Abstract
Background
Insomnia is a prevalent disorder leading to psychological problems such as anxiety and depression.
Methods
This study investigates the effect of a combination of herbs (Melissa officinalis L. and Nepeta menthoides Boiss. & Buhse) on anxiety and depression for insomniacs and on insomnia severity. This study is a double-blind randomized placebo-controlled clinical trial. A total number of 67 participants met the inclusion criteria who were diagnosed as cases of insomnia. The patients were randomized into the herbal treatment or placebo groups. The herbal treatment group received a combination of Melissa officinalis and Nepeta menthoides Boiss. & Buhse. The primary outcomes were insomnia, depression and anxiety. We used per-protocol analysis.
Results
The all outcomes of herbal treatment were significantly improved compared with placebo in the ISI, BAI and BDI scores after four weeks’ treatment (p value: 0.008, 0.005 and <0.001 respectively).
Conclusion
A combination of Melissa officinalis L. and Nepeta menthoides Boiss. & Buhse may improve insomnia and its comorbid depression and anxiety.
Keywords: Anxiety, Depression, Insomnia, Melissa officinalis, Nepeta menthoides
1. Introduction
Insomnia is a very common disorder with considerable long-term health problems. Its prevalence in various communities is reported to be 10–40%. Insomnia is commonly comorbid with other psychiatric and physical disorders. Furthermore, it leads to psychological consequences, including anxiety and depression.1, 2 Bilateral correlation has been shown between depression, anxiety and insomnia.3 Based on World Health Organization (WHO), propensity towards the application of herbal medicine has increased. Many people use medicinal plants (Melissa officinalis and Nepeta menthoides) alone or in a combined formula for the treatment of diseases such as insomnia and mood disorders.4 Melissa officinalis (lemon balm) is known as a heart tonic and removes palpitations.5 It has also anxiolytic and antidepressant effects and improves sleep quality.6, 7, 8 It reduces palpitation in anxious patients.9 In a pilot study using the standard form of Melissa officinalis (Cyracos), mild to moderate anxiety and sleep disturbances are reported to be improved.10 Nepeta menthoides (Persian Lavender) is useful in the treatment of melancholia.5 This herb can decrease anxiety and improve the mood of Major Depressive Disorder (MDD) patients. One study showed that Nepeta menthoides, in comparison with Sertraline, has a higher success in reducing BAI and BDI scores and decreased anxiety in depressed patients.11, 12 Another study reported the superior effect of this on reducing depression compared with usual treatment.13
We selected the combination of these two herbs because traditional texts suggest a synergistic effect for combination therapy and the combination therapy is found more effective on these outcomes from our unpublished pilot study. Additionally, no studies have been done on combined effect of these two medicinal plants on three disorders (anxiety, depression and insomnia).
The aim of this study is to evaluate the effect of a combination of Melissa officinalis L. and Nepeta menthoides Boiss. & Buhse on insomnia severity and on anxiety and depression for insomniacs.
2. Methods
2.1. Study design, participants and screening
The patients were from the Traditional Persian Medicine (TPM) clinic of Tehran University of Medical Sciences from May 2015 to May 2016. The eligible participants with comorbid anxiety and depression who met the study criteria were randomly allocated into herbal combination treatment (Melissa officinalis plus Nepeta menthoides) or placebo groups. The Ethics Committee of the Shiraz University of Medical Sciences approved the protocol (approval number: CT_9378_7428) and informed consent was obtained from all the patients. The study conformed with Helsinki Declaration of 1975 and was registered retrospectively in the Iranian Registry of Clinical Trials (No. IRCT2015040621592N2).
The inclusion criteria were: Diagnosis of insomnia based on ICSD II of people aged 18–60, insomnia severity score (ISI) greater than 7, Persian‐language version of the Beck Depression Inventory‐Second edition (BDI) score above 10, and Persian version of Beck Anxiety Inventory (BAI) score greater than 7. For recruitment, information sheets were distributed and the interested eligible patients from TPM clinic participants were selected from volunteers.
Participants were excluded if they worked night shifts, had other sleep disorders, had other psychological or physical problems, consumed drugs affecting sleep, had mood or anxiety problems, were pregnant, planning to get pregnant or breastfeeding, abused alcohol or substance, had a history of suicidal thought or attempts, were candidates for electroconvulsive therapy or had history of allergy to the Lamniacea family such as Melissa officinalis and Nepeta menthoides. Those who missed more than two doses of intervention were also excluded.
2.2. Intervention
The patients were divided randomly into two groups to receive three 500 mg capsules of treatment (containing 1000 mg Melissa officinalis plus 400 mg of Nepeta menthoides) or placebo every night for four weeks.
Herbal samples were authenticated by the botanist. Voucher numbers PM 798 for Melissa officinalis and PM 799 for Nepeta menthoides were provided. Based on TPM administration, Melissa officinalis was washed, dried, and ground to a powder. Nepeta menthoides was prepared as an aqueous extract by decocting in distilled water (1:10). Then it was concentrated and dried using a freeze-dryer under vacuum conditions to obtain a powder. Considering combination therapy and the maximum daily dosage suggested by TPM manuscripts, the treatment group received three 500 mg capsules with a total dose of 1000 mg Melissa officinalis plus 400 mg Nepeta menthoides. Also, microbial quality control was performed based on the British Pharmacopoeia and other international guidelines with negative results. The placebo was prepared as similar capsules from starch. For creating the same smell, a little lemon balm and Persian lavender powder were added.
2.3. Outcomes
The primary outcome measures were insomnia, depression and anxiety. The medication adverse effects were considered as secondary outcomes.
Patients were evaluated at the baseline and after four weeks of intervention by mean scores of ISI and BAI. Also, the mean BDI score was assessed at the baseline, the second and the fourth weeks. Complications were documented during the study.
2.4. Sample size
Based on prior pilot study, the formula for comparing means between two independent groups is used with α = 0.05 and β = 0.2. The least significant difference of 3.3 scores in ISI index and standard deviation of 4 is considered in each group giving n = 23.04. Assuming 20% dropout, the final sample size is 27.65, defining recruitment of 28 patients in each group.
2.5. Randomization, blinding and allocation
Randomization was generated using Microsoft Excel blocked with four random block sizes. Assignment was performed by the clinic secretary who was not clinically involved in this trial using a list of sequential allocations while the researcher, patients and statistician were blinded. Both groups received capsules with the same shape, box and smell. Randomization codes were released after analysis.
This randomized, double-blind, block design study with placebo control was conducted over a 4-week period, with every 2 weeks’ assessment.
2.6. Statistical method
Demographic and clinical characteristic data were shown as mean ± standard deviation (SD) and number (%) for continuous and nominal variables respectively. The normality of continuous variables was checked by the Kolmogorov-Smirnov test. The chi-square test was used for nominal variables, independent t-test for numerical variables, and repeated measure of analysis of variance and Friedman test for time-treatment interaction during the study period.
3. Results
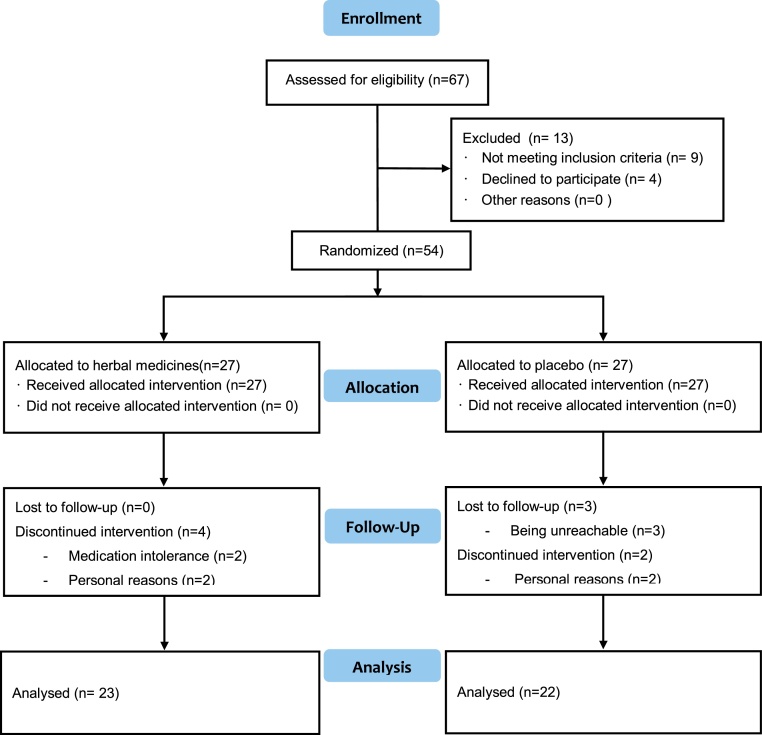
Initially, out of 67 patients complaining of insomnia, thirteen were excluded and 54 eligible cases who met the criteria were randomly allocated into treatment group, (n = 27) and placebo group (n = 27). Finally, 45 patients (23 in treatment group and 22 in placebo group) completed the trial after drop-out and withdrawal (Fig. 1).
Fig. 1.
Flowchart for inclusion and allocation.
The baseline demographic data of patients showed no significant difference in demographic and clinical data between groups. Most of the participants were female in both groups and the mean age of insomniacs was 38.60 ± 12.45 in the treatment group vs 39.68 ± 8.80 in the placebo group.
The mean ISI score decreased significantly in treatment group from baseline to the end compared with placebo group (p value = 0.008). Simultaneously, the mean difference of BAI improved significantly in treatment group from baseline to the fourth week (6.34 ± 7.20) compared with placebo group (1.31 ± 3.50) (p value = 0.005). Besides, the improvement of mean BDI score was significant in treatment group from baseline to the second and fourth weeks compared with placebo group. This difference between the two groups was measured by repeated measure analysis with a p value of <0.001 (Table 1).
Table 1.
Comparison of BAI, BDI and ISI between two groups during four weeks of study
| Groups | Treatment group |
Placebo group |
Between group | ||||
|---|---|---|---|---|---|---|---|
| Outcomes | Baseline | 2nd week | 4th week | Baseline | 2nd week | 4th week | p value |
| ISI | 16.69 ± 4.69 | 11.30 ± 5.24** | 16.09 ± 4.97 | 14.31 ± 5.60* | 0.008 | ||
| BDI | 25.86 ± 10.58 | 17.17 ± 11.72 | 16.26 ± 12.25** | 24.18 ± 12.94 | 21.72 ± 13.11 | 21.40 ± 12.89* | <0.001 |
| BAI | 17.52 ± 7.30 | 11.17 ± 8.64** | 15.27 ± 6.79 | 13.95 ± 7.43* | 0.005 | ||
ISI: Insomnia Severity Index, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory.
*p = 0.05; **p < 0.001: Within group ISI p value (paired t-test).
*p = 0.03; **p < 0.001: Within group BDI p value (paired t-test).
*p = 0.09; **p < 0.001: Within group BAI p value (paired t-test).
Reported adverse events included one person with unbearable itching, three with agitation and intensifying anxiety that improved after few days of treatment.
Melissa officinalis and Nepeta menthoides, in combination, improved the depression and anxiety of insomniacs by the reduction of BDI and BAI scores. Also severity of insomnia was decreased evaluating ISI score. No serious adverse event was reported during 4 weeks of study.
4. Discussion
Anti-oxidant effect of Polyphenols and Flavonoids as the main components of Nepeta menthoides could be responsible for its anxiolytic activity.14 On the other hand, animal studies indicate antidepressant and anxiolytic effects of Melissa officinalis with a high dose in male rats.8 Increasing of brain GABA levels was the hypothesis of some studies conducted on mice.15 A survey showed three mechanisms of Melissa officinalis: inhibition of GABA transaminase, increase of self-rated calmness and inhibition of MAO-A.16
The short duration of study, small sample size, lack of follow-up period after intervention, per protocol analysis and choosing three primary outcomes were limitations for this trial. Future research to overcome limitations is recommended. Additional trials are needed to evaluate the exact underlying mechanism of these herbal effects on insomnia, depression and anxiety.
In conclusion, a combination of Melissa officinalis and Nepeta menthoides may have a beneficial effect on insomnia severity and on depression and anxiety of insomniacs. This combination could be mentioned as a potential alternative treatment with fewer complications for anxious and depressed patients.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This trial was a part of postgraduate thesis by Dr. Maryam Ranjbar supported by a grant from Shiraz University of Medical Sciences for partial fulfilment of the requirements toward a Ph.D. degree in Traditional Persian Medicine. We would like to appreciate the collaboration of colleagues involved in this study with special thanks to Farzad Rafieian and Dr. Arash Najimi for his invaluable helps.
References
- 1.Cunnington D., Junge M.F., Fernando A.T. Insomnia: prevalence, consequences and effective treatment. Med J Aust. 2013;199:36–40. doi: 10.5694/mja13.10718. [DOI] [PubMed] [Google Scholar]
- 2.Mai E., Buysse D.J. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3:167–174. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choueiry N., Salamoun T., Jabbour H., El Osta N., Hajj A., Khabbaz L.R. Insomnia and relationship with anxiety in university students: a cross-sectional designed study. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0149643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvaro P.K., Roberts R.M., Harris J.K. Sleep. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36:1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avicenna . Alaalami Library; Beirut: 2005. Al Qanun Fi Al-Tibb [Arabic] [Google Scholar]
- 6.Saki K., Bahmani M., Rafieian-Kopaei M. The effect of most important medicinal plants on two important psychiatric disorders [anxiety and depression]—a review. Asian Pac J Trop Medcine. 2014;7:534–542. doi: 10.1016/S1995-7645(14)60201-7. [DOI] [PubMed] [Google Scholar]
- 7.Chehroudi S., Fatemi M.J., Saberi M., Salehi S.H., Akbari H., Samimi R. Effects of Melissa officinalis L. on reducing stress, alleviating anxiety disorders, depression, and insomnia, and increasing total antioxidants in burn patients. Trauma Mon. 2016 [Google Scholar]
- 8.Taiwo A.E., Leite F.B., Lucena G.M., Barros M., Silveira D., Silva M.V. Anxiolytic and antidepressant-like effects of Melissa officinalis [lemon balm] extract in rats: influence of administration and gender. Indian J Pharmacol. 2012;44:189–192. doi: 10.4103/0253-7613.93846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alijaniha F., Naseri M., Afsharypuor S., Fallahi F., Noorbala A., Mosaddegh M. Heart palpitation relief with Melissa officinalis leaf extract: double blind, randomized, placebo controlled trial of efficacy and safety. J Ethnopharmacol. 2015;164:378–384. doi: 10.1016/j.jep.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Cases J., Ibarra A., Feuillère N., Roller M., Sukkar S.G. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Mediterr J Nutr Metab. 2011;4:211–218. doi: 10.1007/s12349-010-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firoozabadi A., Kolouri S., Zarshenas M.M., Salehi A., Mosavat S.H., Dastgheib S.A. Efficacy of a freeze-dried aqueous extract of Nepeta menthoides Boiss. & Buhse in the treatment of anxiety in patients with depression: a double-blind, randomized, controlled trial. J Herb Med. 2017 [Google Scholar]
- 12.Kolouri S., Firoozabadi A., Salehi A., Zarshenas M.M., Dastgheib S.A., Heydari M. Nepeta menthoides Boiss. & Buhse freeze-dried aqueous extract versus sertraline in the treatment of major depression: a double blind randomized controlled trial. Complement Ther Med. 2016;26:164–170. doi: 10.1016/j.ctim.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Firoozabadi A., Zarshenas M.M., Salehi A., Jahanbin S., Mohagheghzadeh A. Effectiveness of Cuscuta planiflora Ten. and Nepeta menthoides Boiss. & Buhse in major depression: a triple-blind randomized controlled trial study. J Evid-based Complement Altern Med. 2015;20:94–97. doi: 10.1177/2156587214557359. [DOI] [PubMed] [Google Scholar]
- 14.Hadi N., Sefdkon F., Shojaeiyan A., Šiler B., Jafari A.-A., Aničić N. Phenolics’ composition in four endemic Npeta species from Iran cultivated under experimental field conditions: the possibility of exploitation of Nepeta germplasm. Ind Crops Prod. 2017:475–484. [Google Scholar]
- 15.Ibarra A., Feuillere N., Roller M., Lesburgere E., Beracochea D. Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine. 2010;17:397–403. doi: 10.1016/j.phymed.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Sarris J., Panossian A., Schweitzer I., Stough C., Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21:841–860. doi: 10.1016/j.euroneuro.2011.04.002. [DOI] [PubMed] [Google Scholar]