Abstract
Background
The leaf of Pinus densiflora known as pine needles has been used to treat vascular disease, gastrointestinal diseases, and urinary diseases in traditional medicine. We evaluated anti-osteoporotic effect of water extract of Pinus densiflora (WEPN) on acute bone loss and osteoclastogenesis induced by receptor activator for nuclear factor-κB ligand (RANKL).
Methods
After oral administration of WEPN (0.25 g/kg) for 5 days, femora were collected, and bone parameter [trabecular bone volume/tissue volume (BV/TV), trabecular thickness (Tb. Th), trabecular separation (Tb. Sp), trabecular number (Tb. N), and bone mineral density (BMD)] were analyzed by micro-CT analysis. Anti-osteoclastic effect of WEPN was examined using tartrate-resistant acid phosphatase activity and activation of RANKL signaling pathway.
Results
We found that WEPN significantly attenuated RANKL-induced decrease of BV/TV, Tb.Th., Tb.N, and BMD but increase of Tb. Sp in femora. WEPN dose-dependently decreased osteoclastogenesis accompanied by inhibiting the activation of RANKL signaling components (JNK, p38, and p65) and mRNA expression level of osteoclast specific genes (NFATc1, c-Fos, TRAP, cathepsin K, DC-STAMP, and carbonic anhydrate).
Conclusion
WEPN inhibition on osteoclastogenesis could contribute to attenuate RANKL-induced trabecular bone loss in vivo. Therefore, it might suggest that WEPN could be prescribed in traditional medicine or used in health functional food to prevent or treat osteoporotic bone diseases.
Keywords: Pinus densiflora, Osteoclast, Receptor activator for nuclear factor-κB ligand, Nuclear factor of activated T cells cytoplasmic 1
1. Introduction
Osteoporosis is a skeletal bone disease characterized by degeneration of trabecular structure and decrease of bone mechanical property that increases frequency of fracture and hospitalization.1 Due to increase rate of mortality after osteoporotic fracture and recurrence of osteoporotic fracture in elderly population, health care to prevent or treat osteoporosis is interest in developmental country to decrease social burden of bone diseases on health system.2 The net imbalance of osteoclast bone resorption than osteoblast formation mostly leads to bone loss in osteoporosis and other skeletal bone disease. Since an increase of osteoclast generation or resorption activity is a primary factor of osteoporosis, bisphosphonate drug to directly inhibit osteoclastogenesis is widely prescribed for first line therapy in osteoporosis.3,4 In addition, estrogen or phytoestrogen treatment targeting osteoclastogenesis by regulating stromal cells has been used to prevent postmenopausal osteoporosis.5,6 Osteoclastogenesis is mainly regulated by key cytokine, receptor activator for nuclear factor-κB ligand (RANKL), generated from stromal cells.7 RANKL binding on RANK receptor of osteoclast progenitor cells initiates TRAF/AP-1/NFATc1 axis through NF-κB or MAPK signaling pathway during osteoclastogenesis.8,9 Pharmacological approach against RANKL pathway or bone resorption activity has been investigated to develop prevention or treatment of osteoporotic bone diseases.
Pine needle has been used to eliminate wind-dampness, to relax five zang and muscle, to increase circulation of meridian system for treatment of hypertension, gastrointestinal diseases, and urinary diseases in traditional medicine. Previous studies have shown that pine needle has pharmacological effects including blood circulation improvement, anti-oxidant effect,10 anti-bacterial effect,11 anti-cancer activity,12 and anti-inflammation.13 Major components identified in pine needle are phenolic acids,14 flavonoids such as quercetin, kaempferol, β-pinene, camphene, and pro-anthocyanidin.15,16 In previous study, hexane extract of pine (Pinus densiflora) needle increases alkaline phosphatase (ALP) activity and collagen synthesis in MC3T3-E1 cell,17 suggesting an anabolic effect of pine needle on bone by promoting osteoblast differentiation. In addition, hexane extract of pine needle (P. densiflora) decreases proliferation and tartrate-resistant acid phosphatase (TRAP) activity of osteoclast-like RAW264.7 cells.18 However, the in vivo effect of pine needle on osteoporosis and the molecular mechanism of pine needle on osteoclastogenesis is still unknown. Therefore, we explored the effect of water extract of pine needle (WEPN) on RANKL-induced bone loss model and RANKL signaling pathways using RANKL-induced osteoclastogenesis model.
2. Methods
2.1. Preparation of WEPN
Samples of P. densiflora were purchased from Yeongcheon herb (Yeongcheon, Korea). A specimen was named as W275 and deposited in the herbarium of Korea Institute of Oriental Medicine. P. densiflora (50 g) was immersed in distilled water (1 L) for 1 hour and then extracted by boiling for 3 hours. The extract was filtrated using standard sieves (150 μm), lyophilized, and stored at −20°C. To prepare a water extract of P. densiflora needle (WEPN), the lyophilized extract was re-suspended in distilled water and filtered through a sterile filter (0.2 μm).
2.2. Bone loss model
Animal experiments were handled in accordance with the guidelines of the Korea Food and Drug Administration Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee (IACUC) at the Korea Institute of Oriental Medicine reviewed and approved animal experiments for this study (approval number; 12-121). RANKL-induced bone loss experiment was followed as previously described.19 There were no significant difference of the initial body weight and health status of specific-pathogen-free ICR mice (7-week-old males, 32.16 + 4.1 g, total 24 mice) (Samtako Bio Inc., Korea). The mice were randomly divided into four groups (6 mice per group). The mice were intraperitoneally injected by RANKL (1 mg/kg of body weight) or PBS on days 0 and 1. The RANKL-injected mice were orally administered vehicle (distilled water) or WEPN (0.25 g/kg of body weight) twice daily for five consecutive days. All mice were sacrificed at 7 day by cervical dislocation and the femora were isolated for Micro-CT analysis. Micro-CT scanning of the distal femur was performed using the Quantum FX scanner system (PerkinElmer, Inc., MA, USA). Bone parameters including trabecular bone volume per tissue volume (BV/TV), trabecular thickness (Tb.Th), bone mineral density (BMD), and trabecular separation (Tb.Sp) were measured between 0.54 mm and 1.46 mm distal to the growth plate.
2.3. Cell culture and enzyme assay
Bone marrow-derived macrophages (BMMs) were isolated from femora of mice as previous study. Cytotoxicity of WEPN was evaluated using the Cell Counting Kit-8 (Dojindo, USA) after 2-day incubation with WEPN. Osteoclast differentiation from BMMs (1 × 104 cells/well in a 96-well plate) were induced by BMM cultured with M-CSF and RANKL for 4 days. TRAP activity assay and staining were examined using p-nitrophenyl phosphate as a substrate as previously described.20
2.4. Western blot analysis
After BMMs (4 × 105 cells/well, 6-well plate) were treated with vehicle (distilled water) or WEPN, the cells were lysed in RIPA buffer (Thermo, USA). Total protein (30 μg) were subjected to western blot analysis using specific primary antibodies and secondary antibodies. All antibodies were from Cell Signaling Technology (USA). Chemiluminescent signals were detected with Pierce ECL Western Blotting Substrate (Bio-Rad). The intensities of the bands were analyzed using Image Lab software (version 5.2.1, Bio-Rad).
2.5. Real-time quantitative polymerase chain reaction
Osteoclasts (4 × 105 cells/well, 6-well plate) were treated with vehicle (distilled water) or the indicated concentration of WEPN. Total RNA (1 μg) isolated by spine-column was used for cDNA synthesis with a cDNA synthesis kit (Bioneer Inc., Daejeon, Korea). cDNA were mixed with AccuPower GreenStar qPCR Master mix (Bioneer) and reacted in the CFX96 Touch Real-Time PCR System (Bio-Rad, Hercules, CA, USA). Two qPCR program (CFX manager software Version 3.1, Bio-Rad, USA) was performed. It was consisted of pre-incubation at 95°C activation for 5 minutes, 40 cycles amplification at 94°C for 20 seconds and at 60°C for 40 seconds. An internal control of qPCR was hypoxanthine phosphoribosyltransferase.
2.6. Actin ring staining
To visualize actin rings of mature osteoclasts, the cells were treated with vehicle or sample for 1 hour. The cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and then incubated with phalloidin-TRITC (0.2 μg/mL, Sigma). After PBS washing, the stained cells were observed under an inverted phase contrast fluorescence microscope (IX73, Olympus).
2.7. Statistical analysis
Statistical significance (p < 0.05) in bone parameters were analyzed by Dunnett test after one-way ANOVA. Statistical significance (p < 0.05) in enzyme activity assay and gene expression level were analyzed by Student t-test. Data was presented as means + standard deviation of three independent experiments except the animal experiment.
3. Results
3.1. WEPN suppresses RANKL-induced trabecular bone loss
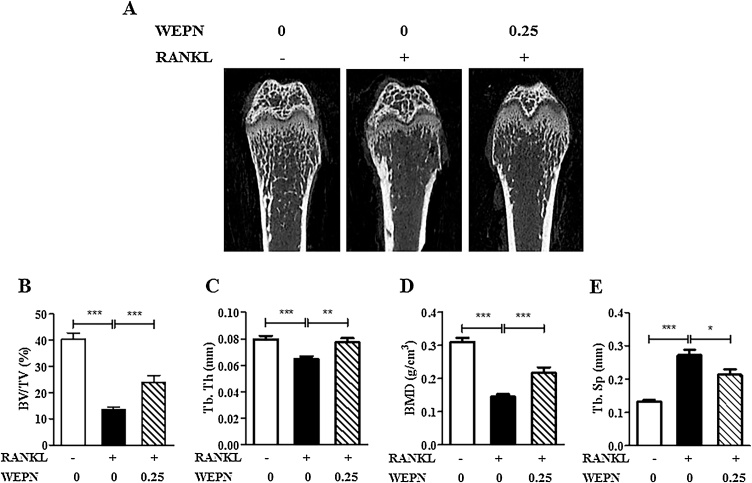
To examine whether WEPN has a protective effect against bone loss, we used RANKL-induced trabecular bone loss model. Intraperitoneal RANKL injections in mouse rapidly induces trabecular bone loss within 5 days. RANKL injections markedly caused acute trabecular bone loss at the distal femoral metaphysis (Fig. 1A). But, oral treatment of WEPN (0.25 g/kg) obviously attenuated RANKL-induced trabecular loss. When we analyzed bone micro-structure by micro-CT analysis, RANKL injections induced significant decrease of bone volume (Fig. 1B), trabecular thickness (Fig. 1C), and bone mineral density (Fig. 1D), whether it induced increase of trabecular separation (Fig. 1E). WEPN (0.25 g/kg) significantly prevented RANKL-induced alteration of bone micro-structure parameters.
Fig. 1.
WEPN attenuates RANKL-induced bone loss in mice. Mice were orally administrated with WEPN (0.25 g/kg) twice a day for 5 days, and RANKL were intraperitoneally injected on days 3 and 4. Trabecular bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), and bone mineral density (BMD) at the distal femoral metaphysis were analyzed by micro-CT scanning. Data are expressed as mean + SD. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. WEPN inhibits RANKL-induced osteoclastogenesis
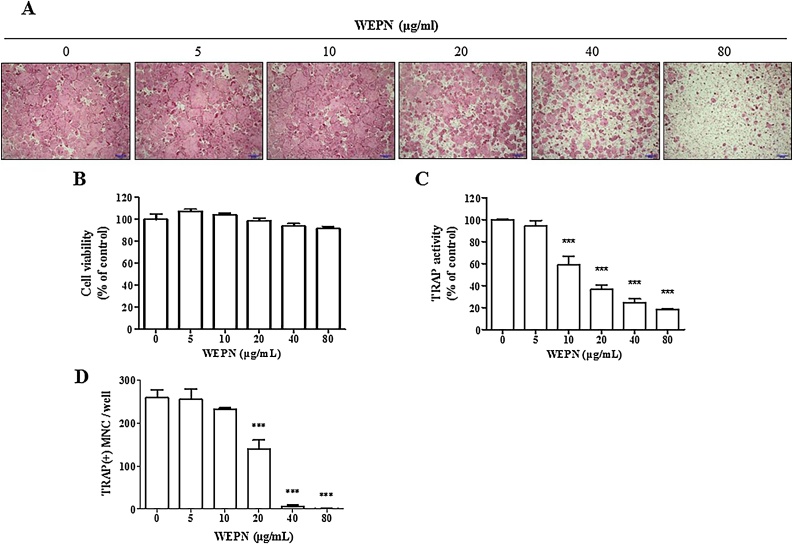
RANKL induces differentiation of bone marrow-derived macrophages (BMMs) into mature osteoclasts. After BMMs were incubated with RANKL for 4 days, tartrate-resistant acid phosphatase (TRAP)-positive stained multinuclear osteoclasts were formed (Fig. 2A). WEPN up to 80 μg/mL obviously decreased TRAP-positive stained osteoclasts without any inhibitory effect on the proliferation of BMMs (Fig. 2A and B). WEPN dose-dependently inhibited RANKL-induced TRAP activity in BMM with 80% inhibition at 80 μg/mL, consistent with TRAP staining results (Fig. 3C). In addition, when we counted number of multinuclear osteoclasts representing mature osteoclasts, WEPN also inhibited osteoclast formation (Fig. 3D). Since we treated WEPN at 0 day, it suggested that WEPN had an inhibitory activity at an early stage of osteoclast differentiation.
Fig. 2.
Effect of WEPN on RANKL-induced osteoclast differentiation in BMMs. BMMs were cultured with vehicle (distilled water) or WEPN (5–80 μg/mL) in the presence of M-CSF (60 ng/mL) and RANKL (100 ng/mL) for 4 days. (A) Cells were stained for TRAP staining. (B) BMMs were cultured with or without WEPN in the presence of M-CSF for 2 days, and cell viability was determined using Cell Counting Kit-8 assay. (C) TRAP activity of osteoclasts were analyzed as described in Section 2. (D) TRAP-positive multinucleated cells containing more than three nuclei and larger than 100 μm in diameter were counted as osteoclasts. ***p < 0.001 vs vehicle-treated control.
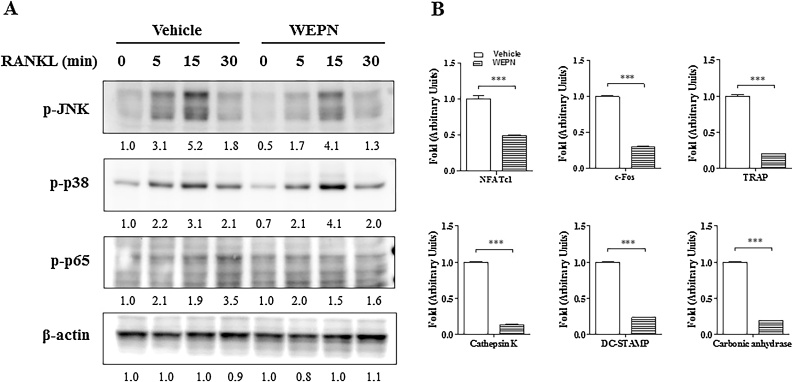
Fig. 3.
Effect of WEPN on RANKL signaling pathway in BMMs. (A) BMMs were pretreated with WEPN (80 μg/mL) in the presence of M-CSF (60 ng/mL) for 1 h and then incubated with RANKL (100 ng/mL) for indicated time points. Total cell lysate (30 μg) was subjected to Western blot analysis with the indicated antibodies. (B) mRNA expression levels of c-Fos, NFATc1, TRAP, cathepsin K, DC-STAMP, and carbonic anhydrate were analyzed by qPCR with specific primers. ***p < 0.001.
3.3. WEPN negatively affects RANKL signaling pathways
RANKL signaling axis consist of MEKK/MAPK or NF-κB/AP-1 pathways to induce or stimulate NFATc1 activation. NFATc1 is an essential transcription factor for all stage of osteoclast differentiation by regulating osteoclast specific transcription. To address the effect of WEPN on RANKL signaling axis, we examined MAPK and NF-κB activation that are upstream of c-Fos and NFATc1 expression in BMMs. We found that WEPN decreased phosphorylation of JNK and p65 (Ser536), but slightly increased p38 (Fig. 3A). Next, we also evaluated NFATc1-regulated osteoclast gene expression (c-Fos, TRAP, cathepsin K, DC-STAMP, carbonic anhydrate) that are specific for osteoclast differentiation. WEPN significantly downregulated those gene expressions (Fig. 3B).
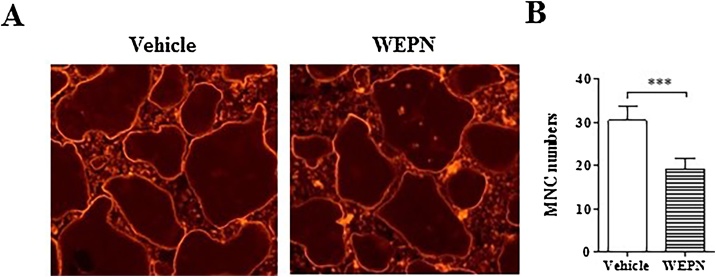
3.4. WEPN inhibits actin ring formation of osteoclasts
Actin ring structure is a specific character observed in mature multinuclear osteoclasts. It is needed for migration, polarization of membrane, and bone resorption of osteoclasts. We evaluated the effect of WEPN on actin ring structure of multinuclear osteoclasts generated by RANKL. Osteoclasts treated with vehicle exhibited a circular ring-like structure of actin rings, however, osteoclasts treated with WEPN generated discontinuous and shrunken actin rings (Fig. 4A). When we counted multinuclear cells (MNC) with actin ring staining, WEPN significantly decreased MNC numbers compared with vehicle (Fig. 4B).
Fig. 4.
Effect of WEPN on actin ring of osteoclasts. (A) Osteoclasts were treated with or without WEPN for 1 h and then stained with phalloidin-TRITC (0.2 μg/mL, Sigma). Representative microscopic images of actin-ring were visualized using an inverted phase contrast fluorescence microscope. (B) Multinuclear cells (MNC) stained were counted.
4. Discussion
Pine needles have been used in Asian countries as a traditional medicine to treat vascular and gastrointestinal diseases. Anti-osteoclastogenic effect of Pine needles extract on TRAP activity of osteoclast-like RAW264.7 cells suggests the potential activity of Pine needles against bone loss. But, the in vivo effect of Pine needles and the molecular mechanism of this activity on osteoclasts remain unknown. In this study, we found the pharmacological effect of WEPN having anti-osteoclastogenic activity on RANKL-induced bone loss model by inhibiting RANKL signaling pathway and osteoclast specific gene expression.
We found that WEPN (0.25 g/kg) significantly attenuated RANKL-induced trabecular bone loss in mice (Fig. 1). An intraperitoneal RANKL injection rapidly induces an increase of osteoclast differentiation as well as resorption of mature osteoclasts resided in trabecular bone.19 It also induces an increase of osteoblast activity for bone formation coupled with increase of osteoclast activity that results in high rate of bone turnover, acceleration of bone remodeling, and deterioration of bone structural parameter in RANKL-induced bone loss model. We found that WEPN inhibited RANKL-induced osteoclast differentiation (Fig. 2). It has also reported an anabolic activity of WEPN to stimulate alkaline phosphatase activity and collagen synthesis of osteoblasts.17 Thus, it could suggest that WEPN activity to inhibit osteoclast differentiation and stimulate osteoblast differentiation might contribute to decrease bone turnover rate by de-coupling of osteoclast and osteoblast activity and thereby, attenuate RANKL-induced trabecular bone loss in vivo. In addition, high dose of WEPN (0.75 g/kg) did not have protective effect on bone loss as much as low dose of WEPN (0.25 g/kg) although it improved some bone micro-parameter (data not shown). It might be resulted from biphasic effect of active components in WEPN having hormone-like activity or having feed-back inhibition activity.21,22 Interestingly, the pathological mechanism of bone cells on RANKL-induced bone loss is similar to ovariectomized-induced osteoporosis which is a golden standard model of menopause or post-menopause-induced osteoporosis.19,23 Regarding the traditional and modern usage of Pine needles as medicinal and edible source in Asia countries,24,25 the pharmacological activity of WEPN against bone loss (Fig. 1) suggest that WEPN could be safe candidate to treat menopause or post-menopause-induced osteoporosis in adult woman. Since we did not directly evaluate the serum level of osteoclast specific marker or osteoclastogenesis from BMM in mice administrated with WEPN, anti-osteoclastogenic activity of WEPN in vivo should be further evaluated more in further study.
We found that WEPN inhibited MAPK/NF-κB activation on RANKL-induced osteoclast differentiation from BMM to osteoclasts (Fig. 3A). WEPN decreased phosphorylation of JNK and p65 (Ser536), but slightly increased p38 (Fig. 3) and did not change phosphorylation of ERK (data not shown). RANKL-induced p38 and JNK phosphorylation by western blot analysis representing its kinase activity is necessary for osteoclast differentiation signaling in BMM.26,27 But, ERK activation is more responsible for osteoclast survival rather than osteoclast differentiation.28,29 In classical NF-κB signaling pathway, IκBα phosphorylation and degradation is followed by nuclear translocation of NF-κB complexes containing the p50 and p65 subunits. Recent study of knock-in mice expressing non-phosphorylation form of p65 (Ser536) suggests that p65 (Ser536) phosphorylation is required for negative regulation of NF-κB signaling by the inhibitor of κB (IκB) kinase (IKK).30 But, it is also reported that MMK6-p38 signaling participates in RANKL-induced transcriptional activity of NF-κB by stimulating p65 phosphorylation on Ser536,26 suggesting the interaction between distinct signaling pathways induced by RANKL. Thus, it could suggest that WEPN negatively affect osteoclastogenesis by altering the cooperated activation of RANKL-stimulated MAPK kinase/MAPK and IKK/NF-κB signaling pathway to regulate NF-κB during osteoclast differentiation.
Previous study reported that water or ethanol extract of pine needle significantly reduces TRAP-positive multinuclear cells from RAW 264.7 cells by stimulating superoxide dismutase activity to scavenge reactive oxygen species (ROS).18 RANKL-induced calcium signaling pathway generates ROS to stimulate cAMP response element-binding protein (CREB) to amplify NFATc1 expression for progress of osteoclast differentiation.31,32 As stimulatory signaling on osteoclast differentiation, MAPK/NF-κB activation is also necessary for an early induction of NFATc1 expression that further stimulates auto-amplification of NFATc1 with other co-stimulatory transcription factors.33, 34, 35 This transcriptional complex cooperate to increase osteoclast-specific gene expression in osteoclasts.8 Thus, the result of this study suggests that WEPN could target different signaling components to inhibit NFATc1 induction (MAPK/NF-κB signaling) as well as NFATc1 amplification (CREB signaling) in RANKL signaling pathway during osteoclast differentiation. Considering WEPN inhibition on stimulatory signaling of NFATc1, it might be possible that WEPN might affect suppressive signaling pathway such as IFN-β to negatively control NFATc1 expression or activation during osteoclast differentiation.36
We found that WEPN inhibited TRAP-positive actin ring formation of multinuclear osteoclasts (Fig. 4A). Actin ring structure is dynamically changed for osteoclast adhesion, migration, and resorption on bone surface. Several actin regulatory proteins such as Rho and c-Src and osteoclast specific genes such as DC-STAMP and cathepsin K play an essential role to generate actin ring and to degrade collagen during resorption process.37 We found that WEPN significantly inhibited RANKL-induced expression of DC-STAMP and cathepsin K (Fig. 3B) as well as inhibited MNC formation of osteoclasts (Fig. 4B). Thus, it could suggest that WEPN inhibition on actin ring formation of multinuclear cells and resorption gene expression might contribute to suppress bone resorption activity of residing osteoclasts to attenuate RANKL-induced bone loss in vivo.
In conclusion, the results of this study demonstrated the inhibitory activity of WEPN against RANKL-induced bone loss and osteoclast differentiation. These data may be helpful for understanding the pharmacological mechanism of pine needle on traditional medicine and provide therapeutic potential of pine needle for treatment of osteoporotic bone diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants (K17281) from the Korea Institute of Oriental Medicine, Ministry of Science, ICT and Future Planning, Korea. We thank to Dr. Hyunil Ha and Dr. Taesoo Kim for in vivo experiments.
References
- 1.Cummings SR, Eastell R. Risk and prevention of fracture in patients with major medical illnesses: a mini-review. J Bone Miner Res. 2016;31:2069–2072. doi: 10.1002/jbmr.3030. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525–535. doi: 10.1359/jbmr.071206. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 5.Poulsen RC, Kruger MC. Soy phytoestrogens: impact on postmenopausal bone loss and mechanisms of action. Nutr Rev. 2008;66:359–374. doi: 10.1111/j.1753-4887.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 8.Song I, Kim JH, Kim K, Jin HM, Youn BU, Kim N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009;583:2435–2440. doi: 10.1016/j.febslet.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL) J Biol Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- 10.Busserolles J, Gueux E, Balasinska B, Piriou Y, Rock E, Rayssiguier Y. In vivo antioxidant activity of procyanidin-rich extracts from grape seed and pine (Pinus maritima) bark in rats. Int J Vitam Nutr Res. 2006;76:22–27. doi: 10.1024/0300-9831.76.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Zeng WC, Zhang Z, Gao H, Jia LR, He Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara) J Food Sci. 2012;77:C824–829. doi: 10.1111/j.1750-3841.2012.02767.x. [DOI] [PubMed] [Google Scholar]
- 12.Tourino S, Selga A, Jimenez A, Julia L, Lozano C, Lizarraga D. Procyanidin fractions from pine (Pinus pinaster) bark: radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J Agric Food Chem. 2005;53:4728–4735. doi: 10.1021/jf050262q. [DOI] [PubMed] [Google Scholar]
- 13.Yen GC, Duh PD, Huang DW, Hsu CL, Fu TY. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem Toxicol. 2008;46:175–185. doi: 10.1016/j.fct.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Pasqualini V, Robles C, Garzino S, Greff S, Bousquet-Melou A, Bonin G. Phenolic compounds content in Pinus halepensis Mill. needles: a bioindicator of air pollution. Chemosphere. 2003;52:239–248. doi: 10.1016/S0045-6535(03)00268-6. [DOI] [PubMed] [Google Scholar]
- 15.Roitto M, Rautio P, Julkunen-Tiitto R, Kukkola E, Huttunen S. Changes in the concentrations of phenolics and photosynthates in Scots pine (Pinus sylvestris L.) seedlings exposed to nickel and copper. Environ Pollut. 2005;137:603–609. doi: 10.1016/j.envpol.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Iwata H, Koya S, Miyamoto K, Wakabayashi K. Plasma triglyceride-decreasing components of pine needles. Biol Pharm Bull. 1997;20:656–661. doi: 10.1248/bpb.20.656. [DOI] [PubMed] [Google Scholar]
- 17.Jeon MHKY, Park YS, Hwang HJ, Kim SG, Lee SH, Choi SI. Effect of pine (Pinus densiflora) needle extracts on synthesis of collagen in osteoblastic MC3T3-E1 cells. J Life Sci. 2010;20:607–613. [Google Scholar]
- 18.Jeon MHPM, Park YS, Hwang HJ, Kim SG, Lee SH, Kim MH. Effect of pine (Pinus densiflora) needle extracts on antioxidant activity and proliferation of osteoclastic RAW 264.7 cells. J Korean Soc Food Sci Nutr. 2011;40:525–530. [Google Scholar]
- 19.Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24:1194–1205. doi: 10.1359/jbmr.090217. [DOI] [PubMed] [Google Scholar]
- 20.Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301:119–127. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Cornelius C, Koverech G, Crupi R, Di Paola R, Koverech A, Lodato F. Osteoporosis and alzheimer pathology: role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front Pharmacol. 2014;5:120. doi: 10.3389/fphar.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono K, Kaneko H, Choudhary S, Pilbeam CC, Lorenzo JA, Akatsu T. Biphasic effect of prostaglandin E2 on osteoclast formation in spleen cell cultures: role of the EP2 receptor. J Bone Miner Res. 2005;20:23–29. doi: 10.1080/14041040510033842. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Crockett JC. Osteoporosis – a current view of pharmacological prevention and treatment. Drug Des Dev Ther. 2013;7:435–448. doi: 10.2147/DDDT.S31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KY, Chung HJ. Flavor compounds of pine sprout tea and pine needle tea. J Agric Food Chem. 2000;48:1269–1272. doi: 10.1021/jf9900229. [DOI] [PubMed] [Google Scholar]
- 25.Kuribara H, Iwata H, Tomioka H, Takahashi R, Goto K, Murohashi N. The anxiolytic effect of Sho-ju-sen, a Japanese herbal medicine, assessed by an elevated plus-maze test in mice. Phytother Res. 2001;15:142–147. doi: 10.1002/ptr.698. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Ryu J, Ha J, Chang EJ, Kim HJ, Kim HM. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006;13:1879–1891. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- 27.David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002;115:4317–4325. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002;277:47366–47372. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- 30.Pradere JP, Hernandez C, Koppe C, Friedman RA, Luedde T, Schwabe RF. Negative regulation of NF-kappaB p65 activity by serine 536 phosphorylation. Sci Signal. 2016;9:ra85. doi: 10.1126/scisignal.aab2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H, Kim T, Jeong BC, Cho IT, Han D, Takegahara N. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 2013;17:249–260. doi: 10.1016/j.cmet.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010;285:6913–6921. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, Kim HH. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006;351:99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 37.Wilson SR, Peters C, Saftig P, Bromme D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J Biol Chem. 2009;284:2584–2592. doi: 10.1074/jbc.M805280200. [DOI] [PMC free article] [PubMed] [Google Scholar]