1. Introduction
Primary percutaneous coronary intervention (P-PCI) is the best available reperfusion strategy in patients with acute ST-segment elevation myocardial infarction (STEMI).1 However establishing myocardial reperfusion by P-PCI is associated with a serious complication called no reflow (NR); defined as final Thrombolysis in myocardial infarction (TIMI) flow <3 or TIMI 3 flow with TIMI myocardial blush grade (TMBG) 0 or 1 in absence of mechanical obstruction.2 NR is considered to be an under-reported complication with a low incidence (1–3%) in large registries, based on TIMI flow grade, MBG and ST resolution.3 Modern more sensitive methods of assessing NR and microcirculatory dysfunction, including myocardial contrast echocardiography (MCE) and cardiac magnetic resonance imaging (CMR), have recorded a higher incidence (10–30%).4 Although these techniques have greater accuracy for detecting post-PCI suboptimal reperfusion, TIMI flow grade is the easiest and most commonly used method of evaluating P-PCI success.5, 6 The objective of the present trial was to identify the prevalence of NR in patients with STEMI undergoing P-PCI in the current era and its predictors with short term outcome.
2. Patients and methods
2.1. Study population
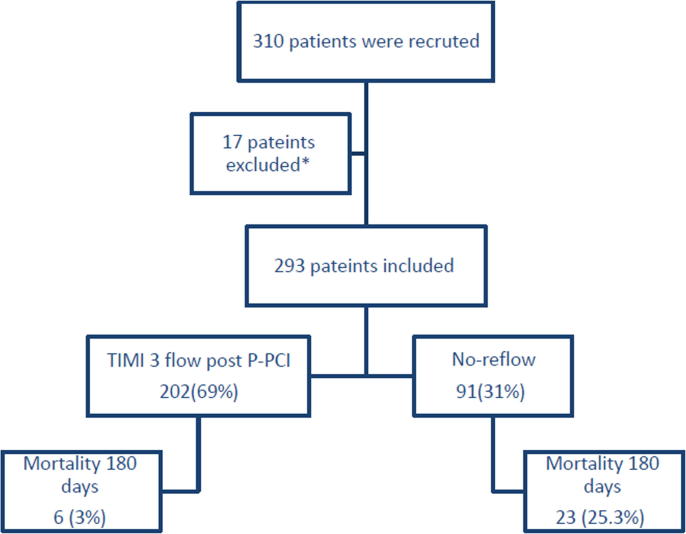
According to the stent for life protocol implemented at Assiut University Hospitals since October 2015,7 all patients from Assiut governorate area with STEMI are subjected to P-PCI. Our hospital provides a round-the-clock service of P-PCI with 16 trained physicians and dedicated nurses. Inclusion criteria were chest pain for >30 min and ST-segment elevation of >0.1 mV in at least 2 contiguous precordial leads, and admission within 12 h from chest pain onset. Exclusion criteria were recent surgery, recent stroke, recent spinal trauma, uncontrolled hypertension, hemorrhagic diatheses, severe liver or kidney failure, cardiogenic shock and known contraindications for therapy with aspirin, clopidogrel or heparin. All patients who fulfilled the P-PCI inclusion criteria according to the stent for life protocol were prospectively included in the study. Patients were divided into 2 groups according to the occurrence of NR. Group I patients with normal flow whose final TIMI flow grade was 3, and group II patients with NR. NR was defined as final TIMI flow grade <3 or with TIMI 3 distal flow but with TMBG 0 or 1 in absence of any spasm or dissection at least 10 min after the end of P-PCI procedure.8 Between 1st of October 2015 and 30th of November 2016, we enrolled 310 consecutive STEMI patients accepted for P-PCI in the study. Seventeen patients were excluded for presence of flow limiting mechanical obstruction either visible dissections or lesions >70% stenosis distal to the implanted stent in the IRA.3 Patient’s medical history, symptoms on arrival, electrocardiographic examination, angiographic, laboratory, echocardiography, clinical follow-up data were recorded in all patients (Fig. 1).
Fig. 1.
Flow chart of the study. P-PCI, primary percutaneous coronary intervention; TIMI, Thrombolysis In Myocardial Infarction; *excluded patients due to flow limiting mechanical obstruction after stenting.
The study was approved by the Assiut University institutional review board and complies with the Declaration of Helsinki. A written informed consent was obtained from all patients.
2.2. Patients medications
All patients received an equivalent of 300 mg of acetylsalicylic acid, 600 mg clopidogrel or 180 mg ticagrilor as a loading dose and heparin given as a bolus of 10,000 IU at the start of the PCI procedure.2 After the procedure, all patients received aspirin (75 mg/d) indefinitely and clopidogrel (75 mg/d) or ticagrilor 90 mg twice daily for one year. Other medications, including beta-blockers, ACE-inhibitors, nitrates, and statins, were prescribed according to standardized protocols.
2.3. Invasive procedure and angiographic evaluation
Coronary angiography was performed by the femoral approach. All patients underwent P-PCI and stenting of the IRA according to standard techniques. Stent implantation was successfully completed in all patients; the choice of stent (bare-metal stent or drug-eluting stent) was left to the operator’s discretion. We performed direct stenting only in cases presenting clear pictures of the arterial lesion. Otherwise, the patient was subjected to balloon angioplasty and stenting was done subsequently. Procedural success was defined as residual stenosis <20% and TIMI flow grade 3. Initial and post procedural TIMI flow grade of the IRA was assessed9. Epicardial coronary blood flow was quantified visually using the TIMI flow grade classification prior to wire insertion and with TMBG after 10 min of P-PCI.2, 8 The TIMI frame count of the coronary artery was obtained using the technique described by Gibson et al.10 Thrombus burden was quantified according to TIMI thrombus grade previously described by Mueller et al.5 The frame rate of a digital cardiac image was calculated at 15 frames/s. Manual thrombectomy was not used routinely but used only as a bailout in high thrombus burden (HTB) patients (Thrombus grade 4–5).11
In case of NR; all patients were managed according to local protocols including intracoronary injection of verapamil, adrenaline and GP 2b/3a inhibitor far distal in the coronary bed.
2.4. Syntax score (SS)
SS was calculated using SS calculator by an expert cardiologist from the baseline angiogram for all patients on discharge as a routine protocol (www.syntaxscore.com).
2.5. Electrocardiographic data
The 12-lead electrocardiogram (ECG) was recorded at presentation and within 90 min after P-PCI. The sum of ST segment elevation was measured 20 ms after the end of the QRS complex (the J point) from leads I, aVL and V1 through V6 for anterior myocardial infarction and leads II, III, aVF, V5 and V6 for inferior myocardial infarction. Patients with bundle branch block who had clear ischemic ST segment elevations were included in this analysis. In addition, subgroups of patients who had a reciprocal ST segment depression ≥0.1 mV were also evaluated. For the total ST segment deviation (no bundle branch block in this group) the sum of ST segment depression in leads II, III and aVF for anterior and that in leads V1-V4 for inferior myocardial infarction were added.
All ECGs were collected and analyzed by an investigator blinded to the assigned treatment. Total ST-segment deviation at inclusion was compared to that taken within 90 min after P-PCI. A complete ST-segment resolution ≥70% of the initial ST-segment deviation was calculated.12
2.6. Clinical outcome
According to the standardized protocol, all patients were seen (1, 3, 6, 9 and 12 months) at a dedicated out-patient clinic for a median of 180 days (90–270 days). Clinical outcome was evaluated through the monitoring of cardiac events at follow up. This included death defined as “all-cause” death at follow-up. Re-infarction was defined as recurrent chest pain (after its complete resolution) with a new elevation in CK (by >50% of the last measured value or ≥2 × normal upper limit) and/or new changes in the ECG (ST-segment elevation, new Q waves). Heart failure was defined as the presence of rales in more than one third of the lung fields that did not clear with coughing or evidence of pulmonary edema on chest radiograph.13 Recurrent typical chest pain on follow up was also documented.
2.7. Sample size calculation
The study was designed to have a 95% power to detect a 30% incidence of NR. With an overall type I error rate of 0.05 (two-sided). Sample size calculated to 278 patients for a population size of 2000 SETMI patients. With regard to drop-out at least 310 patients were planned to be included in the study.
2.8. Statistical analysis
Categorical variables were presented as counts and proportions (percentages) and compared by Pearson chi-square analysis or Fisher exact test. Normal distribution of continuous data was tested using a Kolmogorov-Smirnov test. Continuous and normally distributed data are presented as mean ± 1 SD and were compared by unpaired t test. Not-normally distributed data are expressed as median with interquartile range (IQR), and the Mann-Whitney U test was used to compare differences between two groups. Receiver operator characteristic (ROC) curve analysis was used to determine the cutoff points for significance in different continuous variables. Multivariate logistic and linear regression analysis was performed using all potentially relevant variables to identify baseline independent predictors of NR. All p-values are two-tailed, and statistical significance was defined as a value of p < 0.05. All analyses were performed with SPSS version 24.0 statistical software (SPSS Inc., Chicago, IL, USA).
3. Results
The study population consisted of 293 patients who underwent P-PCI and were treated according to the stent for life protocol. 91(31.06%) patients had NR and 202had normal flow post P-PCI (Fig. 1). Baseline characteristics of the two groups are summarized in Table 1. The NR group were significantly older, were more commonly females, had total ischemic time >4 hours, and more anterior infarctions. No significant difference between the 2 groups regarding mean door to wire time (38.5 ± 13.5 vs. 43.8 ± 20 min, p = 0.09) which comes in agreement with the recent standard guidelines for P-PCI.2 Absence of ST resolution > 70% after 90 min post PCI was a highly significant indicator of NR.
Table 1.
Baseline characteristics and demographic data in patients with and without no-reflow.
| Total N = 293 (100%) |
Occurrence of No-reflow |
P. value | ||
|---|---|---|---|---|
| No N = 202(69%) |
Yes N = 91 (31%) |
|||
| Age > 60 years | 89 (30.4%) | 2 (1%) | 87 (95.6%) | <0.001* |
| Age (Mean ± SD) | 55.6 ± 10.08 | 50.76 ± 7.68 | 66.73 ± 5.49 | <0.001* |
| Female gender | 74 (24.6%) | 46 (21.8%) | 28 (30.77%) | 0.007* |
| Residence | ||||
| From assiut town | 56 (19.1%) | 45 (22.3%) | 11 (14.3%) | 0.108 |
| From near assiut town | 110 (37.5%) | 74 (36.6%) | 36 (39.5%) | |
| >25 Km Far from AUH | 127 (43.34%) | 83 (41.1%) | 44 (48.35%) | |
| Transportation to ED | ||||
| Public | 83 (28.32%) | 51 (25.2%) | 32 (35.16%) | 0.177 |
| Private | 134 (45.7%) | 97 (48%) | 37 (40.65%) | |
| Ambulance | 76 (25.93%) | 54 (26.7%) | 22 (24.18%) | |
| Diabetes mellitus | 82 (27.98%) | 59 (29.2%) | 23 (25.27%) | 0.642 |
| Hypertension | 71 (24.23%) | 56 (27.7%) | 15 (16.48%) | 0.210 |
| History of IHD | 51 (17.4%) | 37 (18.3%) | 14 (15.38%) | 0.909 |
| Smoking | 172 (58.7%) | 128 (63.3%) | 44 (48.35%) | 0.818 |
| Family history of CAD | 71 (24.23%) | 51 (25.2%) | 20 (21.9%) | 0.764 |
| Hypercholesterolemia | 84 (28.7%) | 61 (30.2%) | 23 (25.3%) | 0.888 |
| Pre-infarction angina | 54 (18.4%) | 43 (21.3%) | 11 (12.08%) | 0.233 |
| Symptoms to FMC > 4 h | 154 (52.6%) | 82 (40.6%) | 72 (79.1%) | 0.02 |
| ED to 1st ECG (min) | 8.11 ± 3.05 | 8.12 ± 3.02 | 8.15 ± 3.26 | 0.337 |
| ED to cath. Lab. (min) | 28.3 ± 18.6 | 27.13 ± 19.27 | 31.01 ± 17.94 | 0.784 |
| Door to wire (min) | 40.15 ± 15.32 | 38.5 ± 13.15 | 43.88 ± 20.03 | 0.09 |
| Total ischemic time (hours) | 5.56 ± 3.23 | 4.27 ± 1.76 | 8.55 ± 4.04 | <0.001* |
| Infarction location | ||||
| Anterior Infarctions | 175 (59.7%) | 106 (52.5%) | 69 (75.8%) | 0.009* |
| Inferior infarctions | 110 (37.5%) | 92 (45.5%) | 18 (19.8%) | |
| Others | 8 (2.7%) | 4 (2%) | 4 (4.4%) | |
| Absence of ST resolution > 70% | 91 (20.80%) | 0.00 | 91 (100%) | <0.001* |
Data are presented as mean ± standard deviation or number (%) of patients, MI = myocardial infarction. AUH, Assiut University Hospital; ED, Emergency department; IHD, Ischemic heart disease; CAD, coronary artery disease; FMC, First medical contact; ECG, Electrocardiogram; cath. Lab., catheterization laboratory.
Statistically significant.
Angiographic and procedural results are summarized in Table 2. It reveals that, NR was more frequent in patients who had initial TIMI flow ≤1, mainly in Left anterior descending (LAD), long target lesion ≥21 mm, large vessel diameter ≥3 mm, HTB score ≥4 and high syntax score. Table 2 also reveals that the incidence of NR was significantly lower in the direct stenting group than in stenting with predilation. The NR group had significantly higher mean TIMI frame count (TFC) compared to the normal flow group.
Table 2.
Angiographic and procedural results of the patients with and without no-reflow.
| Total N = 293 (100%) |
Occurrence of No-reflow |
P. value | ||
|---|---|---|---|---|
| No N = 202 (69%) |
Yes N = 91 (31%) |
|||
| Infarct related artery | 0.002* | |||
| Left anterior descending | 174 (59.4%) | 104 (51.5%) | 70 (76.9%) | |
| Circumflex artery | 25 (8.5%) | 20 (9.9%) | 5 (5.5%) | |
| Right coronary artery | 83 (28.3%) | 70 (34.7%) | 13 (14.3%) | |
| Initial TIMI flow grade | ||||
| 0 | 235 (80.2%) | 154 (76.2%) | 81 (89%) | 0.016* |
| 1 | 20 (6.8%) | 14 (6.9%) | 6 (6.6%) | |
| 2 | 20 (6.8%) | 18 (8.9%) | 2 (2.2%) | |
| 3 | 18 (6.1%) | 16 (7.9%) | 2 (2.2%) | |
| Number of diseased vessels (rang) | 1.69 ± 0.76 (1-3) | 1.64 ± 0.77 (1-3) | 1.83 ± 0.73 (1-3) | 0.078 |
| Target lesion location | ||||
| Proximal | 162 (54.4%) | 104 (51.9%) | 58 (63.7%) | 0.257 |
| Mid | 108 (36.9v) | 83 (41.1%) | 25 (27.5%) | |
| Distal | 23 (7.8%) | 15 (7.4%) | 8 (8.8%) | |
| Syntax score | 19.81 ± 6.52 | 18.02 ± 6.17 | 23.45 ± 5.28 | 0.003* |
| Type of occlusion | ||||
| Cut off | 119 (40.6%) | 73 (36.1%) | 46 (50.5%) | 0.014* |
| Tapered | 108 (36.9%) | 76 (37.6%) | 32 (35.2%) | |
| Subtotal | 66 (22.5%) | 53 (26.2%) | 13 (14.3%) | |
| Lesion type | ||||
| Eccentric | 160 (54.6%) | 105 (52%) | 55 (60.4%) | 0.132 |
| Concentric | 133 (45.4%) | 97 (48%) | 36 (39.6%) | |
| Lesion length in mm | 21.5 ± 6.25 | 18 ± 2.1 | 29.04 ± 5.14 | <0.001* |
| Lesion length > 21 mm | 96 (32.8%) | 7 (3.5%) | 89 (97.8%) | <0.001* |
| Thrombus grade ≥ 4 (HTB) | 96 (32.8%) | 13 (6.4%) | 83 (91.2%) | <0.001* |
| Presence of good Collateral flow | 77 (26.27%) | 56 (27.7%) | 21 (28.4%) | 0.914 |
| Reference lumen diameter, mm | 2.94 ± 0.53 | 2.67 ± 0.33 | 3.51 ± 0.34 | 0.015* |
| Reference lumen diameter ≥ 3 mm | 90 (30.9%) | 5 (2.5%) | 85 (93.4%) | <0.001* |
| Method of reperfusion used | ||||
| Balloon Angioplasty | 17 (5.8%) | 8 (3.9%) | 9 (9.9%) | 0.008* |
| Stenting with pre-dilatation | 227 (77.5%) | 150 (74.2%) | 77 (84.6%) | |
| Direct Stenting | 49 (17.8%) | 44 (21.7%) | 5 (6.7%) | |
| Usage of thrombus aspiration | 73 (24.9%) | 50 (24.8%) | 23 (25.3%) | 0.91 |
| Stent length in mm | 27.93 ± 9.16 | 26.41 ± 8.93 | 30.55 ± 8.26 | 0.303 |
| Stent type | ||||
| Bare metal stent | 213 (77.14%) | 156 (77.2%) | 57 (77%) | 0.423 |
| Drug eluting stent | 46 (16.66%) | 37 (18.3%) | 9 (12.2%) | |
| Maximal inflation pressure (atm) | 14.04 ± 2.38 | 14.09 ± 2.21 | 14.06 ± 2.62 | 0.082 |
| Number of stents used | 1.12 ± 0.33 | 1.12 ± 0.34 | 1.09 ± 0.29 | 0.225 |
| TIMI Frame count (TFC) | 21.19 ± 9.12 | 15.99 ± 3.29 | 31.81 ± 6.35 | <0.001* |
| TFC > 22 frames | 90 (30.9%) | 6 (3%) | 84 (92.3%) | <0.001* |
Data are presented as mean ± standard deviation or number (%) of patients. TIMI , Thrombolysis In Myocardial Infarction; mm, millimeter ; HTB, high thrombus burden.
Statistically significant.
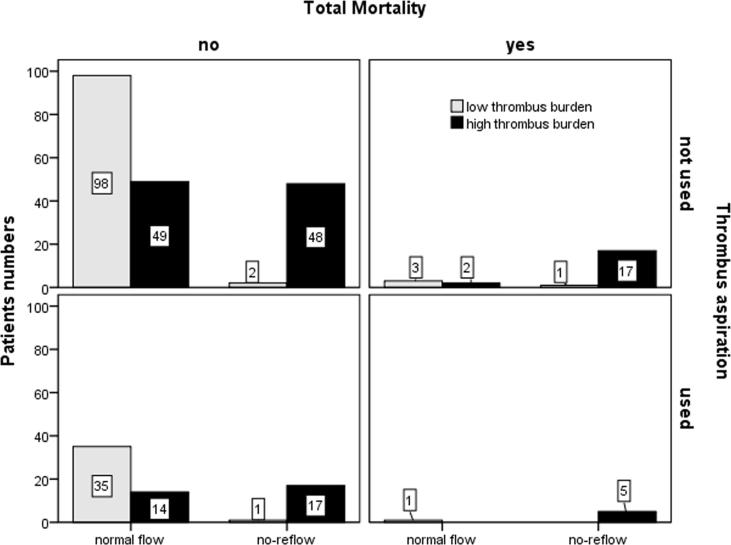
Regarding six-month clinical outcome, all patients were followed for a median of 180 days (range 90–270 days). Total mortality occurred in 29 patients; the incidence of death was higher in the NR group than in patients with the normal flow (25.3% vs. 3%, p = 0.003) (Table 3). The incidence of heart failure was significantly higher in the NR group (36% vs. 8%; p = 0.001). No differences were noted between the two groups with regard to the occurrence of re-infarction. No intracranial bleeding occurred. Determination of cut-off point for significant variables was done using ROC analysis. This revealed the following indicators: age >60 (sensitivity 95.6%, specificity 99%), Total ischemic time both >4 h (sensitivity 97.3%, specificity 95.7%), long lesion length >21 mm (sensitivity 97.8%, specificity 96.5%), RLD ≥3 mm (sensitivity 93.4%, specificity 97.5%), TFC > 22 frames (sensitivity 92.3%, specificity 97.0%), thrombus grade ≥ 4 (sensitivity 91.2%, specificity 93.6%) and low initial TIMI ≤1 (sensitivity 94.7%, specificity 96.5%). Univariate then multivariate binary logistic regression analysis; including all these indicators and risk factors; identified that high thrombus burden ≥4 (OR = 58.7, 95% CI = 15.23–226.7, p < 0.001), reference luminal diameter ≥3 mm, long symptoms to first medical contact time ≥4 h, anterior infarctions and syntax score ≥19 were independent predictors of NR. After adjustment for covariates, the use of thrombus aspiration in a high risk group of patients with high thrombus burden was associated with significant protection against NR (OR = 0.424, 95% CI = 0.22–0.82, p = 0.011) (Table 4, Fig. 2).
Table 3.
Clinical outcome and complications at 180 day follow-up in patients with and without no-reflow.
| Total N = 293 (100%) |
Occurrence of No-reflow |
P. value | ||
|---|---|---|---|---|
| NO N = 202(69%) |
Yes N = 91 (31%) |
|||
| Recurrent chest pain | 60 (18.43%) | 10 (4.95%) | 50 (54.9%) | 0.004* |
| Re-infarction | 8 (6.04%) | 2 (1%) | 6 (6.6%) | 0.05 |
| Heart failure | 50 (17.06%) | 17 (8.4%) | 33 (36.3%) | 0.001* |
| In-hospital mortality | 19 (6.48%) | 3 (1.49%) | 16 (17.6%)) | 0.001* |
| Total mortality 180 days | 29 (9.9%) | 6 (3%) | 23 (25.3%) | 0.003* |
Table 4.
Uni-variate and multi-variate regression analysis for possible predictors of no-reflow.
| Predictors | Uni-variate regression analysis |
Multi-variate regression analysis |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Age ≥ 60 | 1.73 | (1.1–2.86) | 0.032* | 0.99 | (0.41–2.43) | 0.997 |
| Female gender | 1.948 | (1.13–3.36) | 0.016* | 2.272 | (0.83–6.3) | 0.11 |
| Symptoms- FMC ≥ 4 h | 6.91 | (3.39–14.1) | 0.001* | 7.794 | (2.45–24.8) | <0.001* |
| Anterior Infarctions | 2.84 | (1.63–4.9) | 0.001* | 5.951 | (1.942–18.24) | 0.002* |
| Initial TIMI ≤ 1 | 4.40 | (1.51–12.8) | 0.007* | 0.236 | (0.04–1.6) | 0.13 |
| Syntax score ≥ 19.22 | 6.89 | (3.6–13.2) | <0.001* | 6.515 | (2.26–18.78) | 0.001* |
| Lesion length ≥ 21 mm | 2.82 | (1.54–5.2) | <0.001* | 1.049 | (0.36–3.04) | 0.93 |
| Reference lumen diameter ≥ 3 mm | 16.78 | (5.93–47.5) | <0.001* | 31.066 | (7.94–121.5) | <0.001* |
| High thrombus burden | 45.84 | (16.1–130.3) | <0.001* | 58.764 | (15.23–226.7) | <0.001* |
| Direct stenting | 0.12 | (0.03–0.4) | <0.001* | 0.866 | (0.23–3.26) | 0.83 |
| Thrombus aspiration# | 0.42 | (0.22–0.82) | 0.011* | |||
FMC, First medical contact; TIMI, Thrombolysis In Myocardial Infarction; mm, millimeter.
Statistically significant.
After correction of Co-varieties including only patients with high thrombus burden.
Fig. 2.
Shows the relation between thrombus burden, occurrence of no-reflow, usage of thrombus aspiration and incidence of total mortality. Patients with high thrombus burden significantly had higher incidence of no-reflow and the usage of thrombus aspiration significantly reduced the incidence of no-reflow and mortality especially in patients with high thrombus burden.
4. Discussion
Key findings of the present study are: (1) the rate of NR phenomenon after P-PCI was 31%, (2) high thrombus burden in large caliber vessel with higher Syntax score presenting late with anterior MI are independent predictors of NR in our study, (3) total mortality and HF were significantly increased in patients with NR at 6 months follow-up, (4) Thrombus aspiration provides protection against NR in selected patients with high thrombus burden.
The incidence of NR in our study was 31% which comes in agreement with multiples studies with the same sample size. Sahin et al.14 used the same NR definition and reported up to 33% incidence of NR in agreement with our study. Niccoli and his colleges8 presented a detailed review on the NR phenomena and reported an incidence of NR as high as 50%. A low incidence of 1–3% has been recorded in large registries3 based on TIMI flow grade, TMBG and ST resolution while NR in small studies has been reported in up to 30% utilizing TIMI grade.14, 15
In our study, patients with long reperfusion time (≥4 h) had a significantly greater thrombus burden and a significant increase in NR rates than patients with short reperfusion times. It is well established that prolonged ischemia leads to edema of distal capillary beds, swelling of myocardial cells, neutrophil plugging and alterations of capillary integrity. Furthermore, delayed reperfusion can result in an older, more organized intracoronary thrombus, which may increase the risk of distal embolization during P-PCI.16 In our study, the main factor for prolonged total ischemic time was patient's delay. Lack of general awareness concerning differentiation of chest pain, delay in seeking for medical advice especially in females and poverty were factors triggering patients delay in our locality. Transportation also played a role; public transportations prolong symptom-FMC time, while ambulance transportations foreshorten these time delays.17 In our study only 24% of patients with NR were using ambulance transportation. In Analysis of Time Intervals Related to STEMI Management, the key factor affecting the total ischemic time was the patients’ choice of the mode of transportation. Patients who opted for the ambulance, the intervals were significantly shorter. The principal delays were detected in the patients’ delay which is consistent with us.18 In Factors Affecting Time to Presentation in ST-Elevation Myocardial Infarction (FAT STEMI) study, patients who utilized emergency medical system (EMS) were associated with shorter time to presentation than self-presented patients.19
In agreement with our results, Kirma et al.9 showed that high thrombus burden (≥4) was the main driving factor for NR. We found that anterior infarctions were associated with increased risk of NR especially with LAD coronary artery involvement which was involved alone in 46% of NR group in agreement with Ayad et al. study.17 Also, wider RLD ≥3 mm was associated with increased risk of NR (93.4% of NR group had RLD ≥3 mm), this comes consistent with Kirma et al. who showed that large vessel diameter is associated with increased incidence of NR.9
Concerning high syntax score, the cut-off point above which the syntax score became an independent predictor of NR in our study was 19.22. This result was consistent with the study of Sahin et al, in which the mean SS of the NR group was higher than that of the normal flow group and the cutoff value of SS obtained by the ROC curve analysis was 19.75.15
Regarding thrombus aspiration; thrombus aspiration usage in our study, decreased the NR risk in patients with HTB by 58% (OR = 0.42, 95% CI = 0.22–0.82, p = 0.01). Two large randomized controlled trials showed no benefit on clinical outcomes of routine aspiration strategy overall.20 Furthermore, a safety concern emerged in the TOTAL trial with an increase in the risk of stroke.21 In the Taste20 and TOTAL trials21, routine thrombus aspiration is not recommended, but in cases of large residual thrombus burden after opening the vessel with a guide wire or a balloon, thrombus aspiration as a bailout strategy may be considered and it's an area for further studies.21 REMEDIA trial, which was the first randomized trial to assess the role of manual aspiration thrombectomy, reported an improved myocardial perfusion compared to standard PCI22. BCIS-NICOP registry found no significant mortality difference between the overall thrombectomy and the no thrombectomy groups at 30 days or 1 year.23 In our study, thrombus aspiration usage was protective as a bailout strategy only in HTB. This concept comes in agreement with REMEDIA sub-study24 and Svilaas et al study25; both showed that thrombus aspiration significantly reduced the extent of microvascular obstruction and myocardial dysfunction. Hence, NR is associated with increased mortality, thrombus aspiration decrease the mortality risk in these high risk patients.
Final remarks, the high rate of NR in our study was mainly due to patient's delay which necessitates further efforts for community orientation and development of mature EMS with good networking between university hospitals, providing P-PCI, and community hospitals in upper Egypt. This could be the road for minimizing time loss and improving outcomes.
Our study has few limitations including, small sample size and lack of follow up by control angiography, myocardial contrast echocardiography and cardiac magnetic resonance imaging to determine if NR was of transient or persistent type contributed to higher rates of NR in our institute.
5. Conclusion
In the contemporary era of P-PCI, NR is more likely to occur in patients with high thrombus burden presenting late and is still associated with marked increases in adverse outcomes. Thrombus aspiration can prevent NR in patients with high thrombus burden.
Statement of responsibility
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Funding
The authors received no specific funding for this work.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
All authors listed takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.Gupta S., Gupta M.M. No reflow phenomenon in percutaneous coronary interventions in st-segment elevation myocardial infarction. IHJ. 2016;68:539–551. doi: 10.1016/j.ihj.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibanez B., James S., Agewall S. 2017 esc guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevationthe task force for the management of acute myocardial infarction in patients presenting with st-segment elevation of the european society of cardiology (esc) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.Harrison R.W., Aggarwal A., Ou F-S. Registry ACoCNCD. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2013;111:178–184. doi: 10.1016/j.amjcard.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Ito H. Etiology and clinical implications of microvascular dysfunction in patients with acute myocardial infarction. Int Heart J. 2014;55:185–189. doi: 10.1536/ihj.14-057. [DOI] [PubMed] [Google Scholar]
- 5.Mueller H., Dyer A., Greenberg M. The thrombolysis in myocardial infarction (timi) trial. Phase i findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 6.Galiuto L. Optimal therapeutic strategies in the setting of post-infarct no reflow: the need for a pathogenetic classification. 2004 [DOI] [PMC free article] [PubMed]
- 7.Sobhy M., Sadaka M., Okasha N. Stent for life initiative placed at the forefront in egypt 2011. EuroIntervention: J EuroPCR Collaboration Working Group Intervent Cardiol Euro Soc Cardiol. 2012;8:P108–P115. doi: 10.4244/EIJV8SPA19. [DOI] [PubMed] [Google Scholar]
- 8.Niccoli G., Scalone G., Lerman A., Crea F. Coronary microvascular obstruction in acute myocardial infarction. EUR Heart J. 2015;37:1024–1033. doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 9.Kirma C., Izgi A., Dundar C. Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions. Circulation. 2008;72:716–721. doi: 10.1253/circj.72.716. [DOI] [PubMed] [Google Scholar]
- 10.Hamada S., Nishiue T., Nakamura S. Timi frame count immediately after primary coronary angioplasty as a predictor of functional recovery in patients with timi 3 reperfused acute myocardial infarction. JACC: Cardiovasc Intervent. 2001;38:666–671. doi: 10.1016/s0735-1097(01)01424-3. [DOI] [PubMed] [Google Scholar]
- 11.Jolly S.S., James S.K., Džavík V. Thrombus aspiration in st elevation myocardial infarction: an individual patient meta-analysis. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.117.028198. Circulationaha. 116.025371. [DOI] [PubMed] [Google Scholar]
- 12.Durante A., Camici P.G. Novel insights into an “old” phenomenon: the no reflow. IJC Heart Vasculature. 2015;187:273–280. doi: 10.1016/j.ijcard.2015.03.359. [DOI] [PubMed] [Google Scholar]
- 13.McMurray J.J., Adamopoulos S., Anker S.D. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 14.Niccoli G., Burzotta F., Galiuto L., Crea F. Myocardial no-reflow in humans. JACC: Cardiovasc Intervent. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Sahin D.Y., Gür M., Elbasan Z. Syntax score is a predictor of angiographic no-reflow in patients with st-elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24:148–153. doi: 10.1097/MCA.0b013e32835c4719. [DOI] [PubMed] [Google Scholar]
- 16.Nagata Y., Usuda K., Uchiyama A. Pathological analysis of intracoronary thrombus (thrombectomy catheter samples) in acute coronary syndrome. Circulation J: Off J Jpn Circulation Soc. 2003;67:495. [Google Scholar]
- 17.Ayad S.W. Aspiration versus no aspiration during primary pci for st-segment elevation myocardial infarction. Egypt Heart J. 2016;68:147–152. [Google Scholar]
- 18.Francek L., Hlinomaz O., Groch L., Bělašková S. Analysis of time intervals related to stemi management in 2008–2016. Cor et Vasa. 2017 [Google Scholar]
- 19.Hamilton D., Tankazyan H., Khachatryan T., Desai A., Evans J. Factors affecting time to presentation in st-elevation myocardial infarction (fat stemi) J Clin Exp Cardiolog. 2017;8:2. [Google Scholar]
- 20.Fröbert O., Lagerqvist B., Olivecrona G.K. Thrombus aspiration during st-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 21.Jolly S.S., Cairns J.A., Yusuf S. Stroke in the total trial: a randomized trial of routine thrombectomy vs. Percutaneous coronary intervention alone in st elevation myocardial infarction. EUR Heart J. 2015;36:2364–2372. doi: 10.1093/eurheartj/ehv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burzotta F., Trani C., Romagnoli E. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (remedia) trial. JACC: Cardiovasc Intervent. 2005;46:371–376. doi: 10.1016/j.jacc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 23.Sirker A., Mamas M., Kwok C.S., Kontopantelis E., Ludman P., Hildick-Smith D. Outcomes from selective use of thrombectomy in patients undergoing primary percutaneous coronary intervention for st-segment elevation myocardial infarction: an analysis of the british cardiovascular intervention society/national institute for cardiovascular outcomes research (bcis-nicor) registry, 2006–2013. JACC: Cardiovasc Intervent. 2016;9:126–134. doi: 10.1016/j.jcin.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Galiuto L., Garramone B., Burzotta F. Investigators R. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention. JACC: Cardiovasc Intervent. 2006;48:1355–1360. doi: 10.1016/j.jacc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Svilaas T., Vlaar P.J., van der Horst I.C. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]