonist oral anticoagulant; NVAF, non-valvular atrial fibrillation; od, once daily; PE, pulmonary embolism; SAE, serious adverse event; SD, standard deviation; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist
1. Introduction
Atrial fibrillation (AF) is a global disorder affecting an estimated 33.5 million patients worldwide.1 AF is associated with a range of co-morbidities, and patients with AF have an estimated fivefold increased risk of stroke,2, 3 which can be reduced with appropriate anticoagulant therapy. The non-vitamin K antagonist (VKA) oral anticoagulants (NOACs; apixaban, dabigatran, edoxaban and rivaroxaban) are effective alternatives to VKAs for stroke prevention in patients with non-valvular AF (NVAF), with favourable benefit–risk profiles.4 NOACs are recommended as an alternative to VKAs for stroke prevention in patients with AF in the American College of Cardiology/American Heart Association (ACC/AHA) AF guidelines5 and recommended in preference to VKAs in the European Society of Cardiology (ESC) guidelines.6
The ROCKET AF phase III randomised clinical trial demonstrated that rivaroxaban was non-inferior to warfarin for stroke/systemic embolism (SE) prevention in patients with NVAF at moderate to high risk of stroke, with no significant differences in major bleeding.7 The study included 14,264 patients from 1178 participating sites in 45 countries in Western Europe, Eastern Europe, North America, Latin America and Asia-Pacific. However, patients from countries in the Middle East and Africa (with the exception of South Africa) were not represented in ROCKET AF.
There are important regional global differences in clinical characteristics relevant to patients with AF. For example, in Latin America, the incidence of AF-related stroke and associated morbidity is increasing, possibly due to poorly controlled risk factors,8 and coronary artery disease is less common in patients with AF from Latin America than in Western patients.9, 10 In the Middle East, patients with AF are approximately 10 years younger than those in Western countries, with higher rates of diabetes and heart failure,10 but with a relatively high rate (19%) of ‘lone AF’ (i.e. AF with no co-morbidities) compared with that observed in Western countries (∼9%).11, 12 In addition, available evidence suggests that the clinical presentation of AF is more severe in Africa than in developed countries, with higher rates of heart failure, left ventricular hypertrophy and left ventricular systolic dysfunction.10, 13 These differences make it important to validate the safety and efficacy of rivaroxaban in patients with AF observed in ROCKET AF in different geographical regions worldwide.
Real-world evidence is increasingly recognised as an essential component of the clinical evidence base. These data complement phase III data in unselected patient populations, as well as providing additional safety information after the approval of new drugs. The XANTUS programme included XANTUS, XANAP and XANTUS-EL, all of which were prospective, observational studies of rivaroxaban use for stroke prevention in patients with NVAF from different geographical regions.14 XANTUS confirmed low rates of stroke and major bleeding in 6784 rivaroxaban-treated patients with NVAF from Europe, Canada and Israel in routine clinical practice,15 and XANAP demonstrated a safety and efficacy profile consistent with XANTUS in patients from the Asia-Pacific region.16 A pooled analysis of XANTUS, XANAP and XANTUS-EL is also planned.
XANTUS-EL (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation in Eastern Europe, the Middle East and Africa [EEMEA] and Latin America) was the first real-world, prospective, observational study assessing rivaroxaban use in a broad patient population with NVAF in 17 countries across EEMEA and Latin America. The main goal of the study was to investigate the safety of rivaroxaban use in clinical practice and provide data in real-life conditions in these regions. Furthermore, the study aimed to analyse the patterns of use and discontinuation rates of rivaroxaban in patients with different co-morbidities and risk profiles in these regions.
2. Methods
XANTUS-EL was an international, prospective, single-arm, observational, non-interventional cohort study in unselected patients with NVAF. XANTUS-EL was part of the XANTUS programme, and the protocol was aligned with that of XANTUS.14
2.1. Study population and data collection
Eligible patients were adults (≥18 years of age) with NVAF newly prescribed rivaroxaban for the prevention of stroke or non-central nervous system (CNS) SE, who provided written informed consent to participate in the study. Participating investigators were asked to screen all patients with AF receiving pharmacological treatment for stroke prevention in their care, regardless of the prescribed treatment. Each patient was consecutively screened and documented in an anonymous patient log file. The screening documentation was completed before eligible, consenting patients signed the informed consent forms and no patient-related data were permitted to be collected from the remaining ineligible or non-consenting patients. The study was conducted in 17 countries across Eastern Europe (Azerbaijan, Georgia, Kazakhstan, Russia), the Middle East (Bahrain, Jordan, Lebanon, Saudi Arabia, United Arab Emirates), Africa (Egypt, Kenya) and Latin America (Argentina, Chile, Colombia, Mexico, Uruguay, Venezuela).
2.2. Medication and follow-up
It was anticipated that patients would be prescribed rivaroxaban in accordance with their country-specific drug approvals; however, prescription decisions were solely at the discretion of the treating physician. Patients were followed up for 1 year, or until 30 days after permanent rivaroxaban discontinuation (if <1 year) and investigators were asked to collect data at approximately 3-month intervals.
2.3. Study outcomes
Primary outcomes were related to the safety of rivaroxaban and recorded as treatment-emergent adverse events (AEs) or serious AEs (SAEs) and included major bleeding events (defined using the International Society on Thrombosis and Haemostasis [ISTH] criteria), all-cause mortality, and any other AEs and SAEs. Secondary outcomes included symptomatic thromboembolic events, non-major bleeding, treatment satisfaction, treatment persistence, and reasons for treatment switching or interruption. Haemorrhagic strokes were reported as both stroke and as major bleeding events. Major outcomes (major bleeding, stroke, SE, transient ischaemic attack [TIA], pulmonary embolism [PE], deep vein thrombosis, atrial thrombus, myocardial infarction [MI] and all-cause death) were adjudicated by a Central Adjudication Committee who had access to all patient data. Statistical analyses, performed on the safety population, were exploratory and included a descriptive analysis of the primary and secondary outcome variables.
2.4. Study governance
The study complied with the Declaration of Helsinki and was approved by the appropriate Health Authorities, independent Ethics Committees and independent Review Boards as required. An independent academic steering committee oversaw the design, execution and conduct of the study, was responsible for manuscript development, had full access to all of the data and approved all versions of the manuscript.
2.5. Statistical analysis
Statistical analyses included a descriptive analysis of the primary and secondary outcome variables and were performed on the safety population, which included all patients who took at least one dose of rivaroxaban. Events were considered treatment-emergent if they occurred on or after the first dose of rivaroxaban and up to 2 days after the last dose. Statistical analyses were exploratory and descriptive.
3. Results
3.1. Patient population
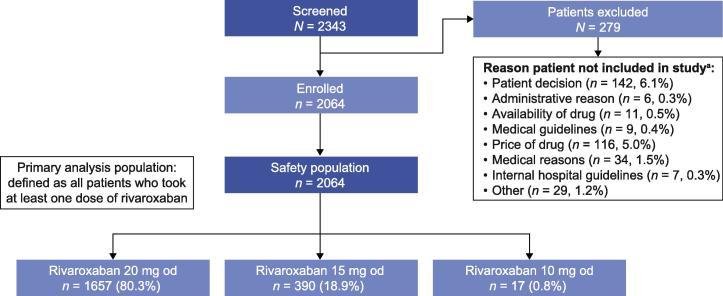
In total, 2343 patients were screened between 14 January 2013 and 16 January 2016; 2064 patients were enrolled and constituted the XANTUS-EL safety population (Fig. 1). The patient population in XANTUS-EL was predominantly Caucasian (75.8%). The remaining patients were Asian (9.2%), black (1.3%) or of mixed ancestry (<0.05%), with no information on race for 13.7% of patients. The baseline demographics and clinical characteristics of these patients are shown in Table 1. Mean age ± standard deviation (SD) was 67.1 ± 11.32 years (>75 years: 25%); first available weight ± SD was 82.9 ± 17.06 kg, and 49.3% of patients were male. In total, 57.4% of patients had a first available creatinine clearance (CrCl) of ≥50 ml/min; 12.7% had CrCl <50 ml/min and values were missing for 29.8%. A total of 14.1% of patients had newly diagnosed AF, 33.8% paroxysmal AF, 14.5% persistent AF and 37.4% permanent AF. Common co-morbidities included hypertension (84.2%), congestive heart failure (30.9%), diabetes mellitus (26.5%), prior stroke/non-CNS SE/TIA (16.2%) and prior MI (10.7%). Patients had mean CHADS2 and CHA2DS2-VASc scores of 2.0 and 3.6, respectively, and a mean HAS-BLED score of 1.6. In total, 59.2% of patients had received prior anticoagulation therapy (VKAs: 26.8%; acetylsalicylic acid: 20.9%). The mean treatment duration was 340 days (SD 108; median 367). Overall, 80.3% and 18.9% of patients received rivaroxaban 20 mg once daily (od) and 15 mg od, respectively. In total, 62 (3%) patients had at least one dose change, of which 11 (0.5%) were due to patient decision.
Fig. 1.
Patient disposition in XANTUS-EL. a Reasons for not continuing in the study included, but were not limited to, patient decision and administrative and medical reasons; some patients could have more than one reason for exclusion. od: once daily.
Table 1.
Baseline demographics and clinical characteristics of patients in XANTUS-EL.
| Rivaroxaban (n = 2064) | |
|---|---|
| Age, years, mean ± SD | 67.1 ± 11.3 |
| <65 years, n (%) | 805 (39.0) |
| ≥65–≤75 years, n (%) | 743 (36.0) |
| >75 years, n (%) | 516 (25.0) |
| Male sex, n (%) | 1018 (49.3) |
| Weight,a kg, mean ± SD | 82.9 ± 17.1 |
| BMI, kg/m2, mean ± SD | 29.5 ± 5.5 |
| Creatinine clearance,a ml/min, n (%) | |
| <15 | 12 (0.6) |
| ≥15–<30 | 36 (1.7) |
| ≥30–<50 | 215 (10.4) |
| ≥50–≤80 | 581 (28.1) |
| >80 | 605 (29.3) |
| Missing | 615 (29.8) |
| Existing co-morbidities, n (%) | |
| Hypertension | 1738 (84.2) |
| Diabetes mellitus | 546 (26.5) |
| Prior stroke/non-CNS SE/TIA | 335 (16.2) |
| Congestive heart failure | 638 (30.9) |
| Prior MI | 220 (10.7) |
| Hospitalisation at baseline, n (%) | 640 (31.0) |
| AF type, n (%) | |
| First diagnosed | 292 (14.1) |
| Paroxysmal | 697 (33.8) |
| Persistent | 299 (14.5) |
| Permanent | 772 (37.4) |
| Missing | 4 (0.2) |
| CHADS2 score, mean ± SD | 2.0 ± 1.23 |
| CHADS2 score, n (%) | |
| 0 | 131 (6.3) |
| 1 | 633 (30.7) |
| 2 | 680 (32.9) |
| 3 | 342 (16.6) |
| 4 | 194 (9.4) |
| 5 | 73 (3.5) |
| 6 | 11 (0.5) |
| Missing | 0 |
| CHA2DS2-VASc score, mean ± SD | 3.6 ± 1.8 |
| CHA2DS2-VASc score, n (%) | |
| 0 | 53 (2.6) |
| 1 | 188 (9.1) |
| 2 | 371 (18.0) |
| 3 | 445 (21.6) |
| 4 | 428 (20.7) |
| 5 | 266 (12.9) |
| 6–9 | 313 (15.2) |
| Missing | 0 |
| HAS-BLED score, mean ± SD | 1.6 ± 1.1 |
| Prior anticoagulation therapy, n (%) | |
| Yes | 1221 (59.2) |
| Naïve | 843 (40.8) |
| VKA | 554 (26.8) |
| Direct thrombin inhibitor | 70 (3.4) |
| Acetylsalicylic acid | 432 (20.9) |
| Dual antiplatelet therapy | 28 (1.4) |
| Factor Xa inhibitor (excluding rivaroxaban) | 7 (0.3) |
| Other | 89 (4.3) |
| Multiple | 41 (2.0) |
AF: atrial fibrillation, BMI: body mass index, CNS: central nervous system, MI: myocardial infarction, SD: standard deviation, SE: systemic embolism, TIA: transient ischaemic attack, VKA: vitamin K antagonist.
First available value.
3.2. Outcomes
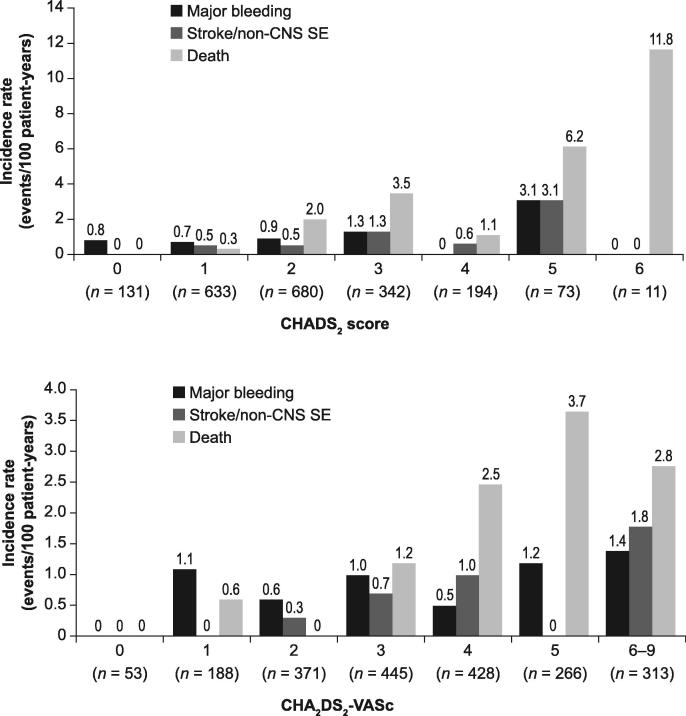
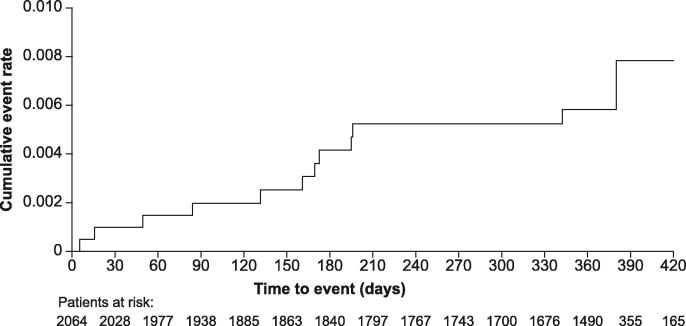
Overall, 0.8% of patients (95% confidence interval [CI] 0.5–1.3) had a treatment-emergent adjudicated major bleeding event, and the incidence rate was 0.9 events/100 patient-years (95% CI 0.5–1.4) (Table 2). The incidence rate of intracranial bleeding was 0.16 events/100 patient-years (95% CI 0.03–0.46), and the rate of major gastrointestinal bleeding was 0.5 events/100 patient-years (95% CI 0.2–1.0). There was one fatality resulting from treatment-emergent adjudicated major bleeding (0.1 events/100 patient-years [95% CI 0.0–0.3]), which was due to haemorrhagic transformation of an ischaemic stroke. In this case, the antithrombotic drug was withdrawn after the major bleeding event. Treatment-emergent AEs occurred in 15.7% of patients (95% CI 14.2–17.3; incidence rate: 18.1 events/100 patient-years [95% CI 16.2–20.1]) and treatment-emergent SAEs occurred in 7.5% of patients ([95% CI 6.4–8.7; incidence rate: 8.3 events/100 patient-years [95% CI 7.0–9.7]). Treatment-emergent adjudicated death occurred in a total of 33/2064 (1.6%) patients (incidence rate: 1.7 events/100 patient-years [95% CI 1.2–2.4]). Adjudicated causes of treatment-emergent death are shown in Table 3. Among the 33 patients who died, the most common causes of death were cardiovascular (36.4%), with cardiac decompensation and heart failure accounting for 21.2% of all deaths, and infectious disease (21.2%). There was a total of 29 symptomatic thromboembolic events in 24 patients (defined as treatment-emergent adjudicated stroke, TIA, non-CNS SE and MI; incidence rate: 1.3 events/100 patient years [95% CI 0.8–1.9]) (Table 2). The incidence rates (events/100 patient-years [95% CI]) for the different events were: stroke 0.6 (0.3–1.1); TIA 0.3 (0.1–0.6); non-CNS SE 0.1 (0.0–0.3) and MI 0.3 (0.1–0.7). Incidences of haemorrhagic and ischaemic stroke were <0.05% and 0.5%, respectively. Rates of major bleeding, stroke/non-CNS SE and death generally rose with increasing CHADS2, CHA2DS2-VASc (Fig. 2) and HAS-BLED scores. The cumulative incidence rate of treatment-emergent adjudicated stroke/non-CNS SE events increased gradually with time on treatment (Fig. 3). The incidence rates (events/100 patient-years [95% CI]) of adjudicated treatment-emergent major bleeding events and death were numerically higher in patients receiving rivaroxaban 15 mg od versus those receiving rivaroxaban 20 mg od (1.1 [0.3–2.9] vs 0.8 [0.4–1.4]) and (3.4 [1.8–6.0] vs 1.2 [0.7–1.9]), respectively. Overall, 58 patients (2.8%) experienced major bleeding, death, stroke or non-CNS SE with an incidence rate of 3.0 events/100 patient-years (95% CI 2.3–3.9), i.e. >97% of patients did not experience any of these events.
Table 2.
Adjudicated treatment-emergent thromboembolic and bleeding events and all-cause death.
| Incidence proportion, n (%) | Incidence rate Number of events/100 patient-years (95% CI) |
|
|---|---|---|
| All-cause death | 33 (1.6) | 1.7 (1.2–2.4) |
| Thromboembolic events (stroke, TIA, non-CNS SE and MI) | 24 (1.2) | 1.3 (0.8–1.9) |
| Stroke/non-CNS SE | 13 (0.6) | 0.7 (0.4–1.2) |
| Stroke | 12 (0.6) | 0.6 (0.3–1.1) |
| Primary haemorrhagica | 1 (<0.05) | – |
| Primary ischaemic | 11 (0.5) | – |
| Haemorrhagic transformationb | 1 (<0.05) | – |
| No haemorrhagic transformation | 10 (0.5) | – |
| Missing | 0 (0.0) | – |
| Uncertain | 2 (0.1) | – |
| Non-CNS SE | 1 (<0.05) | 0.1 (0.0–0.3) |
| TIA | 5 (0.2) | 0.3 (0.1–0.6) |
| MI | 6 (0.3) | 0.3 (0.1–0.7) |
| Major bleeding | 17 (0.8) | 0.9 (0.5–1.4) |
| Fatal | 1 (<0.05) | 0.1 (0.0–0.3) |
| Intracranial haemorrhage | 3 (0.1) | 0.16 (0.0–0.5) |
| Intraparenchymal | 0 (0.0) | NA |
| Subarachnoid | 1 (<0.05) | NA |
| Intraventricular | 1 (<0.05) | NA |
| Subdural haematoma | 0 (0.0) | NA |
| Epidural haematoma | 0 (0.0) | NA |
| Haemorrhagic transformation of ischaemic strokeb | 1 (<0.05) | NA |
| Missing | 0 (0.0) | NA |
| Mucosal bleeding | 11 (0.5) | 0.6 (0.3–1.0) |
| Gastrointestinal bleeding | 10 (0.5) | 0.5 (0.2–1.0) |
| Haemoglobin decrease in ≥2 g/dl | 4 (0.2) | 0.2 (0.1–0.5) |
| Transfusion in ≥2 units of packed red blood cells or whole blood | 9 (0.4) | 0.5 (0.2–0.9) |
| Non-major bleeding events | 118 (5.7) | 6.3 (5.2–7.5) |
| Major bleeding, all-cause death, stroke or non-CNS SE | 58 (2.8) | 3.0 (2.3–3.9) |
CI: confidence interval, CNS: central nervous system, MI: myocardial infarction, SE: systemic embolism, TIA: transient ischaemic attack.
Haemorrhagic strokes were adjudicated as both stroke and as major bleeding events; primary haemorrhagic strokes were defined as stroke with focal collections of intracerebral blood.
Haemorrhagic transformations were adjudicated as both ischaemic stroke and major bleeding.
Table 3.
Summary of adjudicated causes of treatment-emergent death.
| Adjudicated causes of death,an (%) | Deaths (n = 33) |
|---|---|
| Bleeding | 1 (3.0) |
| Extracranial haemorrhage | 0 |
| Intracranial bleeding | 1 (3.0) |
| Cancer | 3 (9.1) |
| Cardiovascular | 12 (36.4) |
| Cardiac decompensation, heart failure | 7 (21.2) |
| Dysrhythmia | 1 (3.0) |
| Myocardial infarction | 2 (6.1) |
| Non-haemorrhagic stroke | 1 (3.0) |
| Sudden or unwitnessed death | 2 (6.1) |
| Venous thromboembolism | 0 |
| Other vascular event | 1 (3.0) |
| Systemic embolism | 0 |
| Infectious disease | 7 (21.2) |
| Other | 6 (18.2) |
| Unexplained | 7 (21.2) |
Multiple reasons were recorded for the cause of adjudicated treatment-emergent death of some patients.
Fig. 2.
Incidence rate of adjudicated treatment-emergent major bleeding, stroke/non-CNS SE and death by (A) CHADS2 score and (B) CHA2DS2-VASc score. CNS: central nervous system, SE: systemic embolism.
Fig. 3.
Cumulative rates (Kaplan–Meier) for treatment-emergent adjudicated stroke/non-CNS SE events. CNS: central nervous system, pts: patients, SE: systemic embolism.
3.3. Additional outcomes
A small proportion of patients had at least one treatment interruption (2.8%), and the most common reasons for treatment interruption were AEs (including but not restricted to bleeding events) and patient decision (Table 4). The median treatment interruption was 8 days (interquartile range 3–20 days). Persistence was 81.9% at the end of the study, with 6.3% of patients discontinuing treatment during the first 3 months. A total of 79.2% of all patients reported being ‘satisfied’ or ‘very satisfied’ with their rivaroxaban treatment at the final visit.
Table 4.
Additional outcomes in XANTUS-EL.
| Rivaroxaban (n = 2064) | |
|---|---|
| Treatment persistence at end of study, n (%) | 1690 (81.9) |
| Total number of treatment interruptionsa | 62 |
| Patients with ≥1 interruption, n (%) | 57 (2.8) |
| Duration of treatment interruption, days, median (interquartile range) | 8 (3–20) |
| Reason for interruption, n (%) | |
| Patient decision | 11 (17.7) |
| Patient convenience | 0 (0.0) |
| Supply issues due to health systems | 6 (9.7) |
| Insufficient therapeutic effects | 0 (0.0) |
| Adverse event (excluding bleeding) | 10 (16.1) |
| Adverse event (bleeding) | 16 (25.8) |
| Surgery | 8 (12.9) |
| Dentistry | 2 (3.2) |
| Other | 9 (14.5) |
| Number of interruptions with bridging therapy, n (%) | 5 (8.1) |
Defined as no treatment for at least 1 day.
4. Discussion
XANTUS-EL is the first real-world, prospective, observational cohort study to describe rivaroxaban use in a broad patient population with NVAF in 17 countries in EEMEA and Latin America. XANTUS-EL can be compared, with consideration of the differences in baseline demographics and clinical characteristics, with the sister studies XANTUS15 and XANAP,16 which investigated rivaroxaban use in patients with NVAF in Europe/Israel/Canada and the Asia-Pacific region, respectively. Results from XANTUS-EL were broadly consistent with those of both XANTUS and XANAP, and also with the phase III ROCKET AF study,7 when differences in baseline characteristics were taken into account (Table 5).
Table 5.
Overview of baseline demographics and major outcomes in ROCKET AF, XANTUS, XANAP and XANTUS-EL.
| ROCKET AF7 (n = 7131) | XANTUS15 (n = 6784) | XANAP16 (n = 2273) | XANTUS-EL (n = 2064) | |
|---|---|---|---|---|
| Baseline demographics | ||||
| Age, years, mean | 73a | 71.5 | 70.5 | 67.1 |
| CrCl <50 ml/min, % | 20.717 | 9.4 (34.4% missing) | 16.3 (48.5% missing) | 12.7 (29.8% missing) |
| CHF, % | 63 | 19 | 20 | 31 |
| Hypertension, % | 90 | 75 | 74 | 84 |
| Diabetes, % | 40 | 20 | 27 | 27 |
| Prior stroke/SE/TIA, % | 55 | 19 | 33 | 16 |
| CHADS2 score, mean | 3.5 | 2.0 | 2.3 | 2.0 |
| CHA2DS2-VASc score, mean | – | 3.4 | 3.7 | 3.6 |
| HAS-BLED, mean | 2.8b18 | 2.0 | 2.1 | 1.6 |
| Use of rivaroxaban 15 mg dose, % | 20.717 | 21 | 44 | 19 |
| Outcomes (events/100 patient-years) | ||||
| Major bleeding | 3.6c | 2.1 | 1.5 | 0.9 |
| Intracranial haemorrhage | 0.5c | 0.4 | 0.7 | 0.2 |
| Fatal | 0.2c | 0.2 | 0.2 | 0.1 |
| GI bleeding | 2.0c19 | 0.9 | 0.5 | 0.5 |
| Stroke/SE | 1.7d | 0.8 | 1.9 | 0.7 |
| Stroke | 1.7d | 0.7 | 1.7 | 0.6 |
| Ischaemic stroke | 1.3d | 0.5e | 0.9e | 0.5e |
| Haemorrhagic strokef | 0.3d | 0.2e | 0.4e | <0.05e |
| MI | 0.9d | 0.4 | 0.5 | 0.3 |
| All-cause mortality | 1.9c | 1.9 | 2.0 | 1.7 |
CHF: congestive heart failure, CrCl: creatinine clearance, GI: gastrointestinal, ITT: intention-to-treat, MI: myocardial infarction, SE: systemic embolism, TIA: transient ischaemic attack.
Median age.
Based on overall ITT to site notification trial population (n = 14,171).
Based on safety population (n = 7111).
Based on safety, as treated, population (n = 7061).
Incidence proportion, not incidence rate.
Haemorrhagic strokes were adjudicated as both stroke and as major bleeding events; primary haemorrhagic strokes were defined as stroke with focal collections of intracerebral blood.
The baseline characteristics of the XANTUS-EL population differed from the XANTUS population, with major differences including: lower age in XANTUS-EL than XANTUS (mean ± SD: 67.1 ± 11.3 years vs 71.5 ± 10.0 years); lower proportion of males (49.3% vs 59.2%); higher incidence of hypertension (84.2% vs 74.7%); higher incidence of diabetes (26.5% vs 19.6%); and higher incidence of congestive heart failure (30.9% vs 18.6%). CHADS2 scores (mean ± SD) were similar between XANTUS-EL (2.0 ± 1.23) and XANTUS (2.0 ± 1.3), as were CHA2DS2-VASc scores (3.6 ± 1.8 and 3.4 ± 1.7, respectively), suggesting that the patients had a similar overall risk of stroke despite the differences in baseline characteristics. The proportion of patients with CrCl <50 ml/min was similar between XANTUS-EL (12.7%) and XANTUS (9.4%), and both were slightly lower than the proportion of patients receiving rivaroxaban 15 mg od (18.9% in XANTUS-EL and 20.8% in XANTUS).
Rates of major bleeding were lower in XANTUS-EL than XANTUS (0.9 vs 2.1 events/100 patient-years), consistent with a lower mean HAS-BLED score in XANTUS-EL versus XANTUS (1.6 vs 2.0). This is also consistent with lower 1-year major bleeding outcomes in patients with AF in South America (2%), Eastern Europe (2%), the Middle East (1%) and Africa (2%) compared with patients from North America, Western Europe or Australia (3%) reported in another study.10 Rates of intracranial haemorrhage were lower in XANTUS-EL than XANTUS: 0.16 events/100 patient-years (95% CI 0.0–0.5) versus 0.4 events/100 patient-years (95% CI 0.3–0.6), as were rates of major gastrointestinal bleeding: 0.5 events/100 patient-years (95% CI 0.2–1.0) versus 0.9 (95% CI 0.6–1.1). Rates of stroke/non-CNS SE were similar between XANTUS-EL and XANTUS (0.7 vs 0.8 events/100 patient-years), as expected based on the similar CHADS2 and CHA2DS2-VASc scores. Incidence proportions of ischaemic stroke were the same in XANTUS-EL and XANTUS (0.5% in both), but the proportion of haemorrhagic strokes was lower in XANTUS-EL (<0.05% vs 0.2%). Rates of all-cause death were also similar between XANTUS-EL and XANTUS (1.7 vs 1.9 events/100 patient-years). The most common causes of death were cardiovascular in both studies, with XANTUS-EL having a lower proportion of cancer and bleeding-related deaths but a higher proportion of deaths related to infectious diseases. The incidence proportions of treatment-emergent AEs (15.7% vs 39.9%) and SAEs (7.5% vs 17.7%) were lower in XANTUS-EL than XANTUS, which may be caused by regional differences in healthcare systems, patient behaviour or reporting habits of the physicians.
Drug persistence is a major concern in stroke prevention worldwide because of discontinuation of anticoagulation leaving patients unprotected from the risk of stroke;15 persistence at 1 year in XANTUS-EL was similar to that in XANTUS (81.9% vs 80%). This is also in line with the Dresden NOAC Registry, in which rivaroxaban persistence was recorded at 85% after ∼1 year.20
4.1. Limitations
XANTUS-EL was a single-arm study and, as with any open-label study, the study design can introduce bias related to knowledge about treatment. Moreover, interference with patient management was not allowed because of the non-interventional nature of the study; this resulted in a relatively low number of patients with available renal function data, which limits any implications these data may have. Another effect of the non-interventional, observational nature of the study was that the reasons for dosing decisions (e.g. a prescription of rivaroxaban 10 mg od) were not recorded. Finally, the outcomes were assessed in the full cohort of patients and not by individual region because of the limited number of patients and low event rates.
5. Conclusions
The results of XANTUS-EL are broadly consistent with those observed in XANTUS when differences in terms of stroke and bleeding risk were taken into consideration, and this study further expands the body of evidence on the safety of patients with NVAF receiving rivaroxaban for stroke prevention. Rates of major bleeding and haemorrhagic stroke were lower in XANTUS-EL than XANTUS, in line with a lower baseline bleeding risk. More than 97% of patients (mean CHADS2 score: 2.0) did not experience any of the outcomes of stroke/non-CNS SE, treatment-emergent adjudicated major bleeding or death. As part of the XANTUS international study programme, with its adjudication of endpoints expected to reduce reporting bias, XANTUS-EL offers a unique real-world evidence dataset of rivaroxaban use in the routine care of patients with NVAF in EEMEA and Latin America.
6. Disclosures
CAAM has acted as a consultant for Bayer. FL has acted as a consultant for Boehringer Ingelheim, Bayer, Bristol-Myers Squibb and Pfizer. GR has received consulting fees and honoraria from Bayer, Sanofi, MSD, Takeda and Servier. SMK has acted as a consultant for Bayer and Pfizer. ML is an employee of Chrestos Concept, which received funding for this analysis from Bayer AG. MALV and NZ are employees of Bayer AG. AGGT has been a consultant for Bayer, Janssen Pharmaceutical Research & Development, Astellas, Portola and Takeda.
Acknowledgments
Acknowledgements
The XANTUS-EL steering committee thanks all patients, carers and families who participated in the study as well as the XANTUS-EL investigators and their associated teams. The authors thank Carole Mongin-Bulewski for editorial assistance in the preparation of the manuscript, with funding from Bayer AG and Janssen Scientific Affairs, LLC.
Funding
This work was supported by Bayer AG and Janssen Research & Development, LLC. Chrestos Concept received funding for this analysis from Bayer AG.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
Contributor Information
Fernando Lanas, Email: fernando.lanas@ufrontera.cl.
Ghazi Radaideh, Email: garadaideh@dha.gov.ae.
Suleiman M. Kharabsheh, Email: skharabsheh@kfshrc.edu.sa.
Marc Lambelet, Email: marc.lambelet.ext@bayer.com.
Marco Antonio Lavagnino Viaud, Email: marcoantonio.lavagnino@bayer.com.
Naser Samih Ziadeh, Email: naser.ziadeh@bayer.com.
Alexander G.G. Turpie, Email: turpiea@mcmaster.ca.
References
- 1.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassand J.P., Accetta G., Camm A.J. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37:2882–2889. doi: 10.1093/eurheartj/ehw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:21320. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruff C.T., Giugliano R.P., Braunwald E. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 5.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 7.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Massaro A.R., Lip G.Y.H. Stroke prevention in atrial fibrillation: focus on Latin America. Arq Bras Cardiol. 2016;107:576–589. doi: 10.5935/abc.20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamra H., Murin J., Chiang C.E. Use of antithrombotics in atrial fibrillation in Africa, Europe, Asia and South America: insights from the International RealiseAF Survey. Arch Cardiovasc Dis. 2014;107:77–87. doi: 10.1016/j.acvd.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Healey J.S., Oldgren J., Ezekowitz M. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388:1161–1169. doi: 10.1016/S0140-6736(16)30968-0. [DOI] [PubMed] [Google Scholar]
- 11.Zubaid M., Rashed W.A., Alsheikh-Ali A.A. Gulf Survey of Atrial Fibrillation Events (Gulf SAFE): design and baseline characteristics of patients with atrial fibrillation in the Arab Middle East. Circ Cardiovasc Qual Outcomes. 2011;4:477–482. doi: 10.1161/CIRCOUTCOMES.110.959700. [DOI] [PubMed] [Google Scholar]
- 12.Kim E.J., Yin X., Fontes J.D. Atrial fibrillation without comorbidities: prevalence, incidence and prognosis (from the Framingham Heart Study) Am Heart J. 2016;177:138–144. doi: 10.1016/j.ahj.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ntep-Gweth M., Zimmermann M., Meiltz A. Atrial fibrillation in Africa: clinical characteristics, prognosis, and adherence to guidelines in Cameroon. Europace. 2010;12:482–487. doi: 10.1093/europace/euq006. [DOI] [PubMed] [Google Scholar]
- 14.Camm A.J., Amarenco P., Haas S. XANTUS: rationale and design of a noninterventional study of rivaroxaban for the prevention of stroke in patients with atrial fibrillation. Vasc Health Risk Manag. 2014;10:425–434. doi: 10.2147/VHRM.S63298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camm A.J., Amarenco P., Haas S. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–1153. doi: 10.1093/eurheartj/ehv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y.H., Thanachartwet T., Camm J. XANAP: real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. J Arrhythmia. 2016;32:j131. doi: 10.1002/joa3.12073. Abstract P2-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox K.A.A., Piccini J.P., Wojdyla D. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 18.Breithardt G., Baumgartner H., Berkowitz S.D. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–3385. doi: 10.1093/eurheartj/ehu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwood M.W., Nessel C.C., Hellkamp A.S. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66:2271–2281. doi: 10.1016/j.jacc.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Hecker J., Marten S., Keller L. Effectiveness and safety of rivaroxaban therapy in daily-care patients with atrial fibrillation. Results from the Dresden NOAC Registry. Thromb Haemost. 2016;115:939–949. doi: 10.1160/TH15-10-0840. [DOI] [PubMed] [Google Scholar]