Abstract
Ripening of the model fruit tomato (Solanum lycopersicum) is controlled by a transcription factor network including NAC (NAM, ATAF1/2, and CUC2) domain proteins such as No-ripening (NOR), SlNAC1, and SlNAC4, but very little is known about the NAC targets or how they regulate ripening. Here, we conducted a systematic search of fruit-expressed NAC genes and showed that silencing NOR-like1 (Solyc07g063420) using virus-induced gene silencing (VIGS) inhibited specific aspects of ripening. Ripening initiation was delayed by 14 days when NOR-like1 function was inactivated by CRISPR/Cas9 and fruits showed obviously reduced ethylene production, retarded softening and chlorophyll loss, and reduced lycopene accumulation. RNA-sequencing profiling and gene promoter analysis suggested that genes involved in ethylene biosynthesis (SlACS2, SlACS4), color formation (SlGgpps2, SlSGR1), and cell wall metabolism (SlPG2a, SlPL, SlCEL2, and SlEXP1) are direct targets of NOR-like1. Electrophoretic mobility shift assays (EMSA), chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR), and dual-luciferase reporter assay (DLR) confirmed that NOR-like1 bound to the promoters of these genes both in vitro and in vivo, and activated their expression. Our findings demonstrate that NOR-like1 is a new positive regulator of tomato fruit ripening, with an important role in the transcriptional regulatory network.
Crop genetics: novel gene involved in tomato ripening
Chinese researchers have identified a new gene which regulates the ripening of tomatoes. Several genes known to control tomato ripening are members of the NAC family of regulators. To identify others, a team led by Daqi Fu of China Agricultural University blocked the expression of candidate NAC genes. The discovered that silencing NOR-like1 repressed ripening, leaving the tomatoes partially green. The team also engineered plants with defective copies of NOR-like1 and found that this delayed ripening and eventually resulted in partially ripe fruit and impaired seed development. RNA sequencing of these lines revealed that NOR-like1 directly regulates genes involved in ethylene synthesis, carotenoid accumluation, chlorophyll metabolism, and cell wall breakdown. These findings clearly demonstrate a key role for NOR-like1 as a positive regulator of tomato ripening and a potential tool for controlling this important process.
Introduction
Tomato fruit ripening is regulated by endogenous hormones, environmental signals and genetic regulators acting in a network that determines the specific expression of ripening-associated genes1–3. Transcriptional regulation has a very important role during tomato fruit ripening and the identification of target genes of transcription factors (TFs) is important for understanding the mechanism of ripening regulation4,5.
Early studies on the transcriptional regulation of fruit ripening were based on the characterization of natural fruit ripening mutants6 such as ripening-inhibitor (rin)7 and colorless non-ripening (Cnr)8. MADS-RIN was identified by the cloning of the rin mutant, which inhibited almost all measured ripening phenomena and the mutation was caused by a deletion of a genomic DNA fragment between RIN and macrocalyx (MC) , forming a chimeric gene (RIN-MC) that was thought to be a loss of function mutation. MADS-RIN was considered to regulate ripening, whereas MADS-MC was thought to affect sepal development and inflorescence determinacy7. However, the role of MADS-RIN in tomato fruit ripening was recently re-assessed in studies that showed the mutated RIN-MC was actually translated into a functional transcription factor to regulate many genes involved in tomato fruit ripening9,10. The Cnr mutant, which is colorless and has altered fruit pericarp texture, results from a spontaneous epigenetic change in the SBP-box gene LeSPL-CNR promoter, whereas the CNR gene sequence, itself remains unaltered8. Map-based cloning showed the no-ripening (nor) mutant was due to the loss of two A bases in the NOR coding sequence, which resulted in the early termination of protein translation (Patent No.: US 6,762,347 B1). Yuan et al.11 compared the proteome of the nor mutant with that in wild-type fruits used isobaric tags for relative and absolute quantitation (iTRAQ) and showed that many proteins involved in fruit ripening, quality and disease resistance were altered. NOR is a NAC family transcription factor, having a global role in tomato fruit ripening, but compared with the other two ripening-related mutants rin and Cnr, the mechanism NOR regulate tomato fruit ripening is unclear. A recent study confirmed that NAC-NOR mutations in tomato Penjar accessions attenuated multiple metabolic processes and prolonged fruit shelf life12.
Identification of other crucial ripening-associated TFs has recently shed more light on the complex mechanism of tomato fruit ripening. TAGL1, a MADS transcription factor, directly activated the expression of ethylene biosynthetic genes SlACS2 in tomato fruits13. TAGL1-silenced fruits remain yellow and ethylene production is decreased significantly14. Two additional MADS family TFs, FUL1/FUL2 also have crucial roles in tomato fruit ripening. FUL1/FUL2-silenced tomato fruits are orange-ripe and do not regulate ethylene biosynthesis15. Fujisawa et al.16 demonstrated that FUL homologs, RIN, and TAGL1 form a DNA-binding complex, and probably regulate tomato fruit ripening through tetrameric complexes. Another MADS TF, SlMADS1, can interact with RIN, but acts as a negative regulator of fruit ripening17. SlAP2a is a negative regulator of ethylene biosynthesis, and SlAP2a-RNAi tomato lines over-produce ethylene18. An additional TF, LeHB-1, encodes a homeobox protein that binds in vitro to the promoter of the ethylene biosynthesis gene LeACO1, which encodes the final enzyme required for ethylene synthesis19.
NAC TFs (comprising NAM, ATAF, and CUC members) are a plant-specific super family and different members have a variety of important functions. Most NAC proteins contain a highly conserved N-terminal DNA-binding domain, and a variable C-terminal domain20. The tomato genome contains 101 NAC TFs21. In addition to the famous NOR6,22, two other NAC family genes SlNAC1 and SlNAC4 have been shown to be involved in the regulation of tomato fruit ripening23–25. In SlNAC1-overexpressing fruits, ethylene production was decreased, fruit softened earlier and remained yellow or orange when fully ripe23. In SlNAC1-RNAi transgenic lines, although ethylene production was delayed, it eventually reached a higher level than that in WT24. Reduced SlNAC4 expression by RNAi delayed fruit ripening by 2–3 days25. Despite the large number of NAC genes in plants, there is no information about the possible role of other NAC family members associated with fruit ripening, and the precise target genes of NOR, SlNAC1, and SlNAC4 are still unclear.
In this report, we used TRV-mediated VIGS to screen NAC TFs candidates and found that silencing NOR-like1 (Solyc07g063420), markedly suppressed tomato fruit ripening. We also obtained two stable knock-out mutants of nor-like1 by CRISPR/Cas9 and they showed a similar phenotype to the VIGS-NOR-like1 silenced fruit. Further study showed that NOR-like1 directly binds to the promoters of several genes involved in tomato ripening processes, including ethylene biosynthesis, color change, and cell wall metabolism, and positively regulates their expression. Our data define important NOR-like1 targets and establish the role of NOR-like1 as a new positive regulator of tomato fruit ripening.
Results
Virus-induced gene silencing of NOR-like1 delayed tomato fruit ripening
In a systematic study of NAC TF genes, potentially related to tomato fruit ripening, we obtained a striking inhibition of ripening in VIGS fruits infected with a TRV-NOR-like1 (Solyc07g063420) construct. Fruit showed mottled green and orange areas, separated by a distinct border, which contrasted with the uniform orange phenotype observed in the control fruit at B + 5 (5 days after color break) (Fig. 1a), suggesting that NOR-like1 may be involved in regulating tomato fruit ripening. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) result showed that the NOR-like1 transcripts in the green sections of TRV-NOR-like1-infected fruit were significantly lower than in the orange sections and the green and orange stage of the control fruits infected by TRV alone (Fig. 1b), confirming that NOR-like1 gene silencing was associated with the uneven color phenotype.
Fig. 1. Virus-induced gene silencing of NOR-like1 delays tomato fruit ripening.
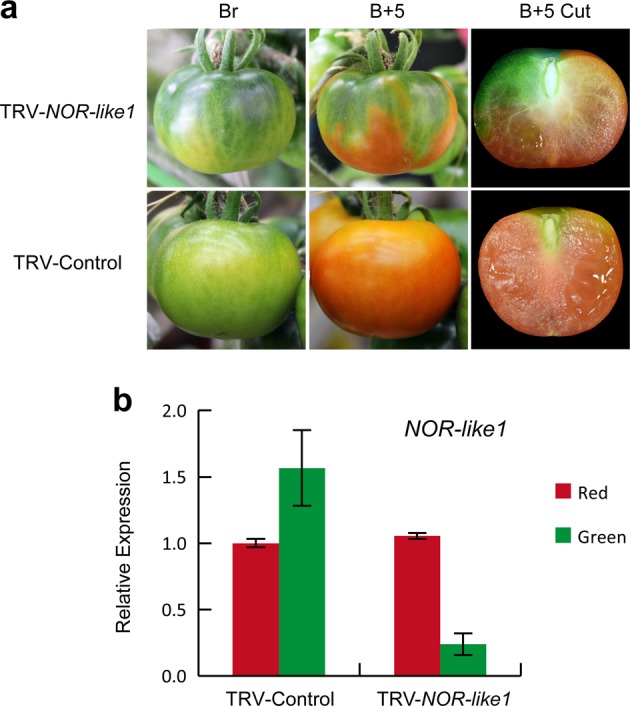
a Virus-induced gene silencing assay in wild-type (WT) tomato fruits (Ailsa Craig, AC) shows the effect of NOR-like1 on fruit ripening. Br, breaker; B + 5, 5 days after breaker. b qRT-PCR analysis of NOR-like1 transcripts in TRV-NOR-like1 and TRV-control tomato fruits at B + 5 stage. Actin was used as the internal control. Values are means ± SD of three independent replicates
NOR-like1 is located in the nucleus and highly expressed in tomato fruit
Phylogenetic analysis of NOR-like1 and other NAC proteins in Arabidopsis and tomato showed that NOR-like1 and NOR (sometimes referred to NAC-NOR) are closely related (Supplemental Figure S1). The NOR-like1 gene is 2811 bp long, comprises three exons and two introns, encodes a protein of approximately 37 KD (329 amino acid), with a conserved NAC domain at the N-terminus and an un-conserved transcriptional activation domain at the C-terminus (Supplemental Figure S2), all typical features of the NAC TF family.
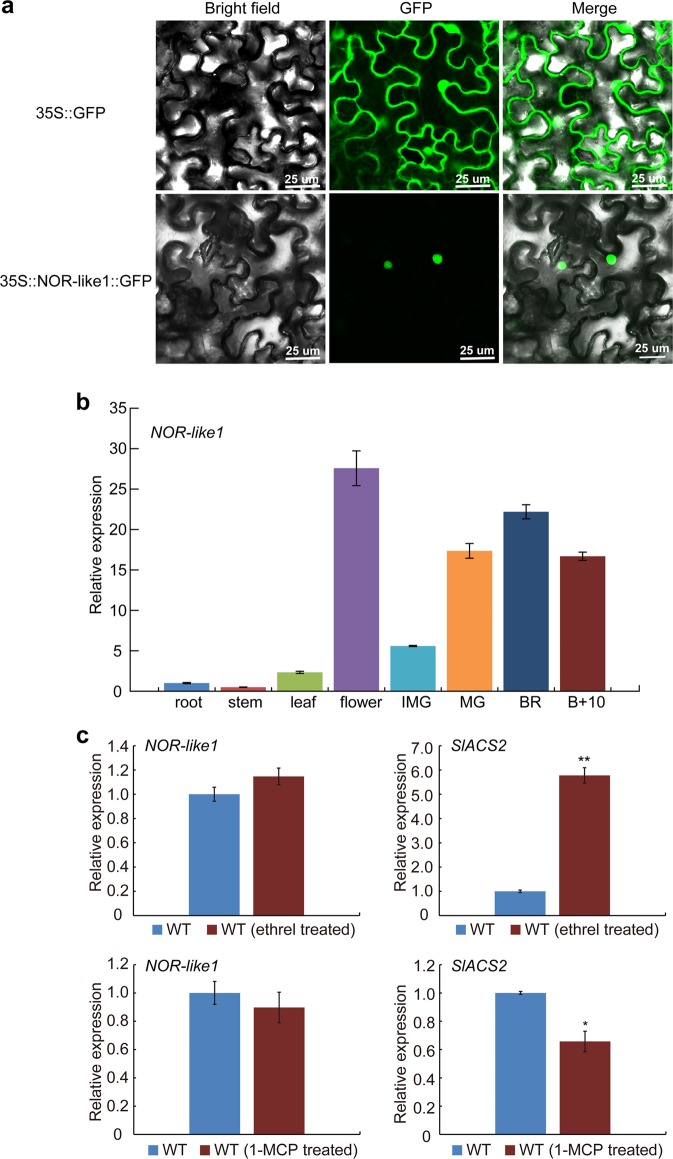
Subcellular localization of NOR-like1 protein clearly indicated that the NOR-like1:GFP fusion protein was exclusively localized in the nucleus (Fig. 2a), consistent with a putative role in transcriptional regulation.
Fig. 2. Subcellular localization of NOR-like1 in nuclei, gene expression pattern of NOR-like1 and the expression of NOR-like1 in WT fruit after treatment with ethrel and 1-MCP.
a Subcellular localization of NOR-like1 in nuclei. Tobacco leaves were used for subcellular localization. Green fluorescence images were taken in a dark field, while the outline of the cell was photographed in a bright field. 35S:NOR-like1:GFP represents NOR-like1 and GFP fusion protein. 35S:GFP represents the control. Bars = 25 μm. b qRT-PCR analyses of NOR-like1 in different tomato organs and fruit ripening stages. IMG, immature green. Actin was used as the internal control. Bars represent ± SD of three independent replicates. c Expression of NOR-like1 in WT fruit after treated with ethrel and 1-MCP. Actin was used as the internal control. SlACS2 was detected as the positive control. Error bars indicate ± SD of three biological replicates. Asterisks indicate significant differences determined by Student’s t-test (**p < 0.01, *p < 0.05)
During tomato plant growth and development, NOR-like1 transcripts were present in much lower amounts in vegetative organs such as roots, stems, and leaves, but were highly expressed in reproductive organs such as flowers and fruits. During fruit ripening, the expression of NOR-like1 increased rapidly from the mature green (MG) stage and reached the highest expression level at the breaker stage (Fig. 2b). But the expression of NOR-like1 was not affected by exogenous ethylene or the ethylene perception inhibitor 1-Methylcyclopropene (1-MCP) (Fig. 2c). The expression pattern and insensitivity to ethylene suggested that NOR-like1 may have an active role in fruit ripening initiation or development.
NOR-like1 CRISPR/Cas9-edited tomato fruits fail to produce NOR-like1 protein and ripening is significantly inhibited
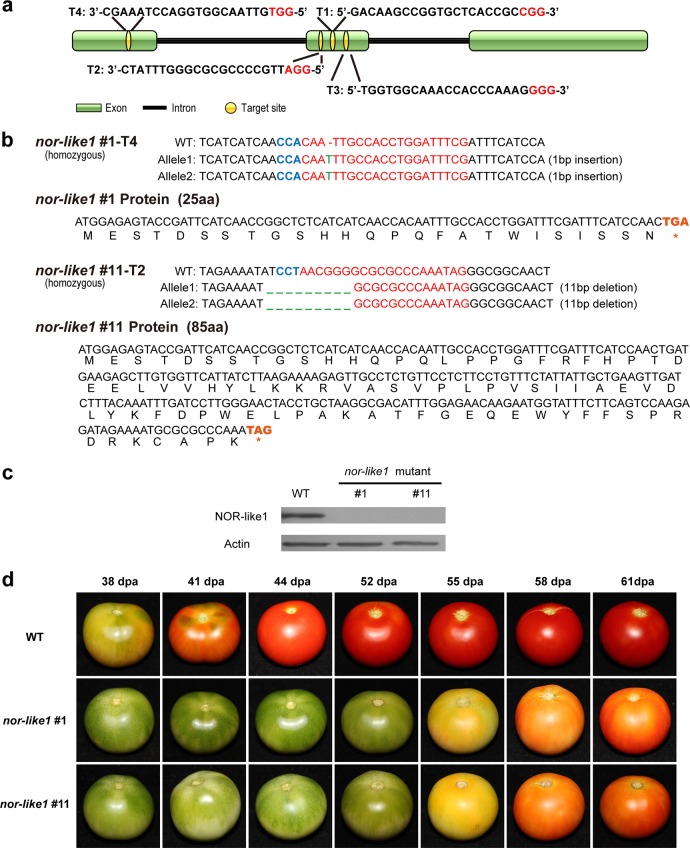
To gain insight into the function of NOR-like1 in tomato fruit, we generated NOR-like1 knock-out mutants in tomato cultivar Ailsa Craig (AC) using CRISPR/Cas9 gene editing technology. In total, sixteen independent T0 transgenic lines were produced (Supplemental Figure S3a). Of these lines, six were genome edited and the editing rates of each target sites are shown in Supplemental Figure S3b. We selected two representative T0 transgenic lines (CR-NOR-like1#1 and CR-NOR-like1#11) for further analysis (the editing details of the two lines are shown in Supplemental Figure S3c). In the two lines, the average number of days from anthesis to the breaker stage of ripening was increased and the rate of ripening after the breaker stage was inhibited (Supplemental Figure S4). Then we obtained two homozygous lines from CR-NOR-like1#1 and CR-NOR-like1#11, named nor-like1#1 and nor-like1#11, respectively (details shown in Fig. 3b). To confirm the premature termination of NOR-like1 protein translation, the expression levels of NOR-like1 protein in nor-like1#1, nor-like1#11, and WT tomato fruit at the breaker stage were examined by western blot using NOR-like1-specific antibody produced from the C-terminus of NOR-like1 protein. The results showed that the mature NOR-like1 protein was absent from nor-like1#1 and nor-like1#11 mutants compared with that in WT fruit (Fig. 3c), suggesting that these two nor-like1 mutant lines were prematurely terminated at the N-terminus of the NOR-like1 protein.
Fig. 3. Tomato fruit ripening was greatly delayed in nor-like1 mutants.
a Schematic illustration of four target sites in NOR-like1 genomic sequence. b Gene editing analysis of nor-like1#1 and nor-like1#11 mutants. Red letters indicate the target sites, green letters represent edited site and editing type, and blue letters represent the protospacer adjacent motif (PAM). c The absence of antibody-detectable NOR-like1 protein in nor-like1#1 and nor-like1#11 mutants. Actin was used as the internal control. d Tomato fruit ripening was greatly delayed in nor-like1#1 and nor-like1#11 mutants compared with that in WT. Dpa, days post anthesis
To test for off-target gene editing in nor-like1#1 and nor-like1#11 mutants, two of the most likely off-target sites were tested (Supplemental Table S7) and the results showed that no off-target activity occurred in either of the two lines. Furthermore, we examined whether there was editing in the genomic DNA of NOR (Solyc10g006880), SlNAC4 (Solyc11g017470), and SlNAC1 (Solyc04g009440), which have been reported to have an important role in tomato fruit ripening6,22–25. The results indicated that the genomic DNA sequences of the three related genes were all unedited (Supplemental Figure S5, S6, and S7), confirming that nor-like1#1 and nor-like1#11 mutants only had the NOR-like1 gene editing.
In order to analyze the ripening phenotype of nor-like1 mutants, the fruit ripening process in mutants and WT were studied in detail. The results showed that ripening in both nor-like1#1 and nor-like1#11 lines was markedly inhibited and fruit reached the breaker stage 14 days or more later than WT. In addition, fruits failed to turn fully red and remained an orange-red color at the final ripe stage (Fig. 3d). The results verified the fruit phenotypes generated from the NOR-like1-VIGS silenced tomato plants (Fig. 1a), suggesting that NOR-like1 regulates tomato fruit ripening. When we tried to harvest seeds for reproduction, however, we were surprised to find that the NOR-like1 mutation seriously affected seed development (Supplemental Figure S8a), reducing the number (by 78% and 74.8%, respectively) and weight (by 54.9% and 61.1%, respectively) of seeds (Supplemental Figure S8b) and the remaining seeds showed poor germination.
NOR-like1 alters expression of key genes involved in tomato fruit ripening
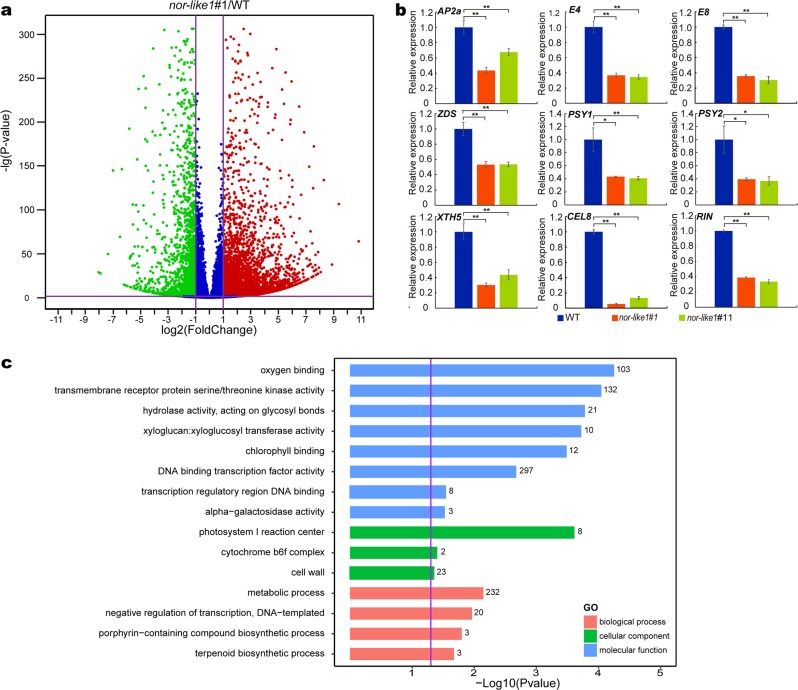
To understand how NOR-like1 mutation affects tomato ripening at the molecular level, RNA-sequencing (RNA-Seq) profiling was performed to evaluate the effect of loss function of NOR-like1 on the entire transcriptome. The reads per kilobase per million mapped reads (RPKM) values of three biological replicates for each sample were highly correlated, indicating that the RNA-seq data were reliable (Supplemental Figure S9a). On the basis of a cutoff threshold of | Log2 (fold change) | > 1 and p-value < 0.05, analysis of differentially expressed genes (DEGs) revealed that 4453 genes were upregulated while 2254 genes were downregulated in the nor-like1#1 mutant compared with that in WT (Fig. 4a; Supplemental Data Set S1; Supplemental Figure S9b). The reliability of the RNA-Seq data was tested by examining the transcript level of 9 genes (SlAP2a; SlE4; SlE8; SlZDS; SlPSY1; SlPSY2; SlXTH5; SlCEL8, and SlRIN) in nor-like1#1, nor-like1#11 and WT by qRT-PCR, the results were highly correlated with the RNA-seq data (R2 = 0.91) (Fig. 4b; Supplemental Figure S10). Gene ontology (GO) analysis indicated that loss of NOR-like1 function affected multiple metabolic pathways, including the cell wall, xyloglucosyl transferase activity, chlorophyll binding, cytochrome b6f complex, DNA-binding transcription factor activity, porphyrin-containing compound biosynthetic process, and terpenoid biosynthetic process (Fig. 4c). Further analysis of DEGs showed that a series of key genes involved in the ethylene biosynthesis and signal transduction pathway, carotenoid biosynthesis pathway, softening pathway, and some crucial TFs were also affected by NOR-like1 gene editing (Table 1). The data suggest that NOR-like1 regulates tomato fruit ripening by regulating the transcription of ripening-associated genes.
Fig. 4. NOR-like1 affects the expression of genes related to tomato fruit ripening.
a RNA-seq data visualized by volcano plots. Each point represents a DEG. Red points represent upregulated genes, and green points represent downregulated genes (nor-like1#1/WT). | Log2 (fold change) | = 1 and p-value = 0.05 are marked with purple lines. b Validation of RNA-seq results by qRT-PCR. Nine genes of different expression levels were selected to be tested by qRT-PCR in nor-like1#1, nor-like1#11 and WT fruits at the B + 3 stage. Actin gene was used as the internal control. Bars represent ± SD of three independent replicates. c GO functional enrichment analysis of genes different in abundance between nor-like1#1 and WT fruits at B + 3 stage. p-value = 0.05 was marked with a purple line
Table 1.
Gene expression changes involved in tomato fruit ripening between nor-like1 mutant and WT fruits at the B + 3 stage
| Gene names | Gene ID | Log2 (fold change) | Annotation |
|---|---|---|---|
| ACS2 | Solyc01g095080 | −3.65 | 1-aminocyclopropane-1-carboxylate synthase |
| ACS4 | Solyc05g050010 | −2.73 | 1-aminocyclopropane-1-carboxylate synthase |
| AP2a | Solyc03g044300 | −1.88 | AP2-like ethylene-responsive transcription factor |
| E4 | Solyc03g111720 | −1.19 | Peptide methionine sulfoxide reductase msrA |
| E8 | Solyc09g089580 | −1.96 | 1-aminocyclopropane-1-carboxylate oxidase-like protein |
| DXS | Solyc01g067890 | −1.21 | 1-deoxy-D-xylulose 5-phosphate synthase 1 |
| Ggpps2 | Solyc04g079960 | −3.03 | Geranylgeranyl pyrophosphate synthase 2 |
| PSY1 | Solyc03g031860 | −1.13 | Phytoene synthase 1 |
| PSY2 | Solyc02g081330 | −1.53 | Phytoene synthase 2 |
| PDS | Solyc03g123760 | −1.06 | Phytoene_desaturase |
| ZDS | Solyc01g097810 | −1.17 | Zeta-carotene desaturase |
| SGR1 | Solyc08g080090 | −4.20 | Senescence-inducible chloroplast stay-green protein 2 |
| PL | Solyc03g111690 | −3.23 | Pectate lyase |
| EXP1 | Solyc06g051800 | −5.31 | Expansin |
| CEL2 | Solyc09g010210 | −10.13 | Endo-glucanase |
| PG2a | Solyc10g080210 | −4.17 | Polygalacturonase A |
| CEL8 | Solyc08g082250 | −4.61 | Endo-glucanase |
| XTH5 | Solyc01g081060 | −2.00 | Xyloglucan endotransglucosylase/hydrolase 14 |
| RIN | Solyc05g012020 | −2.10 | MADS-box transcription factor |
| TDR4/FUL1 | Solyc06g069430 | −1.13 | MADS-box transcription factor |
| TAGL1 | Solyc03g123760 | −1.06 | Agamous MADS-box transcription facto |
| ZFP2 | Solyc07g055920 | −1.36 | Zinc-finger protein 1 |
NOR-like1 binds to the promoters of SlACS2 and SlACS4 to regulate ethylene production
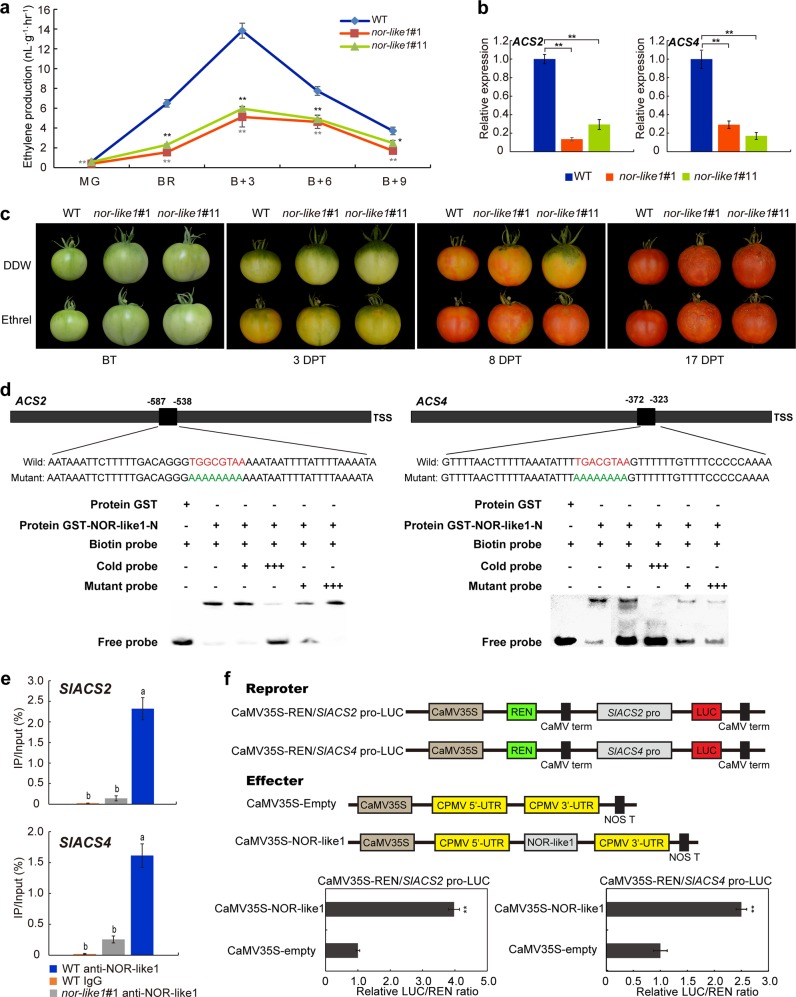
Ethylene has a key role in the regulation of climacteric fruits ripening process22. To test whether NOR-like1 regulates ethylene biosynthesis in tomato fruit, gas chromatography (GC) analysis of ethylene evolution from WT and nor-like1 mutant fruits was performed. We found that ethylene production by the fruit of both nor-like1#1 and nor-like1#11 lines was approximately halved compared with that in WT during fruit ripening (Fig. 5a). Furthermore, qRT-PCR analysis showed that the expression of SlACS2 and SlACS4 genes was markedly reduced by between 70-80% both in nor-like1#1 and nor-like1#11 lines compared to WT (Fig. 5b). To find out whether the inhibited ripening phenotype of nor-like1 mutants could be restored by supplying exogenous ethylene, we treated the mature green nor-like1 mutant and WT fruits with the ethylene-generating compound ethrel (0.4%). Although the maturation process was promoted, the fruit of nor-like1#1 and nor-like1#11 mutants still could not become fully red like WT (Fig. 5c). To understand whether NOR-like1 directly regulates transcription of SlACS2 and SlACS4 during tomato fruit ripening, we performed promoter analysis of SlACS2 and SlACS4 genes and found that there was a NAC recognition sequence (NACRS, [TA][TG][AGC]CGT[GA][TA]) containing the core CGT[GA] motif26 in the 2 -kb upstream regions of the promoters of both genes. To test whether NOR-like1 protein directly binds the promoter regions of SlACS2 and SlACS4 genes, EMSA was performed. The results showed that NOR-like1 could specifically bind to the NACRS motif in the SlACS2 and SlACS4 promoters (Fig. 5d). ChIP-qPCR results showed that NOR-like1 could directly bind to the SlACS2/SlACS4 promoters in tomato fruit (Fig. 5e). In nor-like1 mutants, the expression of SlACS2 and SlACS4 was significantly inhibited, suggesting that NOR-like1 protein positively regulated the transcription of both genes. To test this assumption, DLR assay was performed. The relative LUC/REN ratio in tobacco leaves co-transformed with CaMV35S-NOR-like1 and CaMV35S-REN/pSlACS2-LUC or CaMV35S-REN/pSlACS4-LUC was significantly higher than in leaves co-transformed with CaMV35S-Empty and CaMV35S-REN/pSlACS2-LUC or CaMV35S-REN/pSlACS4-LUC (Fig. 5f), indicating that NOR-like1 could activate the promoter activity of SlACS2 and SlACS4 in tobacco. Taken together, the results demonstrated that NOR-like1 is a transcriptional activator that positively regulates ethylene biosynthesis in tomato fruit by directly targeting the promoter of SlACS2 and SlACS4.
Fig. 5. SlACS2 and SlACS4 are directly regulated by NOR-like1 resulting in the reduction of ethylene production in CRISPR/Cas9 nor-like1 mutant fruits.
a Ethylene production was reduced in nor-like1#1 and nor-like1#11 mutants compared with that in WT. Asterisks indicate significant difference determined by Student’s t-test (*p < 0.05; **p < 0.01), asterisks marked in dark gray indicated significant difference of nor-like1#1 and WT, the significant difference of nor-like1#11 and WT were marked in black. b Expression of SlACS2 and SlACS4 was significantly downregulated both in nor-like1#1 and nor-like1#11 mutants compared with that in WT. Asterisks indicate p < 0.01 (Student’s t-test). c The phenotype of WT and nor-like1#1 and nor-like1#11 mutant fruits after treatment with ethrel. DDW, double distilled water, BT before treatment, DPT, days post treatment. d Binding of NOR-like1 to the promoters of downstream target genes SlACS2 and SlACS4. The sequences of the wild-type probes containing the NACRS were biotin-labeled. Competition for NOR-like1 binding was performed with 50× and 500 × cold probes containing the wild-type NACRS (indicated with red letters) or mutated NACRS (indicated with green letters). The symbols + or – represent presence or absence, respectively, +++ indicates increasing amounts. e ChIP-qPCR assay showing the direct binding of NOR-like1 to the promoters of SlACS2 and SlACS4. Values represent the percentage of DNA fragments in WT fruits that co-immunoprecipitated with anti-NOR-like1 or IgG, or DNA fragments in nor-like1#1 fruit that co-immunoprecipitated with anti-NOR-like1 relative to the input DNA. Bars represent ± SD of three independent replicates. The lowercase indicates significant difference (Duncan’s multiple range test, p < 0.05). f Transient expression assay for NOR-like1 activation of the SlACS2 and SlACS4 promoters. Each value represents the means of three biological replicates. Asterisks indicate p < 0.01 (Student’s t-test)
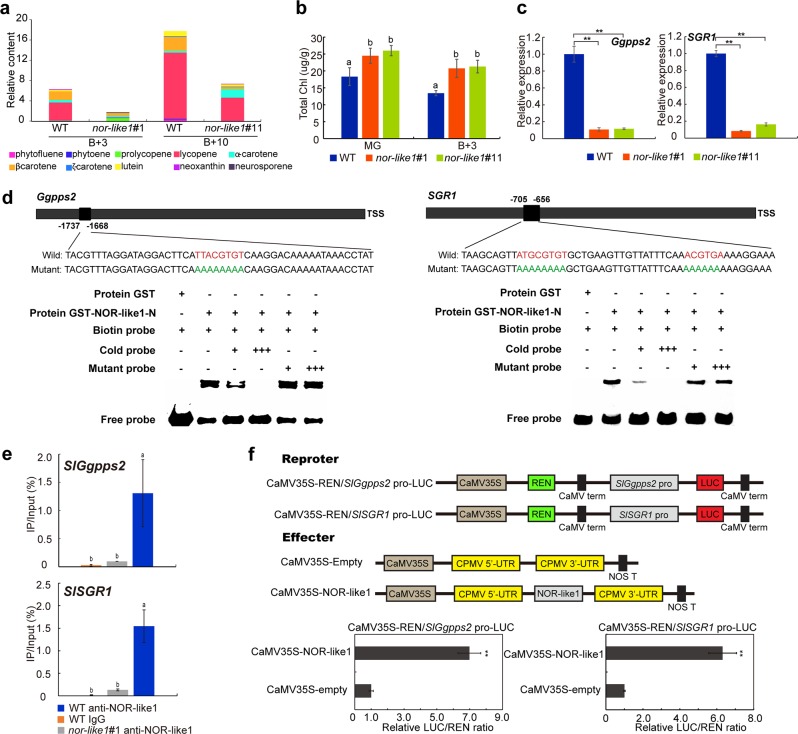
NOR-like1 positively regulates the expression of SlGgpps2 and SlSGR1 involved in color change in ripening tomato fruits
The process of tomato fruit ripening is accompanied by chlorophyll degradation and lycopene accumulation, which is a transcriptionally coordinated regulatory process27. To evaluate the characteristics of tomato fruit color change, carotenoids were measured at the B + 3 and B + 10 stages of nor-like1#1 mutant and WT fruits. The results showed that the level of β-carotene and lycopene decreased significantly in nor-like1 mutant fruit compared with that in WT fruit (Fig. 6a). In addition, we also found that the degradation rate of chlorophyll in the mutants was significantly lower than in WT. Measurement of the total chlorophyll content of nor-like1 mutants and WT at MG and B + 3 stages confirmed that the total chlorophyll content of nor-like1 mutants was higher than that of the control fruit (Fig. 6b). RNA-seq data indicated that SlGgpps2 (encoding geranylgeranyl pyrophosphate synthase 2) and SlSGR1 (encoding senescence-inducible chloroplast stay-green protein 2), which are involved in carotenoid accumulation and chlorophyll degradation, respectively, were markedly downregulated, and promoter analysis suggested that both genes contain a NACRS motif in the promoter region, so we speculated that these two genes could be NOR-like1 targets. Analysis of qRT-PCR confirmed that SlGgpps2 and SlSGR1 expression was markedly inhibited in nor-like1 mutant fruit compared with that in WT at B + 3 stage (Fig. 6c) and results of EMSA (Fig. 6d) and ChIP-qPCR (Fig. 6e) confirmed that NOR-like1 was able to bind to the SlGgpps2 and SlSGR1 promoters both in vitro and in vivo. Furthermore, transient expression analysis demonstrated that NOR-like1 could activate the promoter of SlGgpps2 and SlSGR1 in tobacco leaves (Fig. 6f). Taken together, our data suggest that NOR-like1 regulates the color change of tomato fruit by directly upregulating the transcription of SlGgpps2 and SlSGR1.
Fig. 6. SlSGR1 and SlGgpps2 are directly regulated by NOR-like1, carotenoid accumulation is reduced, and chlorophyll degradation is inhibited in CRISPR/Cas9 nor-like1 mutants.
a Carotenoid accumulation was reduced in tomato fruits of nor-like1#1 mutant at B + 3 and B + 10 ripening stages compared with that in WT. b Total chlorophyll content in nor-like1#1 and nor-like1#11 mutants was greater than that of in WT at MG and B + 3 stages. c Expression of SlGgpps2 and SlSGR1 were significantly downregulated both in nor-like1#1 and nor-like1#11 mutants compared with that in WT. Asterisks indicate p < 0.01 (Student’s t-test). d Binding of NOR-like1 to the promoters of downstream target genes SlGgpps2 and SlSGR1. The sequences of the wild-type probes containing the NACRS were biotin-labeled. Competition for NOR-like1 binding was performed with 50× and 500 × cold probes containing the wild-type NACRS (indicated with red letters) or mutated NACRS (indicated with green letters). The symbols + or – represent presence or absence respectively. +++ indicates increasing amounts. e ChIP-qPCR assay showing the direct binding of NOR-like1 to the promoter of SlGgpps2 and SlSGR1. Values represent the percentage of DNA fragments in WT fruits that co-immunoprecipitated with anti-NOR-like1 or IgG, or DNA fragments in nor-like1#1 fruit that co-immunoprecipitated with anti-NOR-like1 relative to the input DNA. Bars represent ± SD of three independent replicates. Significant differences (Duncan’s multiple range test, p < 0.05) are indicated in lowercase. f Transient expression assay of NOR-like1 strongly activated the SlGgpps2 and SlSGR1 promoters. Each value is the mean of three biological replicates. Asterisks indicate p < 0.01 (Student’s t-test)
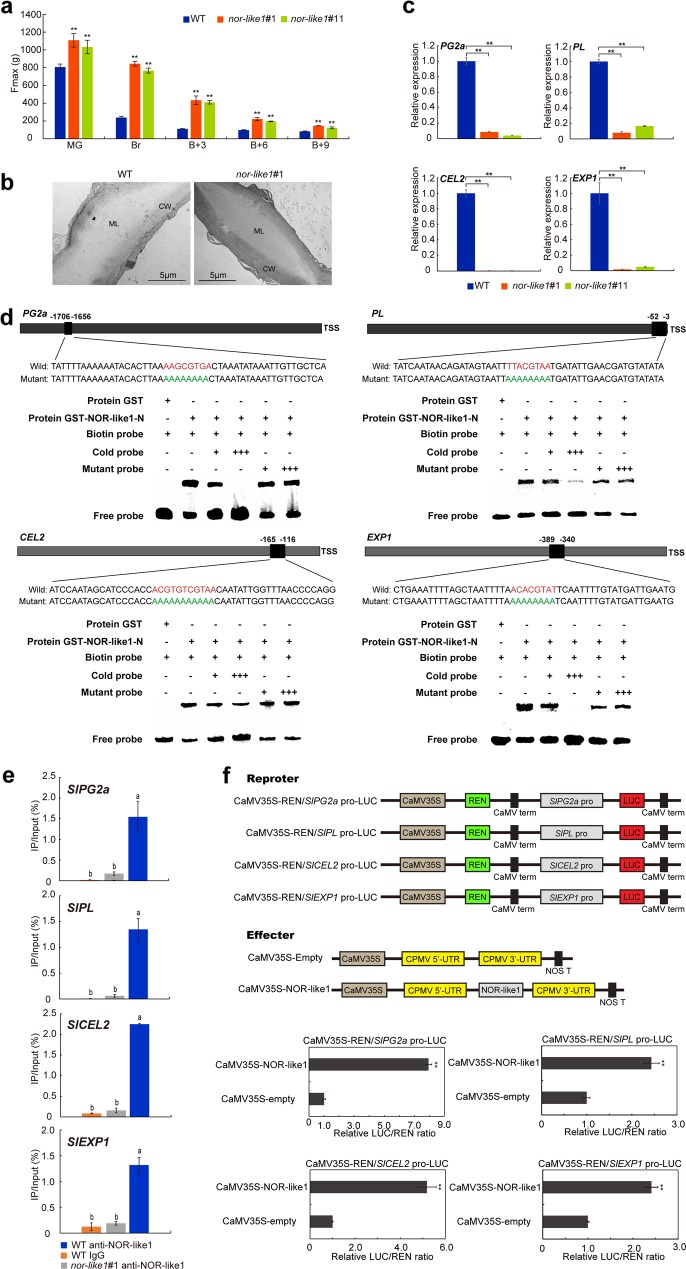
NOR-like1 directly regulates expression of SlPG2a, SlPL, SlCEL2 and SlEXP1 and affects the firmness of tomato fruits
The degradation of the cell wall leads to fruit softening during ripening28,29. To evaluate the effect of the NOR-like1 mutation on tomato fruit softening, the fruit firmness of WT and nor-like1 mutant fruits at five ripening stages (MG, Br, B + 3, B + 6, B + 9) were measured by texture analyzer. The fruit firmness of nor-like1#1 and nor-like1#11 mutant fruits was significantly higher than that in WT fruit at all five ripening stages examined (Fig. 7a). Furthermore, transmission electron microscopy (TEM) indicated that the cell walls of the nor-like1 mutant were dark-colored with a dark middle lamella and microfilaments more closely arranged compared with that in the WT cell walls (Fig. 7b), suggesting that cell wall degradation was reduced in the fruit of nor-like1 mutant. To search for cell wall associated genes directly regulated by NOR-like1, we screened the RNA-seq DEGs to obtain the genes related to cell wall metabolism, and then analyzed their 2-kb promoter regions to detect NACRS motif. The fact that the expression levels of 4 genes related to cell wall metabolism, polygalacturonase 2a (SlPG2a), pectate lyase (SlPL), Endo-glucanase 2 (SlCEL2), and expansin1 (SlEXP1) were downregulated by at least 89% (Fig. 7c) and the promoter regions of these four genes all contain the NACRS motif suggested they could be NOR-like1 targets. The results from EMSA analysis (Fig. 7d), ChIP-qPCR (Fig. 7e) and DLR (Fig. 7f) experiments all demonstrated that NOR-like1 could bind to SlPG2a, SlPL, SlCEL2 and SlEXP1 promoters in vitro and in vivo and activate their expression, suggesting that NOR-like1 positively regulates cell wall metabolism by targeting SlPG2a, SlPL, SlCEL2, and SlEXP1.
Fig. 7. SlPG2a, SlPL, SlCEL2, and SlEXP1 are directly regulated by NOR-like1 resulting in increased tomato fruit firmness in nor-like1 mutants.
a The fruit softening process was delayed and softening degree was reduced in nor-like1 mutant tomato fruits compared with that in WT fruits. b TEM indicating that the nor-like1 mutant fruit cell walls were more deeply stained and had more closely arranged microfilaments and dark middle lamella compared with that in WT fruits. CW, cell wall, ML, middle layer. Bars = 5 μm. c Expression of SlPG2a, SlPL, SlCEL2, and SlEXP1 was significantly downregulated both in nor-like1#1 and nor-like1#11 mutants compared with that in WT. Asterisks indicate p < 0.01 (Student’s t-test). d Binding of NOR-like1 to the promoters of downstream target genes SlPG2a, SlPL, SlCEL2, and SlEXP1. The sequences of the wild-type probes containing the NACRS were biotin-labeled. Competition for NOR-like1 binding was performed with 50× and 500 × cold probes containing the wild-type NACRS (indicated with red letters) or mutated NACRS (indicated with green letters). The symbols + or − represent presence or absence, respectively, +++ indicates increasing amounts. e ChIP-qPCR assay showing the direct binding of NOR-like1 to the promoter of SlPG2a, SlPL, SlCEL2, and SlEXP1. Values represent the percentage of DNA fragments in wild-type fruits that co-immunoprecipitated with anti-NOR-like1 or IgG, or DNA fragments in nor-like1#1 fruit that co-immunoprecipitated with anti-NOR-like1 relative to the input DNA. Bars represent ± SD of three independent replicates. Significant differences (Duncan’s multiple range test, p < 0.05) are indicated in lowercase. f Transient expression assay showing NOR-like1 greatly activated the SlPG2a, SlPL, SlCEL2, and SlEXP1 promoters. Each value represents the means of three biological replicates. Asterisks indicate p < 0.01 (Student’s t-test)
Discussion
NOR-like1 and NOR proteins have homology but low functional redundancy
NOR-like1 has 62.84% amino acid homology to NOR, which is a global ripening regulator22, and they belong to a small branch of the NAC phylogenetic tree (supplemental Figure S1). Despite this, the two mutant phenotypes are different; nor mutant fruits produce no ethylene and remain green6, whereas mutant fruit of nor-like1, although showing an obvious delay in ripening (Fig. 3d), could produce some ethylene and carotenoids but the ripe fruits remained orange-red. In addition, we found that seed development in nor-like1 mutant fruit was defective (supplemental Figure S8), whereas the nor mutant is known to produce normal seeds. Previous research also showed that NOR-like1-RNAi transgenic lines had reduced seed size30. In Arabidopsis, seed development is controlled by two homologous genes NARS1 (ANAC056, AT3G15510) and NARS2 (ANAC018, AT1G52880)31, and their proteins have the closest evolutionary relationship with NOR-like1 and NOR (Supplemental Figure S1). In tomato, however, it seems that only NOR-like1 but not NOR controls seed development. These results indicate that NOR-like1 and NOR may have some overlapping but also different functions in the regulation of tomato fruit ripening and seed development.
NOR-like1 regulates ethylene biosynthesis but is not involved in ethylene feedback regulation
Tomato is classified physiologically as a climacteric fruit, based on the marked induction of respiration and ethylene production at the onset of ripening. Ethylene synthesis is tightly controlled during the plant life cycle and two modes (System-1 and System-2) of ethylene regulation have been proposed32–34. Transition from System-1 to System-2 ethylene during the onset of ripening is correlated with increased ACC synthase (SlACS1A, SlACS2, and SlACS4) expression33,35,36. SlACS2 is largely ethylene-inducible in mature fruit while both SlACS2 and SlACS4 are under additional developmental control33. ACC Oxidase (SlACO1, SlACO3), SlACS2 and SlACS4 are responsible for the production of System-2 ethylene during tomato fruit ripening37 and are regulated by ripening-associated TFs38. It has been shown previously that RIN forms complexes with TAGL1 and FUL1/FUL2 and regulates expression of ripening-related genes including SlACS2 and SlACS415,38. Here, we identified a new NAC TF, NOR-like1, that also positively regulates the expression of SlACS2 and SlACS4 (Figs. 5, 8) during tomato fruit ripening. This suggests that both RIN and NOR-like1 control ACC synthase genes SlACS2 and SlACS4, but does not rule out the possibility of other interactions between RIN and NOR-like1.
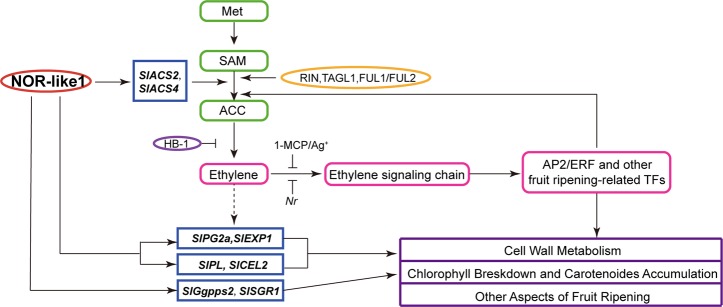
Fig. 8.
Working model of the role of NOR-like1 in the tomato fruit ripening control network and expression of ripening-associated genes
Treating WT fruits with either exogenous ethylene or 1-MCP had no effect on the level of NOR-like1 transcripts (Fig. 2c). This suggests that NOR-like1 acts on ethylene biosynthesis genes, but is not involved in the ethylene signal transduction pathway (Fig. 8). The nor-like1 mutants could produce about 40% ethylene of the WT fruits (Fig. 5a). This is consistent with other TFs, such as RIN, also regulating expression of ethylene biosynthesis genes, and this may explain why the fruit ripening process in the mutants is only partially inhibited (Fig. 3d). The ripening deficiency of nor-like1 mutants is not due simply to lack of ethylene since supplying ethylene externally did not restore full ripening. Although ethylene promoted ripening of nor-like1 mutants to some extent, the ripe phenotype was not fully recovered, and the mutant fruit failed to turn completely red (Fig. 5c), suggesting that NOR-like1 protein has an important role in color development. These data suggest that expression of NOR-like1 occurs at least partly independently of ethylene and that NOR-like1 shares the control of ethylene production with RIN, and perhaps other TFs (Fig. 8).
NOR-like1 influences color change in ripening fruit via a direct effect on both chlorophyll degradation and carotenoid biosynthesis
Ripe tomato fruit color is determined by the accumulation of carotenoids, particularly lycopene, together with the concomitant degradation of chlorophylls, leading to marked color changes39, which are often used as an indicator of the extent of ripening and overall fruit quality. In higher plants, carotenoids are synthesized from IPP, which is converted to GGPP by IPP isomerase (IPI) and GGPP synthase (GGPPS)40, located upstream of the carotenoid synthesis pathway39. In nor-like1 mutants, some genes (DXS, Ggpps2, PSY1, PSY2) involved in the carotenoid biosynthesis pathway were downregulated, the accumulation of carotenoids in mutant fruit was inhibited and the fruit finally exhibited an orange-red phenotype. Further analysis showed that NOR-like1 could bind to the promoter of Ggpps2 and positively regulate its expression (Fig. 6d–f).
Fruit of nor-like1 mutant remained green longer than WT, and it is particularly noteworthy that the process of fruit color transformation was slower after the breaker stage and degradation of chlorophyll was inhibited. The finding that SlSGR1 is a direct target gene of NOR-like1 helps explain this phenotype. Previous discoveries have found that SlSGR1 encodes a STAY-GREEN protein that has a critical role in the regulation of chlorophyll degradation in tomato leaves and fruits41–43. Silencing SlSGR1 in tomato inhibits chlorophyll degradation and causes a stay-green phenotype42. SlSGR1 can also regulate tomato lycopene accumulation through direct interaction with a key carotenoid synthetic enzyme SlPSY1 and inhibit its activity43. This evidence and our results indicate that NOR-like1 controls color formation by directly regulating carotenoids (SlGgpps2) accumulation and chlorophyll metabolism (SlSGR1).
NOR-like1 is a key regulator of tomato fruit softening
Fruit softening during ripening includes a series of modifications to the cell wall which consists of inextensible cellulose microfibrils held together by networks of hemicellulose and pectins, including attached structural glycoproteins and expansins29,44. Changes during ripening lead to pectin solubilization, cellulose and hemicellulose depolymerization, loosening of the xyloglucan-cellulose network and increased wall porosity caused by cell wall swelling, allowing greater access of degradative enzymes to their substrates45.
At least four enzymes have crucial roles in the process of pectin degradation during fruit ripening, polygalacturonase (PG), pectin methylesterase (PME), pectate lyase (PL), and β-galactosidase (TBG)29. PL breaks down cross-linked homogalacturonan polymers, particularly in tricellular junctions, PL and PG are involved in degradation of the pectic polysaccharides in the middle lamella29,46. Silencing PL was shown to increase fruit firmness without affecting other aspects of ripening, and the fruit shelf life was improved46. Previous studies also showed that tomato fruit firmness was increased by TBG4-inhibition47, but RNAi-mediated repression of either PG2a or PME2 singly had little effect on the firmness of tomato fruit although it does affect pectin structure, and overexpression of PG2a in rin mutant could not restore fruit softening48,49. Our findings show that NOR-like1 is involved in the regulation of pectin dissolution during fruit softening by directly targeting PL and PG2a, leading to the inhibition of pectin solubilization in nor-like1 mutant fruit.
During tomato fruit softening, the interaction between hemicellulose and cellulose becomes relaxed, which contributes to cell wall loosing. Xyloglucan endotransglycosylase (XTH) and endo-glucanase (CEL) are involved in the depolymerization of cellulose-hemicellulose framework50. Expansins (EXP), which lack significant hydrolase or transglycosylase activity44, are also thought to loosen cell wall hemicellulose–cellulose interactions51. EXP1 is expressed at high levels specifically during tomato fruit ripening52. Antisense expression of EXP1 resulted in firmer fruits, in contrast, fruits overexpressing high levels of EXP1 were much softer than WT44. Minoia et al.51 demonstrated that a SlEXP1 loss of function mutant yielded firmer and late ripening fruits through modification of hemicellulose structure. In tomato, XTH5 expression is highly related to fruit softening, although the biomechanical properties of plant walls could not be affected by incubation with SlXTH553 and the function of SlXTH5 needs further verification. Previous discoveries have found that the CEL2 product contributed to cell wall disassembly occurring in cell separation during fruit abscission, but softening or textural changes in fruit pericarp was not affected by CEL2 suppression54. NOR-like1 knock-out resulted in firmer fruits, darker-colored and thicker, closely arranged microfilaments in nor-like1 mutant cell walls, indicating that the cell wall relaxation and hemicelluloses-cellulose framework depolymerization were inhibited. NOR-like1 directly targeted SlEXP1, SlCEL2 and activated their expression, which could explain this phenotype. Although the role of CEL2 in tomato fruit softening is still controversial, the extremely low expression of CEL2 in nor-like1 mutants indicates its potential important role in cell wall modification.
Previous studies showed that PG55,56, PME57,58, and EXP152 may be regulated by ethylene during tomato fruit ripening29,37, so the inhibition of pectin degradation and the xyloglucan-cellulose network depolymerization caused by the downregulated PG and EXP1 expression in nor-like1 mutants may be partly because of the reduction in ethylene synthesis (Fig. 8). Therefore, NOR-like1 affects fruit softening by regulating the dissolution of pectin, the depolymerization of cellulose-hemicellulose framework and the relaxation of cell wall and is a key regulator of tomato fruit softening.
Materials and methods
Plant materials and growth conditions
Wild-type tomato (Solanum lycopersicum) cultivars Ailsa Craig (AC) and transgenic lines were grown in the greenhouse. Flowers were tagged at anthesis to record the ripening stages accurately through fruit development. Fruits of wild-type and transgenic lines were collected at different ripening stages (MG, Br, B + 3, B + 6, and B + 9). Pericarp tissues of the fruits were collected after harvesting, frozen in liquid nitrogen immediately, and stored at −80℃ until use.
Plasmid construction and VIGS assay
Tobacco rattle virus (TRV)-based vectors pTRV1 and pTRV2 were used for VIGS59. The NOR-like1 cDNA fragment was amplified using the forward primer (5′-CTGCTTGGATCCCTCTCTCTTCTAAGCTGAAT-3′) and the reverse primer (5′-GACTTAGAATTCCAATATTCAAATCATCTCTT-3′), then inserted into the pTRV2 vector. The pTRV2-NOR-like1 plasmid was verified by sequencing and transformed into GV3101. VIGS was carried out on AC fruits following the protocol as previously described60.
Total RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from the pericarp of tomato fruit according to the RNeasy Mini Kit (Qiagen, Germany) procedures and DNaseI (Qiagen, Germany) digestion was performed to remove genomic DNA. TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, China) was used to synthesize cDNA from 2 μg total RNA. qRT-PCR was conducted using SYBR Green PCR Master Mix (TransGen, China) with a CFX96 Real-Time PCR System (Bio-Rad, USA). The tomato Actin gene (Solyc03g078400) was used as the internal control. Relative gene expression values were calculated according to the 2−△△Ct method61. For each sample, three biological replicates were included. All primers used for qRT-PCR are listed in Supplemental Table S1.
Subcellular localization analysis
The coding sequence (CDS) fragment of NOR-like1 without the stop codon was amplified by PCR (primers used are listed in Supplemental Table S2) and then inserted into the pEAQ-GFP vector to produce the fusion construct pEAQ-NOR-like1-GFP using ClonExpress II One Step Cloning Kit (Vazyme, China). Then pEAQ-NOR-like1-GFP and the control vector (pEAQ-GFP) were transferred to A. tumefaciens strain GV3101 and injected into 4-week-old tobacco leaves62. GFP fluorescence was observed and captured by a laser confocal microscope (Leica, Germany) after 48 h of infiltration.
pYLCRISPR/Cas9Pubi-H-NOR-like1 vector construction and tomato genetic transformation
CRISPR-P (http://cbi.hzau.edu.cn/crispr/) was used to select four sgRNAs that targeted NOR-like1 (Supplemental Table S3, Fig. 3a). The four sgRNAs were cloned into the pYLCRISPR/Cas9Pubi-H binary plasmid using the Golden Gate ligation method63. Oligonucleotide primers used in this section are listed in Supplemental Table S4. pYLCRISPR/Cas9Pubi-H-NOR-like1 vector was transformed into AC using the stable Agrobacterium tumefaciens-mediated transformation method64. The transgenic tomato lines were selected through their hygromycin resistance.
DNA extraction and mutation analysis
Total genomic DNA was extracted from tomato fresh frozen leaves using a DNA secure Plant Kit (Tiangen, China) and used as a template for amplifying the desired gene fragments using primers flanking the target sites. The PCR products were sequenced directly or cloned into the pEASY-T1 vector using pEASY-T1 Cloning Kit (TransGen, China) then sequenced to identify mutations. Oligonucleotide primers used for this analysis are listed in Supplemental Table S5 and Supplemental Table S6. For each target, two most likely off-target sites (Supplemental Table S7) were tested.
Protein extraction and western blot
Proteins were extracted from tomato fruit pericarp as previously described with miner revisions65. Protein concentration was determined by BCA Protein Assay Kit (Solarbio, China). 30 μg of total proteins were separated by 12% SDS-PAGE and transferred to a PVDF membrane (Millipore, USA). The membrane was blocked in 5% nonfat milk in TBST buffer [20 mM Tris–HCl (pH 7.5), 150 mM NaCl and 0.1% Tween-20] at room temperature for 1 h. Immunoblots were performed at 4 °C overnight with affinity-purified rabbit polyclonal anti-NOR-like1 (the specific polyclonal antibodies against NOR-like1 were prepared with peptide antigen, and the peptide sequence is c-PIDHERDDLNIDMM, Abmart; 1:500 dilutions) or mouse monoclonal Anti-β-Actin (CWBIO, China; 1:2000 dilutions). The membranes were then washed by TBST buffer (3 × 10 min) and treated with the corresponding secondary antibodies (CWBIO, China; 1:10,000 dilutions) conjugated to horseradish peroxidase for 1 h at room temperature. Finally, the membranes were visualized using a horseradish peroxidase-enhanced chemiluminescence system (Solarbio, China).
RNA sequencing and bioinformatics analysis
Total RNA was extracted from tomato fruits of WT and nor-like1#1 mutant at B + 3 stage with the RNeasy Mini Kit (Qiagen, Germany), three biological replicates were made for each sample. The mRNA was enriched using oligo-dTs coupled with magnetic beads before being cut into 300 bp fragments (Novogene, China). Next, RNA-seq libraries were constructed and 150 bp pair-end sequencing was performed on HiSeq PE150 (Illumina, USA). The clean data were mapped to the tomato reference genome (version SL2.50) using TopHat software (version 2.0.14). Subsequently, fragments were assigned to genes by feature Counts and count programs, and gene expression abundance was represented by RPKM value. Differences in gene expression between nor-like1 mutant and WT were identified by DESeq2 Library66. The fold change was calculated by RPKMslnac3/RPKMWT. Genes were considered as differentially expressed genes (DEGs) between NOR-like1 mutant and WT if∣fold change∣ ≥ 2 and p-value < 0.05.
GO enrichment analysis
GO enrichment analysis was carried out by the GO seq R package67 based on DEGs, using a threshold of p-value < 0.05. Proteins were filtered based on their grouping to cellular components, molecular functions, and biological functions.
Ethylene measurement
To measure ethylene production, fruit from WT and nor-like1 mutants were harvested at different ripening stages (MG, Br, B + 3, B + 6, and B + 9), weighed, and placed at room temperature for 2 h to avoid measuring “wound ethylene” which was transiently generated by picking. Then the fruits were transferred into 300 ml gastight jars, sealed, and incubated at room temperature for 1 h. Then 1 ml gas samples were withdrawn and analyzed by a gas chromatograph equipped with a flame ionization detector. Ethylene concentrations were calculated by comparing sample peak areas with ethylene standards of known concentration, and normalized for fruit weight. At least three replicates were used for each measurement.
Ethrel and 1-MCP treatment
The WT tomato fruits at the mature green stage were immersed in 0.4% ethrel or double distilled water (DDW) as control for 10 min68, then dried and placed at room temperature for 12 h. Wild-type fruits at breaker stage were treated with the ethylene signaling inhibitor 1-MCP (1.0 mg/l) or air as control for 16 h69. After treatment, the pericarp tissues were sliced, frozen in liquid nitrogen, and then used for RNA isolation and qRT-PCR. For each treatment, three biological replicates from independent sample were included.
Carotenoids extraction and LC–Q-TOF–MS analysis
Carotenoids were extracted as described previously70 with minor revision. WT and nor-like1 mutant fruits at B + 3 and B + 10 stages were used and four independent extractions were performed. Carotenoids were identified and the relative contents were determined as previously described70.
Firmness measurement
The firmness of tomato fruits at five ripening stages (MG, Br, B + 3, B + 6, and B + 9) was measured by compressing a junction of outer and radial pericarp at the equator by using a 4 mm cylindrical probe (test speed 1 mm/s) (Brookfield CT3), avoiding visible vascular bundles, fissures and locular tissue. The maximum force developed during the test was recorded71. Each fruit was measured at two or three sites. The data for each fruit were averaged as one biological replicate and each measurement was performed using a minimum of three biological repetitions.
Transmission electron microscope (TEM)
Pericarp samples were excised from WT and nor-like1#1 mutant fruits at the B + 3 stage, fixed and performed as previously described72. The sections were viewed in an FEI Talos 120C TEM (Hillsboro, OR) and micrographs were taken using the integrated Ceta CMOS camera.
Electrophoretic mobility shift assay
The NOR-like1 CDS region without the termination codon was inserted into the pGEX-GST vector by ClonExpress II One Step Cloning Kit (Vazyme, China), primers used are shown in Supplemental Table S2. Recombinant GST-tagged NOR-like1 protein was expressed in E. coli strain Transetta DE3 (TransGen, China) and purified with Glutathione Sepharose 4B (GE Healthcare). EMSA was performed as previously described73 using the EMSA kit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The oligonucleotide probes containing the NACRS (NAC recognize sequence) [TA][TG][AGC]CGT[GA][TA] and the mutant probes used in this study are listed in supplemental Table S8.
Chromatin immunoprecipitation (ChIP)-qPCR analysis
Tomato fruits of WT and the nor-like1#1 mutant at the breaker stage were harvested and cut into slices, immediately cross-linked with 1% (v/v) formaldehyde and ground into a fine powder in liquid nitrogen. The nuclei were isolated, and then sonicated to shear the DNA into 300–1000 bp fragments. Ten percent of total sonicated chromatin were reverse cross-linked and used as the input control. Polyclonal anti-NOR-like1 antibodies (Abmart, China) or equal amounts of IgG (Solarbio, China) were bound to Pierce™ ChIP-grade Protein A/G Magnetic Beads (Thermo Fisher Scientific, USA), and immunoprecipitated with corresponding sonicated chromatin overnight at 4 ℃ with rotation. Sonicated chromatin from WT fruits immunoprecipitated with IgG and sonicated chromatin from nor-like1#1 mutant fruit immunoprecipitated with anti-NOR-like1 were both used as negative controls. After proteinase K treatment, crosslinking was reversed at 65 ℃ overnight in 0.25 M NaCl. Subsequently, the ChIP DNA was extracted, and the amount of each precipitated DNA fragment was determined by qRT-PCR using gene specific-primers listed in supplemental Table S9. The enrichment of predicted gene promoter fragments was normalized to its respective input DNA value. The error bars represent the SD from three independent experiments.
Dual-luciferase reporter assay
Transcription activation of the SlACS2, SlACS4, SlGgpps2, SlSGR1, SlPG2a, SlPL, SlCEL2, and SlEXP1 promoters by NOR-like1 was performed in tobacco (N. benthamiana) leaves using Agrobacterium-infiltration. The 1–2 kb promoter regions were cloned into pGreenII 0800-LUC double-reporter vector74, while the NOR-like1 coding sequence was cloned into the pEAQ vector75 as effector. All primers used for vector construction are listed in supplemental Table S2. The recombinant vectors were sequenced and transferred into A. tumefaciens strain EHA105 separately. A. tumefaciens containing constructed effector and reporter plasmids were co-transformed into tobacco leaves and the LUC to REN ratio was calculated from the results of at least six transient assays.
Statistical analysis
Significance analysis of corresponding experimental data was conducted using IBM SPSS statistics 20 software. Pairwise comparison was computed using Student’s t-test (*p < 0.05 and **p < 0.01), while multiple comparisons were subjected to ANOVA using Duncan test, statistically significant differences (p < 0.05) were indicated by diverse lowercase.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC 31571898, 31772029, 31572173). We thank Yaoguang Liu (South China Agricultural University) for providing the binary vector pYLCRISPR/Cas9 system, Silin Zhong (The Chinese University of Hong Kong) for providing the protocol for ChIP-qPCR experiments and Ruiheng Wang (Shanghai Jiao Tong University) and Qiang Zhang (Chongqing University) for advice on bioinformatics.
Author contributions
D.Q.F. and Y.G. designed and conceived the research strategy. Y.G. W.W., X.Z., X.T., Z.F., Y.Z., Y.J. and L.M. performed the experiments. Y.G. analyzed the data. J.C., B.Z., H.Z. and Y.L. provided materials and intellectual input for the work. Y.G. and D.Q.F. wrote the article. D.G. suggested additional experiments and with C.Z.J. helped to revise the manuscript and improve the English.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Supplementary Information accompanies this paper at (10.1038/s41438-018-0111-5).
References
- 1.Klee HJ, Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 2.Seymour GB, Chapman NH, Chew BL, Rose JK. Regulation of ripening and opportunities for control in tomato and other fruits. Plant Biotechnol. J. 2013;11:269–278. doi: 10.1111/j.1467-7652.2012.00738.x. [DOI] [PubMed] [Google Scholar]
- 3.Pesaresi, P., Mizzotti, C., Colombo, M. & Masiero, S. Genetic regulation and structural changes during tomato fruit development and ripening. Front. Plant Sci. 5, e14427 (2014). [DOI] [PMC free article] [PubMed]
- 4.Karlova R, et al. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot. 2014;65:4527–4541. doi: 10.1093/jxb/eru316. [DOI] [PubMed] [Google Scholar]
- 5.Giovannoni J, Nguyen C, Ampofo B, Zhong S, Fei Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017;68:61–84. doi: 10.1146/annurev-arplant-042916-040906. [DOI] [PubMed] [Google Scholar]
- 6.Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007;10:283–289. doi: 10.1016/j.pbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Vrebalov J, et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- 8.Manning K, et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006;38:948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, et al. Re-evaluation of the rin mutation and the role of RIN in the induction of tomato ripening. Nat. Plants. 2017;3:866–874. doi: 10.1038/s41477-017-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.Li S, et al. The RIN-MC fusion of MADS-Box transcription factors has transcriptional activity and modulates expression of many ripening genes. Plant Physiol. 2018;176:891–909. doi: 10.1104/pp.17.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan XY, Wang RH, Zhao XD, Luo YB, Fu DQ. Role of the Tomato non-ripening mutation in regulating fruit quality elucidated using iTRAQ protein profile analysis. PLoS ONE. 2016;11:e0164335. doi: 10.1371/journal.pone.0164335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R, Tamboli V, Sharma R, Sreelakshmi Y. NAC-NOR mutations in tomato Penjar accessions attenuate multiple metabolic processes and prolong the fruit shelf life. Food Chem. 2018;259:234–244. doi: 10.1016/j.foodchem.2018.03.135. [DOI] [PubMed] [Google Scholar]
- 13.Itkin M, et al. TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J. 2009;60:1081–1095. doi: 10.1111/j.1365-313X.2009.04064.x. [DOI] [PubMed] [Google Scholar]
- 14.Vrebalov J, et al. Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell. 2009;21:3041–3062. doi: 10.1105/tpc.109.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bemer M, et al. The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell. 2012;24:4437–4451. doi: 10.1105/tpc.112.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisawa M, et al. Transcriptional regulation of fruit ripening by tomato FRUITFULL homologs and associated MADS box proteins. Plant Cell. 2014;26:89–101. doi: 10.1105/tpc.113.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong T, et al. A tomato MADS-box transcription factor, SlMADS1, acts as a negative regulator of fruit ripening. Plant Physiol. 2013;163:1026–1036. doi: 10.1104/pp.113.224436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlova R, et al. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell. 2011;23:923–941. doi: 10.1105/tpc.110.081273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Z, et al. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 2008;55:301–310. doi: 10.1111/j.1365-313X.2008.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen AN, et al. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005;10:79–87. doi: 10.1016/j.tplants.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, et al. The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato. Plant Physiol. 2018;177:1286–1302. doi: 10.1104/pp.18.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16:S170–S180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma N, et al. Overexpression of tomato SlNAC1 transcription factor alters fruit pigmentation and softening. BMC Plant Biol. 2014;14:351. doi: 10.1186/s12870-014-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng C, et al. Suppression of tomato SlNAC1 transcription factor delays fruit ripening. J. Plant Physiol. 2016;193:88–96. doi: 10.1016/j.jplph.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhu M, et al. A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014;55:119–135. doi: 10.1093/pcp/pct162. [DOI] [PubMed] [Google Scholar]
- 26.Malley ORC, et al. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell. 2016;165:1280–1292. doi: 10.1016/j.cell.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giovannoni J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 28.Tucker G, et al. Ethylene† and fruit softening. Food Qual. Saf. 2017;1:253–267. doi: 10.1093/fqsafe/fyx024. [DOI] [Google Scholar]
- 29.Wang D, Yeats TH, Uluisik S, Rose J, Seymour GB. Fruit softening: revisiting the role of pectin. Trends Plant Sci. 2018;23:302–310. doi: 10.1016/j.tplants.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Han QQ, Song YZ, Zhang JY, Liu LF. Studies on the role of the SlNAC3 gene in regulating seed development in tomato (Solanum lycopersicum) J. Hortic. Sci. Biol. Technol. 2014;89:423–429. doi: 10.1080/14620316.2014.11513101. [DOI] [Google Scholar]
- 31.Kunieda T, et al. NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in Arabidopsis. Plant Cell. 2008;20:2631–2642. doi: 10.1105/tpc.108.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature. 1972;237:235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- 33.Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokotani N, et al. Ripening-associated ethylene biosynthesis in tomato fruit is autocatalytically and developmentally regulated. J. Exp. Bot. 2009;60:3433–3442. doi: 10.1093/jxb/erp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science. 1991;254:437–439. doi: 10.1126/science.1925603. [DOI] [PubMed] [Google Scholar]
- 36.Lincoln JE, et al. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum). Expression in Escherichia coli, structural characterization, expression characteristics, and phylogenetic analysis. J. Biol. Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- 37.Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- 38.Fujisawa M, Nakano T, Shima Y, Ito Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell. 2013;25:371–386. doi: 10.1105/tpc.112.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser PD, et al. Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell. 2007;19:3194–3211. doi: 10.1105/tpc.106.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano G, et al. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol. 2008;26:139–145. doi: 10.1016/j.tibtech.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Hörtensteiner S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009;14:155–162. doi: 10.1016/j.tplants.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Hu ZL, et al. Silencing of the LeSGR1 gene in tomato inhibits chlorophyll degradation and exhibits a stay-green phenotype. Biol. Plant. 2011;55:27–34. doi: 10.1007/s10535-011-0004-z. [DOI] [Google Scholar]
- 43.Luo Z, et al. A STAY-GREEN protein SlSGR1 regulates lycopene and beta-carotene accumulation by interacting directly with SlPSY1 during ripening processes in tomato. New Phytol. 2013;198:442–452. doi: 10.1111/nph.12175. [DOI] [PubMed] [Google Scholar]
- 44.Brummell DA, et al. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell. 1999;11:2203–2216. doi: 10.1105/tpc.11.11.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 46.Uluisik S, et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016;34:950–952. doi: 10.1038/nbt.3602. [DOI] [PubMed] [Google Scholar]
- 47.Smith DL, Abbott JA, Gross KC. Down-regulation of tomato beta-galactosidase 4 results in decreased fruit softening. Plant Physiol. 2002;129:1755–1762. doi: 10.1104/pp.011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tieman DM, Handa AK. Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 1994;106:429–436. doi: 10.1104/pp.106.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose JK, Bennett AB. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999;4:176–183. doi: 10.1016/S1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- 51.Minoia S, et al. Induced mutations in tomato SlExp1 alter cell wall metabolism and delay fruit softening. Plant Sci. 2016;242:195–202. doi: 10.1016/j.plantsci.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Rose JK, Lee HH, Bennett AB. Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc. Natl Acad. Sci. USA. 1997;94:5955–5960. doi: 10.1073/pnas.94.11.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saladie M, Rose JK, Cosgrove DJ, Catala C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 2006;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- 54.Brummell DA, Hall BD, Bennett AB. Antisense suppression of tomato endo-1,4-beta-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol. Biol. 1999;40:615–622. doi: 10.1023/A:1006269031452. [DOI] [PubMed] [Google Scholar]
- 55.Nicholass FJ, Smith CJ, Schuch W, Bird CR, Grierson D. High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol. Biol. 1995;28:423–435. doi: 10.1007/BF00020391. [DOI] [PubMed] [Google Scholar]
- 56.Sitrit Y, Bennett AB. Regulation of tomato fruit polygalacturonase mRNA accumulation by ethylene: a re-examination. Plant Physiol. 1998;116:1145–1150. doi: 10.1104/pp.116.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zegzouti H, et al. Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 1999;18:589–600. doi: 10.1046/j.1365-313x.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 58.Lu C, Zainal Z, Tucker GA, Lycett GW. Developmental abnormalities and reduced fruit softening in tomato plants expressing an antisense Rab11 GTPase gene. Plant Cell. 2001;13:1819–1833. doi: 10.1105/tpc.13.8.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313X.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 60.Fu D, Zhu B, Zhu H, Jiang W, Luo Y. Virus-induced gene silencing in tomato fruit. Plant J. 2005;43:299–308. doi: 10.1111/j.1365-313X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- 61.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 62.Luo D, et al. Involvement of WRKY transcription factors in abscisic-acid-induced cold tolerance of banana fruit. J. Agric. Food Chem. 2017;65:3627–3635. doi: 10.1021/acs.jafc.7b00915. [DOI] [PubMed] [Google Scholar]
- 63.Ma X, et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Van Eck J, Kirk DD, Walmsley AM. Tomato (Lycopersicum esculentum) Methods Mol. Biol. 2006;343:459–473. doi: 10.1385/1-59745-130-4:459. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Vignani R, Scali M, Cresti M. A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis. 2006;27:2782–2786. doi: 10.1002/elps.200500722. [DOI] [PubMed] [Google Scholar]
- 66.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breitel DA, et al. AUXIN RESPONSE FACTOR 2 intersects hormonal signals in the regulation of tomato fruit ripening. PLoS Genet. 2016;12:e1005903. doi: 10.1371/journal.pgen.1005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hao Y, et al. Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato. PLoS Genet. 2015;11:e1005649. doi: 10.1371/journal.pgen.1005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fantini E, Falcone G, Frusciante S, Giliberto L, Giuliano G. Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol. 2013;163:986–998. doi: 10.1104/pp.113.224733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu TX, Abbott JA. Firmness and force relaxation characteristics of tomatoes stored intact or as slices. Postharvest Biol. Technol. 2002;24:59–68. doi: 10.1016/S0925-5214(01)00133-8. [DOI] [Google Scholar]
- 72.Russin, W. A. & Trivett, C. L. in Microwave Techniques and Protocols (eds Giberson, R. T. & Demaree, R. S.) 25–35 (Humana Press, Totowa, NJ, 2001).
- 73.Han Y, et al. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 2016;171:1070–1084. doi: 10.1104/pp.16.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant. Methods1, 13 (2005). [DOI] [PMC free article] [PubMed]
- 75.Sainsbury F, Thuenemann EC, Lomonossoff GP. pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant. Biotechnol. J. 2009;7:682–693. doi: 10.1111/j.1467-7652.2009.00434.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.