Abstract
Background
Coronary no-reflow (NR) is a dreadful complication of primary percutaneous coronary intervention (pPCI) that is seen in nearly 50% of cases. A great effort is being done to discover simple tools that could predict such a complication. We aimed primarily to study the predictive power of R-wave peak time (RWPT) on NR.
Methods
From October 2017 to March 2018, we enrolled 123 patients with STEMI treated with pPCI at Benha University Hospital and National Heart Institute. We measured RWPT from infarct-related artery (IRA) leads and assessed the development of NR in all finally included 100 patients (after exclusions).
Results
Based on occurrence of NR, patients were divided into 2 groups; Group I (n = 39) with NR and group II (n = 61) without NR. Smoking, DM, HTN, longer reperfusion times and higher thrombus burden were significantly associated with NR. Both pre- and postprocedural RWPT were significantly higher in group I than Group II. Preprocedural RWPT > 46 ms predicted NR with a sensitivity and specificity of 79.5% and 86.9% respectively (AUC 0.891, 95% CI 0.82–0.962, P < 0.001). In adjusted multivariate analysis, preprocedural RWPT was found to be among independent predictors for NR (OR: 26.2, 95% CI: 6.5–105.1, P < 0.001). The predictive power of preprocedural RWPT was statistically non-inferior to ST-resolution (STR)% (difference between area under curves = 0.029, P = 0.595).
Conclusion
RWPT is strongly associated with and significantly predicts the development of NR. This association was statistically non-inferior to the well-known association between STR% and NR.
Keywords: Intrinsicoid deflection, ST-elevation myocardial infarction, No-reflow
1. Introduction
Primary percutaneous coronary intervention (pPCI) is by far the management strategy of choice for patients presenting with ST-elevation myocardial infarction (STEMI), however an Achilis heel of pPCI is that it could not succeed in restoration of optimal myocardial perfusion despite epicardial coronary patency in some cases, a phenomenon known as no-reflow (NR).1 NR comes with many poor consequences, including but not limited to; larger infarct size, adverse remodeling, poor left ventricular systolic function and even higher rates of mortality.2 The exact pathophysiologic mechanism for NR remains largely unknown, with many theories pointing to a multitude of factors. It may be due to ischemia-reperfusion injury, endothelial injury or larger infarct sizes.3 The true incidence of NR differs according to the specific diagnostic methods used, but generally is estimated to occur in about 60% of STEMI cases.1, 2, 3 In daily practice, the most commonly used methods to define NR are thrombolysis in myocardial infarction (TIMI) grade, corrected TIMI frame count, myocardial blush grade (MBG), and ST-segment resolution (STR).4
First described in 1930,5 the duration from onset of the QRS complex to the peak of the R wave in the ECG is called ‘R-wave peak time’ (RWPT), otherwise known as intrinsicoid deflection time. It was found to have many clinical applications in the diagnosis of left ventricular hypertrophy, some conduction deficits and in the differential diagnosis of wide complex tachyarrhythmias.6, 7, 8
Considering that ECG is a simple, non-invasive and readily available tool in daily routine practice, we found that it may be of considerable interest if we could study the relation between RWPT and the occurrence of angiographic NR in patients with STEMI treated by pPCI.
2. Methods
2.1. Study population
From October 2017 to March 2018, 123 patients with STEMI who underwent pPCI at Benha University Hospital and National Heat Institute (NHI) were prospectively enrolled in this cross-sectional study. STEMI was defined according to the most recent ESC 2017 STEMI guidelines as follows: ongoing ischemic symptoms (within 12 h) together with ST-segment elevation (measured at the J-point) in at least two contiguous leads of 2.5 mm in men < 40 years, 2 mm in men > 40 years, or 1.5 mm in women in leads V2–V3 and/or 1 mm in the other leads [in the absence of left ventricular (LV) hypertrophy or left bundle branch block (LBBB)].9
Patients with a history of prior MI, heart failure, valvular heart diseases (insufficiency, or stenosis more than the mild degree except ischemic mitral regurgitation), cardiomyopathy (hypertrophic or dilated), renal replacement therapy, cardiogenic shock and failure of reperfusion therapy were excluded. We also excluded patients with technical ECG problems as poor image quality, bundle branch blocks, second-degree and third-degree AV blocks, and QRS duration (QRSD) of more than 120 ms. After exclusions, a total of 100 patients we left for the final analysis.
All baseline clinical criteria and medical histories were recorded. Dual antiplatelet therapy, ß-blockers, Angiotensin converting enzyme inhibitors and statins were given per guidelines if there are no contraindications. Patients were followed in coronary care units for at least 48 h after pPCI. The study design was approved by the local ethical committees and all patients gave informed consents.
2.2. Coronary angiography and pPCI
According to the American Heart Association (AHA) 2016 criteria for STEMI systems of care,10 for the operator to participate in the study by performing pPCI, he/she should have done at least 11 cases of pPCI and a total of 75 PCI procedures per year. Both centers providing cases for the study are experienced pPCI centers with a minimum of 36 cases of pPCI and a total of 200 cases of PCI done per year. All patients received, on a routine basis, 300 mg acetylsalicylic acid and a 600 mg loading dose of clopidogrel (per local protocol) before the intervention. Un-fractionated heparin (UFH) of 10,000 units bolus dose was given after sheath insertion. The procedure was done according to the standard technique for coronary angiography and PCI. Trans femoral approach was done in all patients using 6 Fr sheaths. Diagnostic coronary angiography was done to explore non-infarct related artery. XB or Judkin left guide catheters were used for lesions in the left system, while Judkin right catheters for lesions in right coronary artery (RCA). Thrombus aspiration and glycoproteins inhibitors (Eptifibatide or Tirofiban intracoronary bolus followed by intravenous infusion for 12 h) were left at the operators’ discretion. The operator determined the length and diameter of implanted stents. Sheaths were removed 4 h post procedure. Coronary angiograms were recorded in digital media for quantitative analysis (Dicom-viewer; Germany). Digital angiograms were analyzed by two independent and experienced cardiologists, who were blinded to all data. In case of variabilities, the final decision was made by a consensus.
Coronary blood flow patterns before and after pPCI were evaluated based on TIMI flow grade using grades 0, 1, 2, and 3.4 Thrombus burden was assessed according to the TIMI thrombus grading scale ranging from grade 0 (no thrombus) to grade 5 (very large thrombus causes vessel occlusion). Patients with grade 5 thrombus were reclassified from grade 0 to grade 4 after recanalization with guide wire or small balloon.11 We defined the angiographic NR phenomenon as a coronary TIMI flow grade of 2 or less after a vessel was recanalized.11
2.3. ECG analysis
Twelve-lead ECG (recorded at a speed of 25 mm/s and a voltage of 10 mm/mV) was obtained from all patients at admission and 60 min after pPCI, and all measurements were obtained from these ECG papers. Preprocedural and postprocedural (at 60 min) ECG papers were scanned and analyzed using digital image processing software (http://imagej.nih.gov/ij/). All measurements were performed by two independent cardiologists blinded to other patients’ data. QRSD and RWPT were measured from the beginning of the QRS complex to the J point and from the beginning of the QRS complex to the R-peak, respectively; the average of three consecutive beats from V5 to V6 leads in anterior STEMI, leads II and AVF in inferior STEMI, and leads I and AVL in high lateral STEMI that had the longest duration was recorded. The durations were given as milliseconds (ms). ST segment deviation was analyzed with lens intensified calipers to the nearest of 0.025 mV 20 ms (ms) after the end of QRS complex with the TP segment as reference baseline from leads I, aVL, and V1–V6 for anterior infarction, leads II, III and AVF for inferior infarction and leads I, aVL, V5 and V6 for lateral infarction. Single lead ST resolution (STR) was measured by the ST segment deviation on the single ECG lead which showed maximum deviation at baseline shortly after end of PPCI. Resolution was expressed as percentage from baseline.
3. Statistical analysis
Data management and statistical analysis were done using SPSS vs.25. Numerical data was summarized as means and standard deviations. Categorical data was summarized as numbers and percentages. Comparisons between two groups as regard numeric variables were done using independent t test or Mann Whitney test for normally and non-normally distributed variables respectively. Categorical variables were compared using Chi-square test or Fisher exact test if appropriate. ROC analysis was done for RWPT and ST resolution. Best cutoff points and diagnostic indices were calculated. Logistic regression analysis was done for prediction of no reflow. Odds ratio with 95% confidence interval were calculated for predictors. The De Longs test was used to compare the ROC curve of single-lead STR% with preprocedural RWPT.
4. Results
4.1. Baseline characteristics
For the whole study population, the mean age was 54 ± 11 years, 72% were males, 49% had HTN, 38% were diabetics, 47% were smokers, 72% had history of treated dyslipidemias, 42% were obese, 29% had family history of premature CAD, the mean baseline heart rate (HR) was 76 ± 15 bpm, the mean baseline systolic blood pressure (SBP) was 127 ± 33 mmHg and the mean baseline left ventricular ejection fraction (LVEF) measured by Simpson’s method was 49 ± 6%. Patients were divided into 2 groups based on the development of angiographic NR; group I (n = 39) consisted of those who developed NR and group II (n = 61) consisted of those who did not develop NR. Between groups analysis showed that group I patients were significantly more smokers, hypertensives, diabetics and had significantly lower baseline LVEF than group II patients. Table 1.
Table 1.
Baseline characteristics of study groups.
| G-I (n = 39) | G-II (n = 61) | P value | ||
|---|---|---|---|---|
| Age (years) | Mean ± SD | 56 ± 9 | 52 ± 12 | 0.105 |
| Gender | Male n (%) | 29 (74.4) | 43 (70.5) | 0.674 |
| Female n (%) | 10 (25.6) | 18 (29.5) | ||
| HTN | Yes n (%) | 25 (64.1) | 24 (39.3) | 0.016 |
| DM | Yes n (%) | 20 (51.4) | 18 (29.5) | 0.029 |
| Smoking | Yes n (%) | 26 (66.7) | 21 (34.4) | 0.002 |
| Dyslipidemia | Yes n (%) | 28 (71.8) | 46 (75.4) | 0.688 |
| Obesity | Yes n (%) | 16 (41.0) | 26 (42.6) | 0.875 |
| FH of premature CAD | Yes n (%) | 11 (28.2) | 18 (29.5) | 0.889 |
| Baseline HR (bpm) | Mean ± SD | 77 ± 15 | 76 ± 15 | 0.698 |
| Baseline SBP (mmHg) | Mean ± SD | 129 ± 32 | 126 ± 33 | 0.709 |
| Baseline LVEF (%) | Mean ± SD | 44 ± 5 | 52 ± 5 | <0.001 |
bpm = beats per minute, CAD = Coronay Artery Disease, DM = Diabetes Mellitus, FH = Family History, HTN = Hypertension, HR = Heart Rate, LVEF = Left Ventricular Ejection Fraction, SBP = Systolic Blood Pressure.
4.2. STEMI and procedural data
For the whole study population, 65% had anterior STEMI, 33% had inferior STEMI and 2% had lateral STEMI. The culprit vessel was identified to be the LAD in 60% of patients, RCA in 29% and LCX in 11%. The mean first medical contact (FMC)-to-device activation time was 61 ± 12 min. Thrombectomy was used in 4% of cases. Pre-dilatation balloons were used in 66% of cases with stents implanted in 95% of them. Ninety four percent of the stents were bare metal stents (BMSs) and 6% were drug eluting stents (DESs). The average mean stent(s) length was 26 ± 5 mm and the average mean stent(s) diameter was 3.15 ± 0.34 mm. TIMI thrombus grade ≥ 3 was identified in 27% of cases. The median preprocedural TIMI flow was 0 (range 0–2) and the median postprocedural TIMI flow was 3 (range 1–3). Between groups analysis showed that patients in group I had significantly longer FMC-to-device times, higher use of balloons, longer stent(s) implanted, higher prevalence of TIMI thrombus grade ≥ 3 and lower both pre-and postprocedural TIMI flows. Table 2.
Table 2.
Procedural and angiographic characteristics of study groups.
| G-I (n = 39) | G-II (n = 61) | P value | ||
|---|---|---|---|---|
| Location of STEMI | Anterior n (%) | 27 (69.0) | 38 (62.3) | 0.702 |
| Lateral n (%) | 1 (2.6) | 1 (1.6) | ||
| Inferior n (%) | 11 (28.2) | 22 (36.1) | ||
| Culprit vessel | LAD n (%) | 27 (69.2) | 33 (54.1) | 0.314 |
| LCX n (%) | 3 (7.7) | 8 (13.1) | ||
| RCA n (%) | 9 (23.1) | 20 (32.8) | ||
| FMC to device time (min) | Mean ± SD | 66 ± 16 | 57 ± 8 | 0.002 |
| Thrombectomy | Yes n (%) | 3 (7.7) | 1 (1.6) | 0.296 |
| Balloon | Yes n (%) | 35 (89.7) | 31 (50.8) | <0.001 |
| Stent | Yes n (%) | 39 (100.0) | 56 (91.8) | 0.153 |
| Stent type | BMS n (%) | 38 (97.4) | 56 (91.8) | 0.4 |
| DES n (%) | 1 (2.6) | 5 (8.2) | ||
| Stent length (mm) | Mean ± SD | 28 ± 3 | 24 ± 6 | <0.001 |
| Stent diameter (mm) | Mean ± SD | 3.14 ± 0.38 | 3.16 ± 0.32 | 0.836 |
| Thrombus grade ≥ 3 | Yes n (%) | 19 (48.7) | 8 (13.1) | <0.001 |
| Preprocedural TIMI-flow | Median (range) | 0 (0–1) | 0 (0–2) | 0.002 |
| Postprocedural TIMI-flow | Median (range) | 2 (1–2) | 3 (3–3) | <0.001 |
BMS = Bare Metal Stent, DES = Drug Eluting Stent, FMC = First Medical Contact, STEMI = ST-Elevation Myocardial Infarction, TIMI = Thrombolysis In Myocardial Infarction, LAD = Left Anterior Descending, LCX = Left CicumfleX, RCA = Right Coronary Artery.
4.3. ECG data
For the whole study population, Q waves on admission ECG were identified in 22% of cases. The maximum preprocedural ST elevation (in leads with highest elevation) was 3.6 ± 1.9 mm. The mean single-lead STR was 69 ± 19%. The mean pre- and postprocedural QRSD were 88 ± 18 and 81 ± 17 mm respectively. The mean pre- and postprocedural RWPT were 48 ± 15 and 43 ± 17 mm respectively. Between groups analysis showed that group I had significantly more Q waves on admission ECGs, higher maximum preprocedural ST elevation, lesser single-lead STR, longer pre- and postprocedural QRSD and loner pre- and postprocedural RWPT. Table 3.
Table 3.
ECG characteristics of study groups.
| G-I (n = 39) | G-II (n = 61) | P value | ||
|---|---|---|---|---|
| Q waves on admission | Yes n (%) | 13 (33.3) | 9 (14.8) | 0.029 |
| Preprocedural Maximum ST elevation (mm)a | Mean ± SD | 4.2 ± 1.9 | 3.2 ± 1.8 | 0.01 |
| Single-lead STR (%) | Mean ± SD | 54 ± 20 | 79 ± 11 | <0.001 |
| Preprocedural QRSD (ms) | Mean ± SD | 96 ± 22 | 83 ± 12 | 0.001 |
| Postprocedural QRSD (ms) | Mean ± SD | 90 ± 20 | 75 ± 12 | <0.001 |
| Preprocedural RWPT (ms) | Mean ± SD | 60 ± 17 | 40 ± 6 | <0.001 |
| Postprocedural RWPT (ms) | Mean ± SD | 58 ± 18 | 34 ± 6 | <0.001 |
Measured on lead with the highest elevation, ms = millisecond, QRSD = QRS complex duration, STR = ST Resolution, RWPT = R-wave Peak Time.
4.4. Predictors of no reflow
ROC analysis showed that (1) a cut-off value of preprocedural RWPT of >46 ms predicted the occurrence of NR with a sensitivity and specificity of 79.5% and 86.9% respectively (AUC 0.891, 95% CI 0.82–0.962, P < 0.001) (2) a cut-off value of single-lead STR% of <72.5% was associated with the occurrence of NR with a sensitivity and specificity of 87.2% and 73.8% respectively (AUC 0.862, 95% CI 0.783–0.942, P < 0.001). ROC curves for single-lead STR% (AUC 0.862, P < 0.001) and preprocedural RWPT (AUC 0.891, P < 0.001) were compared. There was no statistically significant difference between both (difference between area = 0.029, P = 0.595). Fig. 1. Moreover, a multivariate logistic regression analysis model adjusted for all other possible covariates using the occurrence of NR as a dependent factor showed that preprocedural RWPT > 46 ms is among the significant independent predictors for occurrence of NR, together with HTN, smoking, stent length more than 20 mm, presence of TIMI thrombus grade ≥ 3. Table 4.
Fig. 1.
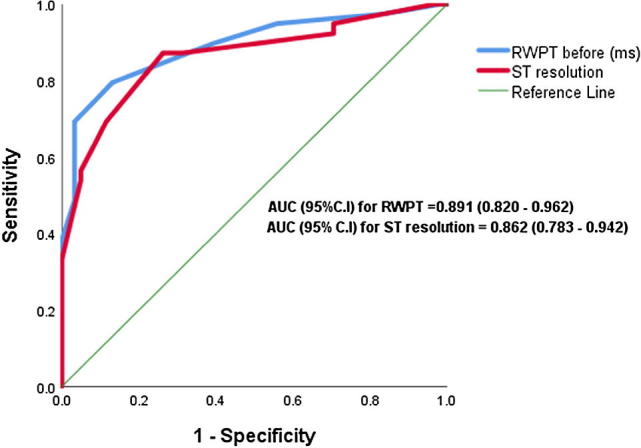
ROC curve comparison between single-lead STR% and preprocedural RWPT. Difference between AUC for single-lead STR% (0.862, P < 0.001) and preprocedural RWPT (0.891, P <0 .001) was 0.029, with a P value of 0.595. AUC = Area Under Curve, RWPT = R-wave Peak Time, ROC = Receiver-Operating Characteristic, STR = ST Resolution.
Table 4.
Independent predictors of no reflow with multivariate P value, odds ratio and 95% confidence interval.
| B | S.E. | OR | 95% C.I. for OR | P value | |
|---|---|---|---|---|---|
| HTN | 1.325 | 0.674 | 3.763 | (1.004–14.108) | 0.049 |
| Smoking | 1.913 | 0.713 | 6.772 | (1.67–27.4) | 0.007 |
| Thrombus grade ≥ 3 | 1.415 | 0.704 | 4.118 | (1.04–16.37) | 0.044 |
| Stent length more than 20 | 2.44 | 1.27 | 11.477 | (0.95–138.27) | 0.055 |
| Pre-procedural RWPT > 46 | 3.265 | 0.709 | 26.184 | (6.52–105.13) | <0.001 |
| Constant | -6.335 | 1.571 | 0.002 | <0.001 |
B = Regression coefficient, CI = Confidence interval, HTN- Hypertension, ms = millisecond, SE = Standard Error, OR = Odds Ratio, RWPT = R-wave Peak Time.
5. Discussion
In a time of confusing advances of complex diagnostic modalities, it’s fascinating to show how simple, non-invasive, readily available and reproducible tool the ECG is. We found that both pre- and postprocedural RWPT are significantly associated with the development of angiographic NR, and that preprocedural RWPT is a significant independent predictor for the occurrence NR. Moreover, the predictive power of preprocedural RWPT for angiographic NR is statistically non-inferior to single-lead STR% in ROC curves comparison. Interestingly, the fact that RWPT is a pre-procedural tool (not a post-procedural one like STR%) is advantageous in such a way that it could alert pPCI operators beforehand to the possibility of such a complication.
Angiographic NR is usually defined in routine clinical care as postprocedural TIMI flow grade of 2 or less in the presence of epicardial patency of coronaries.11 According to this definition, 39% of out population were found to have NR. Similar to previous findings,1, 3 we showed that smoking and DM are significantly associated with the development of NR. Despite lack of consensus about the relation between HTN and NR, we showed that HTN is more prevalent among patients who developed NR like the findings by Cagads et al.12 This could be explained by the strong link observed between HTN and endothelial dysfunction13 and coronary slow flow14 in patients with CAD. Our study showed that patients who developed NR had longer FMC-to-device times and higher thrombus burden as shown in some previous research work.2, 15 Of note, we found that higher thrombus burden than TIMI grade 3 is an independent predictor for NR.
ECG is an exceedingly fundamental tool in diagnosing STEMI and assessment of adequacy of reperfusion. In daily practice, the most commonly used parameter to assess myocardial tissue-level reperfusion is STR. It has been shown that incomplete STR (i.e. less than 70%) after reperfusion of STEMI is a marker for NR.16 We showed that single-lead STR% shortly after pPCI is significantly lower in patients who developed NR and that single-lead STR% < 72.5% is significantly associated with the occurrence of NR.
In the present study, we assessed both pre- and post procedural RWPT and QRSD and found that all these parameters were significantly higher in the NR group. Similar to findings by Cagads et al.,12 we showed also that preprocedural RWPT (and not QRSD) is a significant independent predictor for the occurrence of NR, despite that fact that QRSD itself has been shown in some research work to be associated with the development of NR.17 The nuances between QRSD and RWPT in prediction for NR could be explained on a pathophysiological basis if we consider that STEMI causes a localized segmental myocardial ischemia and hence a localized conduction delay in various ECG infarct-related artery (IRA) leads, therefore RWPT (which reflects early intra-ventricular conduction) could be more sensitive in this regard than QRSD (which reflects the conduction status of the Purkinje system as a whole).
To the best of our knowledge, this is the 1st study on a national base and the 2nd work in the literature that examines RWPT specifically for prediction of NR occurrence in patients with STEMI. The 1st work was done by Cagads et al.12 who showed that a preprocedural RWPT value of >28.2 ms is the best cut-off value to predict NR with a sensitivity and specificity of 61.6% and 56% respectively (AUC 0.679, P > 0.001). Our best cut-off value for prediction of NR has been found to be >46 ms with a sensitivity and specificity of 79.5% and 86.9% respectively (AUC 0.891, 95% CI 0.82–0.962, P < 0.001). Our cut-off value (46 ms) is a little bit higher than what had been reported by Cagads et al.12 (28.2 ms), and reasons for this discrepancy remains elusive. However, explanations might lie in the fact that our sample size is smaller than Cagads et al.12 and/or some ethnic differences between populations of both studies. This implies that utility of RWPT for prediction of such a dreadful complication should be validated in a larger cohort of patients with more diverse ethnicities before it could be provided for routine use in daily practice.
6. Conclusion
RWPT is strongly associated with and significantly predicts the development of NR. This association was statistically non-inferior to the well-known association between STR% and NR. Validity of RWPT utilization for prediction of NR should be furtherly corroborated.
7. Limitations
-
1.
Small sample size.
-
2.
Evaluation of reperfusion success was made visually. We did not use more sensitive and specific methods such as contrast echocardiography or cardiac MRI.
Conflict of interest
None declared.
Acknowledgement
Authors would like to thank Dr. Mohamed Bendary for his contribution to statistical analysis of data.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.Morishima I., Sone T., Mokuno S. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130:239–243. doi: 10.1016/0002-8703(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 2.Ndrepepa G., Tiroch K., Keta D. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Clin Perspect Circ: Cardiovasc Intervent. 2010;3:27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225. [DOI] [PubMed] [Google Scholar]
- 3.Bouleti C., Mewton N., Germain S. The no-reflow phenomenon: state of the art. Arch Cardiovasc Diseases. 2015;1(108):661–674. doi: 10.1016/j.acvd.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Gibson C., Cannon C., Murphy S., Marble S., Barron H., Braunwald E. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–1913. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 5.Macleod A., Wilson F., Barker P. The form of the electrocardiogram I Intrinsicoid electrocardiographic deflections in animals and man. Proc Soc Exp Biol Med. 1930;27:586–587. [Google Scholar]
- 6.Maldonado B., Calderón J., Rijlaarsdam M., Casanova J.G., Attie F., Buendia A. Electrocardiography and echocardiography aspects of hypertrophic myocardiopathy in pediatrics. Arch del Instituto de Cardiologia de Mexico. 2000;70:247–260. [PubMed] [Google Scholar]
- 7.Horwitz S., Lupi E., Hayes J., Frishman W., Cárdenas M., Killip T. Electrocardiographic criteria for the diagnosis of left anterior fascicular block: left axis deviation and delayed intraventricular conduction. Chest. 1975;68:317–320. doi: 10.1378/chest.68.3.317. [DOI] [PubMed] [Google Scholar]
- 8.Pava L., Perafán P., Badiel M. R-wave peak time at DII: a new criterion for differentiating between wide complex QRS tachycardias. Heart Rhythm. 2010;7:922–926. doi: 10.1016/j.hrthm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 10.Recommendations for criteria for STEMI systems of care [internet]. American Heart Association; 2016. Accessed June 2018. Available at: <http://www.heart.org/HEARTORG/Professional/MissionLifelineHomePage/EMS/Recommendations-for-Criteria-for-STEMI-Systems-of-Care_UCM_312070_Article.jsp#. Wziut9Iza00>.
- 11.Niccoli G., Burzotta F., Galiuto L., Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Çagdas M., Karakoyun S., Rencüzogullari I. Relationship between R-wave peak time and no-reflow in ST elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coron Artery Disease. 2017;28:326–331. doi: 10.1097/MCA.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y., Nakagawa K., Kimura M. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and non-dippers. J Am Coll Cardiol. 2002;40:2039–2043. doi: 10.1016/s0735-1097(02)02535-4. [DOI] [PubMed] [Google Scholar]
- 14.Evola S., Cuttitta F., Evola G. Early detection of coronary artery flow and myocardial perfusion impairment in hypertensive patients evidenced by myocardial blush grade (MBG) and thrombolysis in myocardial infarction (TIMI) frame count (TFC) Internal Med. 2012;51:1653–1660. doi: 10.2169/internalmedicine.51.7385. [DOI] [PubMed] [Google Scholar]
- 15.Sahin D., Gür M., Elbasan Z. SYNTAX score is a predictor of angiographic no-reflow in patients with ST-elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coron Artery Disease. 2013;24:148–153. doi: 10.1097/MCA.0b013e32835c4719. [DOI] [PubMed] [Google Scholar]
- 16.Schröder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation. 2004;110:e506–e510. doi: 10.1161/01.CIR.0000147778.05979.E6. [DOI] [PubMed] [Google Scholar]
- 17.Maden O., Kaçmaz F., Selçuk M. Relation of admission QRS duration with development of angiographic no-reflow in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous interventions. J Electrocardiol. 2008;41:72–77. doi: 10.1016/j.jelectrocard.2007.07.004. [DOI] [PubMed] [Google Scholar]


