Abstract
Background
The aim of this study was to evaluate survival of metastatic bone disease of an upper extremity, and to identify the prognostic factors that influence survival.
Methods
Patients with metastatic bone disease of an upper extremity between 2008 and 2015 were reviewed from the database of a tertiary university hospital.
Results
Of 102 patients, 48 males and 54 females with a median age of 61 (range, 28–82 years), the humerus (64.7%), clavicle (13.7%), and scapula (12.7%) were the common sites for bone metastasis of an upper extremity. Fifty-nine (57.8%) presented with pathologic fracture. No history of cancer was found in 76.5% of patients. The mean onset of metastatic bone disease after the first diagnosis of primary cancer was 4.74 ± 14.07 months (range, 0–84 months). Lung (31.4%) was the most common primary cancer followed by liver (14.7%), breast (12.7%), thyroid (7.8%), and renal (3.9%). Eighty-two cases (80.39%) died from the disease such that the median survival was 4.08 months (95% CI 2.57–6.17). The significant risk factors were the type of primary tumor (P < 0.001, HR = 4.44; 95% CI, 1.99–9.90) and ECOG performance status (P = 0.021, HR = 2.11, 95% CI 1.12–3.99).
Conclusions
Patients with metastatic bone disease of an upper extremity have a limited life expectancy. The type of primary tumor and ECOG performance status were the important prognostic factors that influenced overall survival. Our data help in the management of patients, families, and doctors, so as to avoid over- or under-treatment.
Keywords: Metastatic bone disease, Upper extremity, Survival, Prognostic factors
1. Introduction
As treatment of cancer improves, patients have an increasing life-expectancy but also an increased risk of metastatic bone disease. Patients with metastatic bone disease can present with or without a history of cancer. When they present with bone symptoms—i.e., pain or pathological fracture—the existence of a primary tumor must be ruled out. Examples of typical primary tumors frequently associated with metastasis to bones are lung, breast, kidney and prostate [1].
The treatment of metastatic bone disease includes surgery, chemotherapy, and radiation. The choice of surgical procedures in metastatic cancer depends upon the estimated survival of the patient. In patients with a relatively long predicted survival, aggressive treatment(s) and durable implants are appropriate. In cases where life expectancy is short, less invasive or palliative treatment are indicated [2]. Previous studies have indicated that the prognostic factors for metastatic bone disease include age, primary tumors, onset of bone symptoms, pathological fracture, metastasis to other organs, performance score, and pre-operative hemoglobin level [1], [3], [4], [5], [6], [7], [8], [9]. Previous studies regarding the prognostic factors and survival of metastatic bone disease were, however, not specific to metastatic bone disease of an upper extremity [6].
To the best of our knowledge, this is the first study to describe prognostic and risk factors in patients with the metastatic bone disease of an upper extremity—with the primary tumor being a solid organ. The present study, set at a tertiary university hospital, was conducted (a) to evaluate survival of metastatic bone disease of an upper extremity, and (b) to identify the prognostic factors that influence survival.
2. Methods
The ethics committee at our institution reviewed and approved the protocol. The authors reviewed all records of patients diagnosed with metastatic bone disease of an upper extremity between 2008 and 2015 at the Musculoskeletal Oncology Unit, at our tertiary university hospital. The inclusion criteria were patients who presented initially with metastatic bone lesions only in an upper extremity. The exclusion criteria were primary bone tumors. The latter being a hematologic malignancy (i.e., multiple myeloma or lymphoma).
The data reviewed were from the Cancer Registry, Srinagarind Hospital, Khon Kaen University, Thailand. The data from 102 patients with metastatic disease of an upper extremity alone were obtained. The retrieved data were age at diagnosis of bone metastasis, history of cancer, type primary tumor, onset of metastatic bone disease after the first diagnosis of primary tumor, presenting with or without pathologic fracture, performance score, location of bone lesion, and visceral and skeletal metastases. Investigations included plain X-ray of the affected limb, chest X-ray, and computed tomography (CT) scan of chest, abdomen, pelvis, and bone. All investigations were performed before a biopsy was done. All pathology slides were reviewed by a single musculoskeletal oncology pathologist.
3. Statistical analysis
Overall survival time was calculated from the time of admission to our hospital to death or last follow-up visit. The survival analysis was calculated using life table analysis and Kaplan–Meier method. The following parameters: age, sex, onset of metastatic bone disease, primary tumors, Site of metastasis, visceral metastasis, pathological fracture, and performance score were analyzed for validity as prognostic factors. Each prognostic factor was categorized for statistical analysis. Age group was categorized into <60 or ≥60 years. Onset of metastatic bone disease was categorized into no known history of tumor, 1–12 months and >12 months after diagnosis of primary tumor. Primary tumors were categorized according to median survival time into slow (>20 months), moderate (10–20 months) and rapid growth (<10 months) [10]. Visceral metastasis was divided into two groups: with or without metastasis. Pathological fracture was divided into two groups: present or absent fracture. Eastern Cooperative Oncology Group Performance Status (ECOG PS) was used to evaluate the performance status of the patients, and was categorized into two groups: ECOG PS 0–2 and 3–4 [10]. Site of metastasis was divided into proximal and distal to the elbow joint.
The log–rank test was used to screen for potential prognostic value; if any test was significant (P < 0.2) then a Cox regression analyses was performed. P values < 0.05 were considered to be statistically significant.
All statistical analyses were conducted using SPSS 19.0 statistical software (SPSS, Chicago, IL, USA).
4. Results
4.1. Patient characteristics
The demographic data are presented in Table 1. Of the 102 patients, 48 were males (47.1%) and 54 were females (52.9%). The median age was 61 years (range, 28–82 years). Fifty-nine (57.8%) patients presented with pathologic fracture. No history of cancer was found in 78 (76.5%) cases. The mean onset of metastatic bone disease after the first diagnosis of primary cancer was 4.74 ± 14.07 months (range, 0–84 months). The rank of site of bone metastasis was the humerus (64.7%), clavicle (13.7%), and scapula (12.7%). Acral metastasis (lesion below the elbow) occurred in 7.8% (Table 1).
Table 1.
Demographic data.
| Characteristic | Patients (N = 102) | (%) |
|---|---|---|
| Sex | ||
| Male | 48 | 47.1 |
| Female | 54 | 52.9 |
| Age | ||
| <60 years | 50 | 49 |
| ≥60 years | 52 | 51 |
| History of cancer | ||
| Present | 24 | 23.5 |
| Not present | 78 | 76.5 |
| Onset of bone metastasis after diagnosis of primary tumor | ||
| 0 month | 78 | 76.5 |
| 1–12 months | 14 | 13.7 |
| >12 months | 10 | 9.8 |
| Pathologic fracture | ||
| Present | 59 | 57.8 |
| Absent | 43 | 42.2 |
| Tumor location | ||
| Scapula | 13 | 12.7 |
| Clavicle | 14 | 13.7 |
| Humerus | 67 | 65.7 |
| Radius | 3 | 2.9 |
| Ulna | 2 | 2 |
| Hand | 3 | 2.9 |
| Visceral metastases | ||
| Present | 62 | 60.8 |
| Not present | 36 | 35.3 |
| Unknown | 4 | 3.9 |
| ECOG performance status | ||
| 1 | 28 | 27.5 |
| 2 | 49 | 48 |
| 3 | 23 | 22.5 |
| Unknown | 2 | 2 |
The most common primary tumor was lung (31.4%) followed by liver (14.7%), breast (12.7%), thyroid (7.8%), kidney (3.9%), bile duct (cholangiocarcinoma) (2.9%), nasopharynx (2%), and 1% each for the colon, cervix, bladder, endometrium, and esophagus. Adenocarcinoma of unknown origin was 19.6% (Table 2). In 8 cases of acral metastasis, the primary tumor was lung in 7 cases and adenocarcinoma of unknown origin in 1 case.
Table 2.
Primary tumors and median survival time.
| Primary tumors | N | Median survival (months) (95% CI) |
|---|---|---|
| Slow growth | ||
| Endometrium | 1 | 127.13 |
| Moderate growth | ||
| Breast | 13 | 11.67 (1.98–32.58) |
| Thyroid | 8 | 11.63 (4.3–14.0) |
| Nasopharynx | 2 | 10.12 |
| Rapid growth | ||
| Lung | 32 | 5.4 (2.27–8.8) |
| Adenocarcinoma of unknown origin | 20 | 1.97 (1.32–7.90) |
| Liver | 15 | 2.5 (0.92–4.08) |
| Renal | 4 | 4.31 |
| Bile duct (cholangiocarcinoma) | 3 | 2.1 (0.13–4.07) |
| Colon | 1 | 0.33 |
| Cervix | 1 | 6.53 |
| Bladder | 1 | 4.53 |
| Esophagus | 1 | 2.23 |
The treatments were surgery in 50% of cases, palliative treatment in 44.2% and radiation alone in 5.8%. The surgical procedures included excision (7.8%), cementing after tumor curettage and internal fixation with intramedullary nailing (32.4%), plate and screw (7.8%), endoprosthesis (2%).
4.2. Outcome
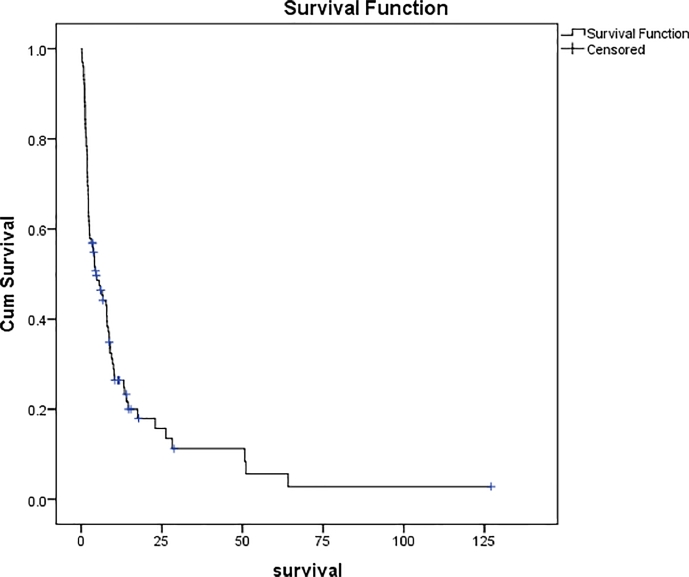
No patient was lost to follow-up. Eighty-two (80.4%) patients died from the disease with a median survival of 4.08 months (95% CI 2.57–6.17) (Fig. 1). Further analysis was done of 101 of the total 102 cases who had a survival time <64 months. The median survival time was 4.53 months (95% CI 1.84–7.22). None of the patients died from any unrelated causes. The cumulative 1- and 2-year survival was 15.2% and 10.5%, respectively.
Fig. 1.
Overall survival in 102 patients with metastatic bone disease of an upper extremity.
Based on the univariate analysis, 6 prognostic factors warranted further analysis. Age (P = 0.046), primary tumor (P < 0.001), ECOG performance score (P = 0.008), visceral metastasis (P = 0.184), pathological fracture (P = 0.193) and onset of metastatic bone disease (P = 0.026) were found to be statistical significant. In the multivariate analysis, the significant risk factors were the type of primary tumor (P < 0.001, HR = 4.44; 95% CI, 1.99–9.90) and ECOG performance status (P = 0.021, HR = 2.11, 95% CI 1.12–3.99) (Table 3).
Table 3.
Univariate and multivariate analyses.
| Variable | Univariate P-value | HR | 95% CI | Multivariate P-value | HR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.046 | 1.57 | 1.01–2.47 | 0.343 | 1.27 | 0.77–2.09 |
| Sex | 0.508 | 0.86 | 0.55–1.34 | |||
| Primary tumors | <0.001 | 4.43 | 2.23–8.45 | <0.001 | 4.44 | 1.99–9.90 |
| Site of metastasis | 0.643 | 0.82 | 0.36–1.90 | |||
| ECOG performance status | 0.008 | 2.02 | 1.24–3.30 | 0.021 | 2.11 | 1.12–3.99 |
| Visceral metastasis | 0.184 | 0.73 | 0.46–1.16 | 0.139 | 0.67 | 0.40–1.14 |
| Pathological fracture | 0.193 | 1.35 | 0.86–2.13 | 0.169 | 1.41 | 0.86–2.31 |
| Onset of metastatic bone disease | 0.026 | 0.66 | 0.45–098 | 0.727 | 1.10 | 065–1.88 |
HR = Hazard ratio, CI = confidence interval.
5. Discussion
Most previous studies on survival and prognostic factors in metastatic bone disease focused on the spine, a combination of upper and lower extremities, or included hematologic malignancy [3], [5], [6], [11] With respect to upper extremity, only the humerus has been studied [3], [12]. In the present study, we excluded hematologic malignancy because it has a different prognosis than bone metastasis from solid malignancies [13].
Bone is the third most common site of metastasis after the lung and liver. The spine, femur, humerus, and pelvis are the most common locations [14]. Metastatic bone disease of the upper extremity alone is rare. Ratasvuori et al. [6] studied skeletal metastasis (excluding the spine) and found the site of metastases occurred less frequently in an upper extremity (24%) than a lower extremity (76%).
The most commonly affected upper extremity bone is the humerus. Only rarely are the ulna, scapula, and radius affected [6]. Similarly, in our study two-thirds of cases occurred in the humerus, and far less frequently in the clavicle, scapula, radius, hand, and ulna.
Katagiri et al. [15] and Ratasvuori et al. [6] reported skeletal lesions can be the first manifestation of malignancy in 30% and 13.5% of patients, respectively. In our study, 76.5% of patients presented with this condition. This number is high and may be due to the upper extremity being a non-weight bearing bone, such that the pain may not be as explicit as early as in lower extremity. These differences should thus be considered in metastatic bone disease before treatment begins even if no history of cancer has been found.
We categorized primary tumors into slow, moderated, and rapid growing, as per Katagiri et al. [10]. Our findings differ from that report as we excluded hematologic malignancy and did not divide breast cancer into hormone-dependent subgroups or lung cancer treated with molecularly targeted drugs vs. not.
In the current study from Thailand and the reported by Muramatsu et al. [16] from Japan, the liver was the second most common primary tumor. By contrast, in western countries the primary tumors commonly metastasizing to bone are breast, prostate, kidney, and lung. The liver is not mentioned in western studies [3]; while the prostate is not listed in the current study.
In the current study, cholangiocarcinoma (CCA) was found as a primary tumor in 3 cases. CCA is a cancer of the biliary system with a poor prognosis, and CCA metastasizing to bone is rare [17]. The incidence of CCA varies worldwide and the highest incidence is in Khon Kaen province Thailand where our institute is located [18], [19].
Bone metastases of unknown origin occur in between 10% and 15% of patients with bone lesions. Adenocarcinoma is the most common histological type; in up to 70% of cases [20], [21]. In the current study, adenocarcinoma of unknown primary tumor was found in 19.6% of cases; notwithstanding a thorough investigation, and all of these cases had a short survival time.
The treatment options depended upon the life expectancy of the patient. The estimated survival time was based on Katagiri's score [22]. The choice of treatment was discussed among the physicians, each patient, and their respective family. The appropriate treatment for each case was discussed again in the Musculoskeletal Tumor Board group of Khon Kaen University, Thailand. In general, radiotherapy was indicated for painful metastatic lesion of flat bone (scapula), or painful, inoperable, bone metastasis. Meanwhile, surgical treatment was indicated for pathological fracture of long bones (i.e., of the humerus, radius, and ulna) in patients with a long life expectancy.
In the current study, a metastatic lesion in the humeral shaft was treated with tumor curettage and internal fixation, using intramedullary nailing or plating and cementing. Metastases to the humeral head were reconstructed with an endoprosthesis. Metastases to the scapula were treated with radiation alone. Muramatsu et al. [16] described a retrospective study of 20 cases with metastatic bone lesions in an upper extremity; the indications for surgery were pathological fracture, painful bone lesion, and uncontrollable tumor size. The choice of treatment was the same as in our study.
In the current study, we found that 80.4% of patients with metastatic bone disease of an upper extremity died with a median survival time of 4.08 months. The respective cumulative 1- and 2-year survival was 15.2% and 10.5%. Wedin et al. [12] reported that in 208 patients with metastatic lesions of the humerus, the respective cumulative survival at 1, 2, and 3 years was 40%, 21%, and 16%. Dijkstra et al. [23] reported a series of 37 patients with metastatic lesions of the humeral shaft. The respective survival rate at 3, 6, and 12 months was 61%, 44%, and 16%. We compared the current study to one of patients with metastatic bone disease of the lower extremity. In that study, Schneiderbauer et al. [24] reported that in 299 patients with metastatic hip disease, the respective survival rate was 40%, 21.5%, and 6% at 1, 2, and 5 years. Mavrogenis et al. [25] studied 110 patients with femoral metastases, and the respective survival rate was 54%, 30%, 20%, and 16% at 1, 2, 3, and 5 years. All of these reports confirm that patients with metastatic bone disease of the lower extremity have better survival than patients with metastatic bone disease of the upper extremity. Further studies should be performed to identify the cause of this difference.
Primary tumor was considered the most important prognostic factor for survival in the current study. Rapid growth of the primary tumor group resulted in the shortest survival time (i.e., colon followed by bile duct [CCA]), adenocarcinoma of unknown origin, esophagus, liver, renal, lung, bladder, and cervix). Hansen et al. [3] studied 460 patients with non-spinal skeletal metastases and reported that the negative and positive prognostic factors for survival were lung cancer and myeloma, respectively.
The ECOG performance status had risk factor for survival. Patients with a high ECOG score (i.e., 3 or 4) was an indication of poorer general health than patients with a low ECOG score (i.e., 0, 1, or 2). In the current study, we found that patients with a high ECOG score had a shorter survival time than patients with a low ECOG score. Our finding was consistent with studies by Ratasvuori et al. [6], Katagiri et al. [10], and Hill et al. [11].
The study had limitations. First, this was a retrospective study, so some of the data were incomplete and the number of cases was constrained. Second, we only used CT and bone scan to search for visceral and other bone metastases while MRI and PET scan were not available. The number of cases in the visceral and multiple bone metastases groups might have been greater if all of the investigations had been performed. Third, the treatment of metastatic bone disease of an upper extremity varied from conservative to surgical, but this factor was not considered in our study.
6. Conclusions
Metastatic bone disease of the upper extremity is still a disease with an extremely poor prognosis. Most of the patients present without any history of cancer. The type of primary tumor and ECOG performance status were the important prognostic factors that influenced the overall survival. Our information is useful for the patients, families and doctors to prevent over- or under treatment in patients with metastatic bone disease of the upper extremity.
Acknowledgments
Acknowledgments
This study was funded by the Faculty of Medicine, Khon Kaen University, Thailand. The authors thank (a) Cancer Registration Unit, Srinagarind Hospital, Khon Kaen University, Thailand; (b) Musculskeletal oncology research group, Khon Kaen University, Thailand; (c) Mr. Saksin Simsin, Department of Anesthesiology, Faculty of Medicine, Khon Kaen University for statistical analysis; (d) Mr. Bryan Roderick Hamman for assistance in English-language presentation of the manuscript via Publication Clinic KKU, Thailand.
Conflict of interest
No author has any conflict of interest.
Ethical approval
IRB approval from Khon Kaen University: HE591470.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.09.007.
Contributor Information
Taweechok Wisanuyotin, Email: tawwis@kku.ac.th.
Permsak Paholpak, Email: permpa@kku.ac.th.
Kamonsak Sukhonthamarn, Email: kamolsu@kku.ac.th.
Weerachai Kosuwon, Email: weera_ko@kku.ac.th.
Appendix. Supplementary materials
References
- 1.Budczies J., von Winterfeld M., Klauschen F., Bockmayr M., Lennerz J.K., Denkert C., Wolf T., Warth A., Dietel M., Anagnostopoulos I., Weichert W., Wittschieber D., Stenzinger A. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6(1):570–583. doi: 10.18632/oncotarget.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forsberg J.A., Eberhardt J., Boland P.J., Wedin R., Healey J.H. Estimating survival in patients with operable skeletal metastases: an application of a Bayesian belief network. PloS One. 2011;6(5):e19956. doi: 10.1371/journal.pone.0019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen B.H., Keller J., Laitinen M., Berg P., Skjeldal S., Trovik C., Nilsson J., Walloe A., Kalen A., Wedin R. The Scandinavian sarcoma group skeletal metastasis register. survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop. Scand. 2004;75(311):11–15. doi: 10.1080/00016470410001708270. Supplementum. [DOI] [PubMed] [Google Scholar]
- 4.Kirkinis M.N., Lyne C.J., Wilson M.D., Choong P.F. Metastatic bone disease: a review of survival, prognostic factors and outcomes following surgical treatment of the appendicular skeleton. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2016;42(12):1787–1797. doi: 10.1016/j.ejso.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Kirkinis M.N., Spelman T., May D., Choong P.F.M. Metastatic bone disease of the pelvis and extremities: rationalizing orthopaedic treatment. ANZ J. Surg. 2017;87(11):940–944. doi: 10.1111/ans.13615. [DOI] [PubMed] [Google Scholar]
- 6.Ratasvuori M., Wedin R., Keller J., Nottrott M., Zaikova O., Bergh P., Kalen A., Nilsson J., Jonsson H., Laitinen M. Insight opinion to surgically treated metastatic bone disease: Scandinavian sarcoma group skeletal metastasis registry report of 1195 operated skeletal metastasis. Surg. Oncol. 2013;22(2):132–138. doi: 10.1016/j.suronc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Weiss R.J., Forsberg J.A., Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83(1):74–79. doi: 10.3109/17453674.2011.645197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss R.J., Tullberg E., Forsberg J.A., Bauer H.C., Wedin R. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23(3):286–290. doi: 10.1016/j.breast.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Hwang N., Nandra R., Grimer R.J., Carter S.R., Tillman R.M., Abudu A., Jeys L.M. Massive endoprosthetic replacement for bone metastases resulting from renal cell carcinoma: factors influencing patient survival. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014;40(4):429–434. doi: 10.1016/j.ejso.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri H., Okada R., Takagi T., Takahashi M., Murata H., Harada H., Nishimura T., Asakura H., Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3(5):1359–1367. doi: 10.1002/cam4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill T., D'Alessandro P., Murray K., Yates P. Prognostic factors following pathological fractures. ANZ J. Surg. 2015;85(3):159–163. doi: 10.1111/ans.12830. [DOI] [PubMed] [Google Scholar]
- 12.Wedin R., Hansen B.H., Laitinen M., Trovik C., Zaikova O., Bergh P., Kalen A., Schwarz-Lausten G., Vult von Steyern F., Walloe A., Keller J., Weiss R.J. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J. Shoulder Elbow Surg. 2012;21(8):1049–1055. doi: 10.1016/j.jse.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Willeumier J.J., van der Linden Y.M., van der Wal C., Jutte P.C., van der Velden J.M., Smolle M.A., van der Zwaal P., Koper P., Bakri L., de Pree I., Leithner A., Fiocco M., Dijkstra P.D.S. An easy-to-use prognostic model for survival estimation for patients with symptomatic long bone metastases. J. Bone Joint Surg. Am. 2018;100(3):196–204. doi: 10.2106/JBJS.16.01514. [DOI] [PubMed] [Google Scholar]
- 14.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri H., Takahashi M., Inagaki J., Sugiura H., Ito S., Iwata H. Determining the site of the primary cancer in patients with skeletal metastasis of unknown origin: a retrospective study. Cancer. 1999;86(3):533–537. doi: 10.1002/(sici)1097-0142(19990801)86:3<533::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Muramatsu K., Ihara K., Iwanagaa R., Taguchi T. Treatment of metastatic bone lesions in the upper extremity: indications for surgery. Orthopedics. 2010;33(11):807. doi: 10.3928/01477447-20100924-29. [DOI] [PubMed] [Google Scholar]
- 17.Lahrach K., Chbani B., Amar F., Bennani A., Marzouki A., Boutayeb F. Humerus pathological fracture revealing biliary carcinoma. Orthop. Traumatol. Surg. Res. 2010;96(8):910–912. doi: 10.1016/j.otsr.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Luvira V., Nilprapha K., Bhudhisawasdi V., Pugkhem A., Chamadol N., Kamsa-ard S. Cholangiocarcinoma patient outcome in Northeastern Thailand: single-center prospective study. Asian Pac. J. Cancer Prev. 2016;17(1):401–406. doi: 10.7314/apjcp.2016.17.1.401. [DOI] [PubMed] [Google Scholar]
- 19.Dowsiriroj P., Paholpak P., Sirichativapee W., Wisanuyotin T., Laupattarakasem P., Sukhonthamarn K., Kosuwon W., Jeeravipoolvarn P. Cholangiocarcinoma with spinal metastasis: single center survival analysis. J. Clin. Neurosci. 2017;38:43–48. doi: 10.1016/j.jocn.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki K., Riihimaki M., Sundquist K., Hemminki A. Site-specific survival rates for cancer of unknown primary according to location of metastases. Int. J. Cancer. 2013;133(1):182–189. doi: 10.1002/ijc.27988. [DOI] [PubMed] [Google Scholar]
- 21.Hemminki K., Bevier M., Hemminki A., Sundquist J. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012;23(7):1854–1863. doi: 10.1093/annonc/mdr536. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri H., Takahashi M., Wakai K., Sugiura H., Kataoka T., Nakanishi K. Prognostic factors and a scoring system for patients with skeletal metastasis. J. Bone Joint Surg. Br. 2005;87(5):698–703. doi: 10.1302/0301-620X.87B5.15185. [DOI] [PubMed] [Google Scholar]
- 23.Dijkstra S., Stapert J., Boxma H., Wiggers T. Treatment of pathological fractures of the humeral shaft due to bone metastases: a comparison of intramedullary locking nail and plate osteosynthesis with adjunctive bone cement. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 1996;22(6):621–626. doi: 10.1016/s0748-7983(96)92450-6. [DOI] [PubMed] [Google Scholar]
- 24.Schneiderbauer M.M., von Knoch M., Schleck C.D., Harmsen W.S., Sim F.H., Scully S.P. Patient survival after hip arthroplasty for metastatic disease of the hip. J. Bone Joint Surg. Am. 2004;86-A(8):1684–1689. doi: 10.2106/00004623-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Mavrogenis A.F., Pala E., Romagnoli C., Romantini M., Calabro T., Ruggieri P. Survival analysis of patients with femoral metastases. J. Surg. Oncol. 2012;105(2):135–141. doi: 10.1002/jso.22061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.