Abstract
The budding yeast kinesin-8 Kip3 is a highly processive motor protein that walks to the ends of cytoskeletal microtubules and shortens them in a collective manner. However, how exactly Kip3 reaches the microtubule end is unclear. Although rotations of microtubules in multimotored Kip3 gliding assays implied directed sideward switching between microtubule protofilaments, two-dimensional, single-molecule, optical-tweezers assays indicated that Kip3 randomly switched protofilaments. Here, we topographically suspended microtubules such that Kip3 motors could freely access the microtubules in three dimensions. Tracking single-motor-driven microspheres with a three-dimensional, zero-load, optical-tweezers-based force clamp showed that Kip3 switched protofilaments in discrete steps equally frequent in both directions. A statistical analysis confirmed the diffusive sideward motion of Kip3, consistent with the two-dimensional single-molecule results. Furthermore, we found that motors were in one of three states: either not switching protofilaments or switching between them with a slow or fast sideward-stepping rate. Interestingly, this sideward diffusion was limited to one turn, suggesting that motors could not cross the microtubule seam. The diffusive protofilament switching may enable Kip3 to efficiently bypass obstacles and reach the microtubule end for length regulation.
Introduction
Motor proteins translocate along cytoskeletal filaments, generating force and motion (1). They are involved in various biological processes like intracellular transport, cell motility, or cell division. Motor proteins from the superfamily of kinesins move along cytoskeletal filaments called microtubules. Microtubules consist of circularly arranged protein chains, so-called protofilaments, assembled from tubulin dimers (1). Kinesin-1 transports cargo by moving in 8 nm steps on microtubules in a rotary hand-over-hand mechanism (2, 3, 4, 5, 6). Other kinesins have other biological functions. Members of the kinesin-8 family are known to regulate microtubule dynamics (7, 8, 9, 10, 11, 12). The budding yeast kinesin-8 Kip3 has been shown to depolymerize microtubules in a length-dependent manner (10). This depolymerization is important during mitosis (9) and only occurs in a collective fashion at high Kip3 concentrations. At low motor concentrations, for example, after a catastrophe, small numbers of Kip3 are proposed to stabilize microtubules promoting their growth via the second microtubule binding domain in the Kip3 tail (13). Because of this additional microtubule binding site, Kip3 can cross-link and “slide” between different microtubules (14). Thus, Kip3’s biological function is thought to be microtubule spindle assembly and spindle length control during mitosis. Furthermore, the motor mechanism of kinesin-8 is important because, e.g., an overexpression of the human kinesin-8 Kif18A is correlated to breast and colorectal cancer (15, 16), and a better understanding of how kinesin-8 works might enable drug development to target the motor for cancer treatment (17, 18).
Kip3 shortens microtubules only at the microtubule plus end (10). Thus, it is essential for Kip3 to reach these ends. Therefore, the question of how the motor gets there efficiently arises. One essential property is that Kip3 is very processive, i.e., it takes many steps before dissociating from the microtubule (8), increasing the probability of reaching the end. Its high run length originates from the second microtubule binding site (13) in addition to a weakly bound slip state (19). Because the lattice of microtubules is crowded with many interacting proteins, another property that Kip3 should have is the ability to bypass obstacles on the microtubule, i.e., to be able to switch between different protofilaments. In multimotored gliding assays, Kip3 rotated microtubules in a left-handed manner, indicating protofilament switching (20). Previously (21), we performed two-dimensional (2D) single-motor stepping assays on immobilized microtubules with alternating sideward loads on Kip3 using high-precision optical tweezers (22, 23). We found that Kip3 switched between protofilaments with an asymmetrical force dependence. This asymmetry may explain the left-handed rotations in multimotored gliding assays. Nevertheless, 2D assays have limitations: surface-immobilized microtubules do not allow motor proteins to walk around the whole microtubule, in particular when coupled to a microsphere for optical trapping. Furthermore, inferring the three-dimensional (3D) motor motion around the microtubule cylinder based on the 2D measurements was nontrivial (21). To overcome these limitations, here we performed Kip3 stepping assays on microtubules that were suspended on topographic structures providing full access to the microtubule. Using optical tweezers, we tracked the 3D motion of Kip3-coated microspheres. Previously, we tracked kinesin-1 using a zero-force-feedback mode with a 3D positional and angular tracking precision quantified by the Allan deviation of better than 10 nm and 2°, respectively, over three orders of magnitude in bandwidth (∼0.2–200 ms) (24, 25). Using this method, we confirmed protofilament tracking of conventional kinesin-1 without any protofilament switches (25). Based on the microtubule supertwist, we could measure the microtubule composition in terms of the number of protofilaments the microtubules are composed of. Furthermore, the tracking precision allowed us to measure individual defects on the microtubule lattice. Here, for single Kip3 motors, we could resolve individual angular steps corresponding to protofilament switches that occurred randomly in both directions. Interestingly, motors seemed to be unable to switch protofilaments across the seam.
Materials and Methods
Microtubule preparation
Porcine tubulin was polymerized in BRB80 buffer (80 mM piperazine-N,N′-bis[2-ethanesulfonic acid], 1 mM EGTA, 1 mM MgCl2 (pH 6.9)) with 5% dimethyl sulfoxide, 4 mM MgCl2, and 1 mM GTP for 1.5–2 h at 37°C. Afterwards, microtubules were diluted with BRB80 containing 10 μM taxol (BRB80T), spun down in an Airfuge (Beckman Coulter, Brea, CA), and resuspended in BRB80T. If not noted otherwise, all chemicals are from Sigma (St. Louis, MO). Microtubules were visualized with differential interference contrast employing a light-emitting diode (26).
Functionalized microsphere preparation
To specifically bind green-fluorescent-protein (GFP)-tagged proteins, carboxylated polystyrene microspheres (mean diameter 0.59 μm; Bangs Laboratories, Fishers, IN) were coated covalently with a small fraction of heterobifunctional polyethylene glycol (hPEG, 3 kDa) linkers to which a GFP antibody was covalently bound, surrounded by many monofunctional PEG molecules (mPEG, 2 kDa), which had no additional molecules bound to their ends as described in (27). The shorter mPEG forms a polymeric brush that suppresses unspecific binding.
Sample preparation and assay
Experiments were performed in flow cells that were constructed using micropatterned coverslips. Using ultraviolet nanoimprint lithography, micropatterned coverslips were fabricated with a repeating pattern of ridges with a width of 5 μm and a height of 1.4 μm and canyons with a width of 10 μm as described in (25). Full-length budding yeast Kip3 (Kip3-eGFP-His6, 805 amino acids without tags) and rat kinesin-1 (His6-rkin430-eGFP, 430 amino acids without tags) were expressed and purified according to (19, 28). Even though Kip3 has many more residues than rkin430, both motors have about the same calculated length of 15 nm (without tags) assuming that the tail of Kip3 is a globular domain including the second microtubule binding domain. The motility buffer for Kip3 stepping assays was BRB80T supplemented with 1 mM ATP, 0.1 mg/mL casein, 112.5 mM KCl, 0.1% Tween-20, and an antifading mix (10 mM dithiothreitol, 20 mM glucose, 20 μg/mL glucose oxidase, 8 μg/mL catalase) (19, 21). As a control, we used a truncated GFP-tagged rat kinesin-1 rkin430 in a BRB80T motility solution with 0.1 mg/mL casein, 10 μM ATP, 0.1% Tween-20, and the abovementioned antifade cocktail (25). Functionalized microspheres were mixed with motor proteins in motility buffer to a motor/microsphere ratio for which every third microsphere showed motility, implying single-molecule conditions with 95% confidence (29). The channels of flow cells were washed with BRB80, filled and incubated successively with anti-β-tubulin I (monoclonal antibody SAP.4G5 from Sigma in BRB80), Pluronic F-127 (1% in BRB80), and microtubules in BRB80T. Finally, the kinesin-microsphere mix was flowed in.
Optical tweezers setup
Measurements were performed in a single-beam optical tweezers setup as described in (22, 30, 31). The setup is equipped with a millikelvin precision temperature control, a laser stabilization system, and a 3D force feedback using piezo tilt mirrors in the lateral directions and the sample stage in the axial direction with a feedback rate of 1 kHz and a sampling rate of 4 kHz, as described in detail in (24). Using Kip3-coated microspheres in a zero-load mode, the response time of the feedback was ∼15 and 50 ms in the lateral and axial directions, respectively. Furthermore, using these microspheres, the 3D force clamp had a measurement precision quantified as the SD of the filtered data of better than 10–20 nm in all dimensions and an angular precision of 3–4°. This measurement precision was determined by tracking immobilized Kip3 on microtubules, as described previously for kinesin-1 (25). For motile motors, we expect a somewhat larger noise caused by the forward movement of the motor, fluctuations of the suspended microtubule, and possibly defects in the microtubule lattice (25) that may lead to significant, directed angular motion. For stable trapping, the objective temperature was set to 29.200°C (22). The optical trap was calibrated by applying a known drag force and analyzing the height-dependent power spectral density as described in (31, 32). We used a lateral and axial trap stiffness of 0.03–0.05 and 0.004–0.007 pN/nm, respectively. Previous controls ruled out any significant influence of the axial and lateral position dependence of the calibration factors as well as thermal drift and laser fluctuations (25). Within the diffusive volume explored during one step, any force bias should be less than 0.1 pN. The drag coefficient of a sphere depends on the proximity to a surface (31). For our microsphere size and dimensions of the topographic structures, the ratio between the drag coefficient of a microsphere under and above the microtubule was 1.07 and 1.17 for the lateral and axial directions, respectively. We did not observe and do not expect a bias away or toward the coverslip based on these differences. Also, because the time between sideward steps is much larger compared to the relaxation time of the microsphere in the trap, we do not expect any entropic bias due to this difference in diffusion coefficients.
3D optical tweezers tracking of Kip3
Kip3 and kinesin-1 control experiments were performed in the way described previously for kinesin-1 (25). Briefly, motor-coated microspheres were trapped and positioned on top of suspended microtubules to await kinesin-initiated motility. When motors started to pull on the microsphere, the feedback was switched on with no net loads applied (Fig. 1 A). The 3D positions of the microsphere center were calculated from the mirror and stage positions, respectively, and rotated to align the microtubule axis along the x axis if needed. The angular position ϕ around the microtubule axis was defined such that positive ϕ values correspond to left-handed rotation. For convenience, ϕ is given in units of degrees (° or deg). The angle ϕ was calculated from a circular fit to the zy projection. All position signals were smoothed with a running median filter over 500 data points for all further analysis. Experiments were performed with 1 mM ATP under single-molecule as well as multiple-molecule conditions and with 10 μM ATP under Kip3 multiple- and rkin430 single-molecule conditions. For the low-ATP-concentration experiments, motor speeds did not change significantly during the course of the experiments such that an ATP regenerating system was not necessary. Multimotor conditions were established by mixing Kip3 and microspheres with a 100–1000× higher Kip3/microsphere ratio than used for single-motor conditions. Under multimotor conditions, nearly every microsphere showed motility. Note that we do not know the number of motors that were interacting simultaneously with the microtubule and that this number may have been limited by the antibody surface density on the microspheres. With a ∼100× lower number of antibodies on the microsphere surface, one out of nine microspheres showed motility, indicating single-molecule conditions independent of the motor concentration used during the microsphere preparation (27). We included ∼80% of all recorded traces in the analysis. We excluded short (<10 s) traces and traces recorded on loosely attached microtubules. On the freely suspended microtubules, we measured a mean microsphere speed for single Kip3 at high ATP of 62 ± 1 nm/s (mean ± standard error (SE) if not noted otherwise, N = 122), for multiple Kip3 at high ATP of 56 ± 2 nm/s (N = 100), for multiple Kip3 at low ATP of 20 ± 2 nm/s (N = 26), and for rkin430 at low ATP of 187 ± 5 nm/s (N = 65).
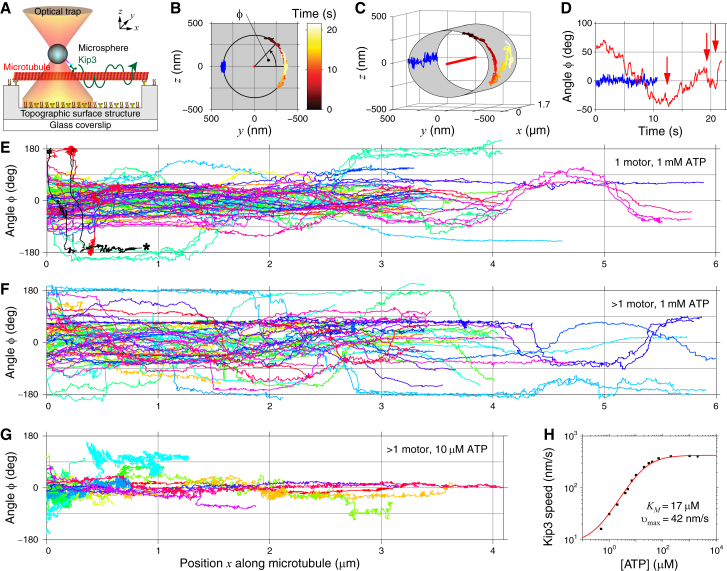
Figure 1.
3D tracking of Kip3 with optical tweezers. (A) A schematic of the 3D stepping assay (side view, not to scale). Suspended microtubules provided free 3D access to kinesin-coated microspheres that were trapped and tracked with optical tweezers. (B) An exemplary 3D trace of a microsphere powered by a single Kip3 (red heat map) or kinesin-1 (blue) is shown in a zy projection with the microtubule drawn as the central red point. The traces from (B) are shown in (C) as a 3D projection with the microtubule drawn as a red line and in (D) as the angular position as a function of time. Red arrows indicated changes of rotation direction. For kinesin-1, we subtracted an offset of 180°. Angle ϕ is shown as a function of forward position x of (E) 84 traces of single Kip3-powered microspheres on 58 different microtubules (the asterisk-marked trace has a 360° phase jump at ∼0.2 μm), (F) 62 traces of multimotored Kip3 microspheres on 47 different microtubules, and (G) 16 traces of multimotored Kip3 microspheres at reduced ATP concentration on seven different microtubules. For each trace in (E)–(G), an offset equal to the mean of the maximal and minimal angle was subtracted. (H) Kip3 forward speed along the microtubule axis (black squares) as a function of ATP concentration. The red line is a Michaelis-Menten fit with the fit parameters KM = 17 ± 1 μM and υmax = 41.7 ± 0.09 nm/s. To see this figure in color, go online.
ATP dependence of Kip3 speed
To determine how Kip3 motility depends on ATP concentration, we measured the speed of single Kip3 molecules on surface-immobilized microtubules in a total internal reflection fluorescence microscope as previously described in (8) at a setup temperature of 29°C. Microtubules were stabilized with guanosine-5′-[(α,β)-methyleno]triphosphate (GMPCPP; Jena Bioscience, Jena, Germany). The motility solution was identical to the one used in the optical tweezers assays with the exception that no Tween-20 or taxol was used. The ATP concentration was varied in the range of 0.5–2000 μM.
Results
Kip3 showed unbiased angular motion that was limited to one turn
3D stepping assays with single Kip3-powered microspheres were performed on suspended microtubules using topographic structures (Materials and Methods; Fig. 1 A). The 3D microsphere motion was tracked with high precision using optical tweezers operated as a zero-force, 3D force clamp. The zy-projected angular position ϕ of a Kip3-powered microsphere corresponded to a circular arc with a radius of 375 ± 9 nm (N = 181), corresponding approximately to the sum of the microsphere and microtubule radii plus the protein and linker contour length not differing significantly from kinesin-1 with a radius of 370 ± 18 nm (N = 32) (25) (Fig. 1 B). In contrast to previous 3D measurements with the nonprotofilament switching kinesin-1 that showed either no (blue trace in Fig. 1, C and D) or unidirectional angular motion consistent with the microtubule supertwist (25), the 3D projection and angular traces as a function of time for Kip3 (heat-map-colored and red trace in Fig. 1, C and D, respectively) showed bidirectional motion with multiple changes of the rotation direction (red arrows in the exemplary trace of Fig. 1 D). Most recorded traces showed such changes in the rotation direction, consistent with the notion that motors can step sidewards in both directions (Fig. 1 E). The mean slope of the angular position ϕ as a function of forward position x of all 84 recorded traces shown in Fig. 1 E was −0.01 ± 0.02°/nm and not significantly different from zero. Based on the microtubule composition measured using kinesin-1 (25), microtubules were on average not supertwisted (average inverse pitch 0.06 ± 0.03 μm−1 (N = 93)), consistent with the mean zero angular slope measured here. Thus, the angular motion of Kip3 in the absence of loads had no directional bias, suggesting an unbiased random sideward walk of Kip3 on the microtubule lattice.
Surprisingly, the peak-to-peak angular motion for most traces was limited to 360°, i.e., to one turn, with the exception of one trace (black trace in Fig. 1 E marked with an asterisk). This trace showed angular motion over two turns, demonstrating that the one-turn limitation was not a limitation of the method itself. We considered this trace to be an outlier because it also had a very low speed and run length. The limitation to one turn implies some kind of barrier to the angular motion of Kip3. To test whether this barrier could be crossed by multiple Kip3 molecules bound to the same microsphere, we performed a multimotor assay under otherwise identical conditions. Also, with several Kip3 motors interacting simultaneously with the microtubule (Fig. 1 F), all traces were limited to one turn and had no bias, with a mean angular slope of −0.007 ± 0.009°/nm. Taken together, the data suggest that Kip3 motors performed a bounded, random sideward walk.
Single Kip3 motors switched protofilaments in a discrete manner
If Kip3 walks on different protofilaments, switching should occur in discrete angular steps in analogy to its discrete 8 nm forward steps on the microtubule lattice (19). Previous 2D stepping assays under sideward loads showed discrete lateral steps normal to the microtubule axis that were consistent with discrete protofilament switching (21). However, because of the geometrical limitations in the 2D stepping assay, lateral sideward steps varied in size over a broad range and the attribution to a discrete angular step with identified direction was impossible. Because our angular tracking precision in our 3D assay should allow us to resolve individual angular sideward steps, we analyzed the angular traces with a step-finding algorithm (30) (Fig. 2 A). The detected steps had a mean absolute step size of 22 ± 8° (SD), shown in the histogram of Fig. 2 B. 84 steps were to the left and 60 to the right, found in 39 traces. Note that these selected traces had a leftward mean angular slope causing an apparent leftward bias of steps, which, based on a binomial distribution, was, however, not even significantly different from an equal left/right stepping probability with a p-value of 0.055. The expected angular step size depends on the number of protofilaments in the microtubule. As our microtubules preparation mostly contained microtubules with 13 protofilaments, we expected a step size Δϕ = 360°/13 ≈ 28° (inset Fig. 2 B), in reasonable agreement with the measured step size. The mean angular dwell time τside was 2.17 ± 0.03 s (Fig. 2 C). The corresponding sideward stepping rate was 0.46 ± 0.01 s−1. This rate implies that at saturating ATP concentrations and in the absence of loads, about every 18th step that the motor took was a sideward step.
Figure 2.
Kip3 switches protofilaments in a discrete manner. (A) Angular traces (black) with detected steps (red lines). For clarity, traces are offset arbitrarily. (B) A histogram of absolute angular step sizes with Gaussian fit (red line). The center of the Gaussian, its SD, and number of data points are given. The inset shows a schematic of an angular step of size Δϕ corresponding to a protofilament switch. (C) A histogram of angular dwell times with exponential fit (red line). The inset shows a cumulative distribution function plot with exponential fit, mean step time, SE, and number of data points. To see this figure in color, go online.
Low ATP concentrations reduced the angular motion of Kip3
To investigate whether protofilament switching depended on the ATP concentration, we performed assays at a reduced ATP concentration of 10 μM (Fig. 1 G). Under these conditions, ATP was rate limiting (Fig. 1 H) and, as noted previously, the stall force of Kip3 was decreased significantly (21). This low stall force made optical tweezers assays especially difficult to perform because it was difficult to identify whether a motor-coated microsphere under single-molecule conditions was interacting with a microtubule. Therefore, we used multimotor conditions for which higher stall forces enabled us to perform experiments at low ATP concentrations. Although the mean stall force at high ATP (1 mM) was 1.50 ± 0.02 pN (N = 77) in a static trap for single Kip3 motors and 3.1 ± 0.2 pN (N = 41) for our specific multimotor conditions, respectively, we measured a stall force of only 0.8 ± 0.1 pN (N = 20) for the multimotor conditions at low ATP (10 μM). With respect to the sidewards motion, the low-ATP, multimotor 3D traces (Fig. 1 G) also had no bias based on the within-error-bars, zero-mean-angular slope of 0.01 ± 0.04°/nm. Yet, compared to the high ATP conditions, traces qualitatively showed far less angular motion. The data suggest that when ATP binding becomes rate limiting, the number of sideward steps per forward step was reduced.
Statistical analysis reveals three distinct states of motion
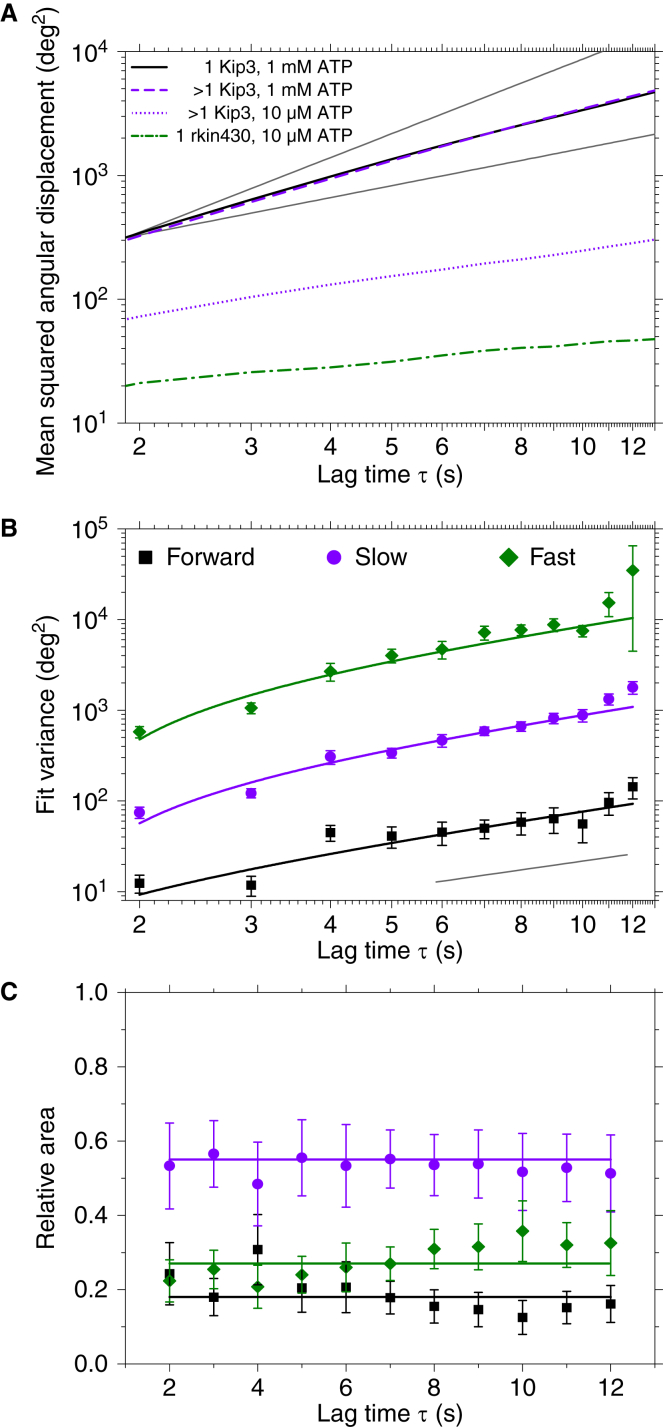
To analyze the angular motion of all Kip3 traces and compare the different experiments quantitatively, we performed a mean-squared angular displacement (MSAD) analysis (Fig. 3 A; Supporting Materials and Methods, Section 1). Although the MSAD curves for Kip3 at single and multimotor conditions for high ATP concentrations were nearly identical, the MSAD curve obtained for the multimotor Kip3 assay at low ATP concentration was significantly smaller, as already qualitatively seen in Fig. 1 G. Even accounting for the threefold slower forward speed at low ATP by normalizing the number of sideward steps to the distance traveled on the microtubule does not affect that conclusion (Fig. S1). For comparison, we also analyzed 36 angular traces of the truncated kinesin-1 rkin430 on straight, 13-protofilament microtubules that we recorded previously (25). For kinesin-1, the MSAD curve was very low and nearly constant (Fig. S1), reflecting the angular tracking precision.
Figure 3.
Statistical analysis of angular motion. (A) Mean-squared anglular displacements (MSADs) as a function of lag time τ for single Kip3 at 1 mM ATP (black line), multiple Kip3 at 1 mM ATP (purple dashed line), multiple Kip3 at 10 μM ATP (purple dotted line), and single kinesin-1 at 10 μM ATP (green dashed/dotted line). Gray lines are guides to the eye with power-law exponents of one and two, respectively. (B) Variances and (C) relative areas with weighted means (horizontal lines) of the three states of motion for Kip3 are shown, using the same color legend. Error bars are SEs. The gray lines in (B) represent the slope of a power law with an exponent of one. Note that because of an offset included in the MSAD fits, they are curved at low lag times. To see this figure in color, go online.
Interestingly, the MSAD curve for single Kip3 deviated from an expected linear dependence on lag time (Fig. 3 A; Fig. S1). When we fitted the MSAD curve with a parabola, we obtained a directed component of 2.9 ± 0.2°/s, corresponding to an absolute rotational pitch of 7.7 ± 0.5 μm using a forward speed of 62 nm/s. Thus, the MSAD analysis indicates an apparent directed motion inconsistent with the observation that the mean angular slope of all traces in Fig. 1 E was zero within error bars.
A closer look at the distributions of the angular displacements as a function of lag time showed that the distributions could not be fitted well by a single Gaussian, i.e., the angular displacements were not normally distributed (Fig. S2). Instead, a sum of three Gaussians fitted the data best (Figs. S2 and S3; Section S2). Thus, there were three different states of Kip3 with respect to its sideward stepping behavior. A similar analysis was performed previously for different states in the diffusive motion of kinesin-13 (33). An averaged MSAD like in Fig. 3 A does not reflect the distinctness of different states. Therefore, we plotted the variance obtained from the Gaussian fits to the angular displacement distributions as a function of lag time. For the individual variances, we indeed observed the expected linear behavior (Fig. 3 B). The relative area of the three Gaussians remained constant within error bars (Fig. 3 C), reflecting that the probability of motors being in the states, i.e., their abundance, did not depend on time. A linear fit to the angular variances as a function of lag time resulted in three very different angular diffusion coefficients (Table 1). For comparison, we also calculated a diffusion constant for kinesin-1 from a linear fit to the initial MSAD data in Fig. S1. The smallest angular diffusion constant of Kip3 was comparable to the value for the protofilament tracker kinesin-1. Thus, this state of Kip3 represents exclusive forward motion with no sideward steps. The other two states correspond to random-sideward-stepping modes with a small and large angular diffusion coefficient, respectively, which we refer to as slow and fast diffusive states.
Table 1.
Diffusional States of Kip3
| Single Kip3, 1 mM ATP, υ = 62 nm/s | |||
|---|---|---|---|
| State of motion | Forward | Slow | Fast |
| Diffusion constant D (deg2/s) | 4.2 ± 0.7 | 52 ± 4 | 490 ± 54 |
| Relative abundance (%) | 18 ± 2 | 55 ± 3 | 27 ± 2 |
| Weighted diffusion constant (deg2/s) | 162 ± 28 | ||
| Single rkin430, 10 μM ATP, υ = 187 nm/s | |||
| Diffusion constant (deg2/s) | 5.0 ± 0.5a | ||
The value was normalized to the speed of single Kip3 (Supporting Materials and Methods, Section 1).
Close inspection of the angular traces (Fig. 1 E) shows that individual Kip3 motors switched between phases of exclusive forward motion and mixed forward and diffusive angular motion (see Fig. S3 for selected traces). Thus, a single molecule could switch between the different states reducing the possibility that three different populations of Kip3 molecules existed in our purification.
For the angular displacement distribution analysis, we also measured a significant but smaller bias of the center positions of all three fits of the Gaussian functions over time of 0.3 ± 0.1°/s (Fig. S5). This bias corresponds to a pitch of ∼75 μm, much larger than typical values for supertwist pitches of non-13-protofilament microtubules but of similar magnitude as a residual shallow supertwist pitch reported for 13-protofilament microtubules (34). Also, the corresponding inverse pitch of 0.013 ± 0.004 μm−1 is consistent with the overall inverse supertwist pitch of the used microtubule mix of 0.06 ± 0.03 μm−1 (25). Taken together, these data suggest that there is an apparent small directed component of angular motion and that Kip3 has at least two different modes of sideward stepping, one with a slow and one with a fast sideward-stepping rate.
To compare our data to ensemble Kip3 measurements, we calculated an average sideward diffusion coefficient. Using the relative abundances of the three Kip3 states, the weighted average of the diffusion constant was 162 ± 28deg2/s (Table 1). From this effective diffusion constant D, we can calculate the mean sideward stepping rate k = 2D/Δϕ2, where Δϕ is the angular step size. For a 13-protofilament microtubule with Δϕ = 28°, the mean sideward stepping rate is 0.42 ± 0.07 s−1; for the measured mean angular step size of 22°, the corresponding sideward stepping rate is 0.66 ± 0.15s−1. Both values are consistent with the directly measured rate based on the mean angular step dwell time (Fig. 2 C) of 0.46 s−1. The rates are also in good agreement with previous simulation of 2D stepping assays that matched 2D experiments under sideward loads extrapolated to zero load of 0.59 ± 0.07 s−1 (21). Overall, the 3D results are in agreement with previous studies and provide more details about the ability of Kip3 to switch protofilaments.
Discussion
We successfully used optical tweezers in a 3D force-feedback mode to track the motion of kinesin-coated microspheres with high precision. Our experiments show that Kip3 1) switches protofilaments in a discrete, diffusive, and ATP-dependent manner, possibly with a small bias; 2) has different states of angular diffusion; and 3) was limited to one turn in angular motion. The protofilament switching under no load is consistent with a simulation matching 2D experiments under sideward loads (21). Moreover, for the first time, to the best of our knowledge, we directly resolved discrete angular sideward steps for a motor protein. Surprisingly, Kip3’s angular motion was limited to one turn. One possible explanation could be the presence of the so-called microtubule seam. This seam is a break in the symmetry of the microtubule lattice where the typical lateral α-α- and β-β-tubulin bonds are shifted to α-β- and β-α-tubulin bonds (1). Consequently, the next kinesin binding site on the neighboring protofilament across the seam is much further away than on the normal microtubule lattice. Therefore, even though Kip3 has a longer neck linker compared to kinesin-1 (20), Kip3 may not be able to reach this binding site and thus not be able to cross the seam. The one-trace exception of Fig. 1 E that showed more than 360° rotation may have been recorded on a rare, non-3-start helix microtubule that does not have a seam (35). In support of a seam, we observed traces in which Kip3 moved sideward until a certain angle, stopped sideward motion, and then turned in the opposite direction. For several traces that were recorded after one another with the same microsphere on the same microtubule with the same starting position, this point of return was at the same angular position, suggesting that it was related to a structural feature of the microtubule like the seam. Interestingly, coating the microspheres with multiple Kip3 motors did not enable the motors to move more than one turn around the microtubule. We hypothesize that both motor heads need to detach from the microtubule to cross the seam. The motor could then reattach beyond the seam. However, because of the high processivity of single Kip3 motors, a complete motor detachment is also unlikely in the presence of multiple motors. Alternatively, a single motor could potentially cross the seam by binding to the microtubule with its second, tail-based microtubule binding site and detaching both heads. In our assay, the rather large microsphere may hinder such a mechanism. Yet, Kip3’s second microtubule site may affect the sideward switching itself.
The MSAD analysis revealed not only diffusive angular motion but also an apparent directed component (Fig. 3 A). This directed angular motion might be caused by an intrinsic bias in Kip3’s random sideward walk or an overall, i.e., average, nonzero supertwist of the microtubules. Although in the angular-displacement-histogram analysis, directed angular motion on positive and negative supertwisted microtubules averages out, in the MSAD analysis, these directed components are squared and thus do not average out. Therefore, we expect a larger directed component in the MSAD analysis compared to the angular-displacement-histogram analysis. Because the MSAD-directed component corresponded to a pitch somewhat larger than the pitch of −5.5 and +5.6 μm for microtubules with 16 or 14 protofilaments, respectively (25), we attribute the directed component—irrespective of the analysis method—to Kip3 motors that walk on supertwisted microtubules of the mixture rather than to an intrinsic sideward stepping bias. If there were an intrinsic sideward-stepping bias, e.g., purely to the left, both methods should result in the same magnitude of the directed component, which we did not observe. Thus, the difference in the two methods supports the notion that the directed component is due to the microtubule structure and mixture and not a motor property. This conclusion is also supported by the net zero angular slope of the angular traces in Fig. 1 E.
What is the structural origin of the three distinct motor states? Although the overall measured sideward stepping rate of 0.46 s−1 with about every 18th step being a sideward step is in good agreement with predictions from our previous work (21), the origin of the different states is unclear. The average sideward switching rate is comparable to the rate at which Kip3 switches to a weakly bound Kip3 “slip” state (19). A weakly bound state may enable a larger reach of the heads, favoring a sideward step. Sideward stepping may also be related to the rotary hand-over-hand mechanism reported for kinesin-1 “shuffling” its heads along the microtubule lattice (6). During the rotation around the stalk, the stepping head may be electrostatically guided along the microtubule lattice and bind to the neighboring protofilament instead of the canonical forward binding site. In our assay with the kinesin tail bound to a microsphere, the tail may be supertwisted if both tail domains of the homodimer are bound and rotationally constrained. Torsion on the tail may affect sideward stepping. Sideward stepping may also be related to the microtubule structure. The exclusive-forward-motion state could be due to microtubules having multiple, i.e., an odd number of seams (36, 37), and Kip3 might be trapped between them. However, even if every microtubule would have multiple seams with only one protofilament between two seams, the probability of Kip3 landing on this protofilament would only be 8% (1/13). This value is less than the abundance of 18% of the forward state. Thus, confinement between two seams cannot explain this motion state alone. Kip3’s second microtubule binding site may explain all three states with the caveat that the trapped microsphere, under zero-load feedback conditions, may have interfered with microtubule binding of the second microtubule binding site. With an unbound tail domain, sideward stepping may be unimpeded, corresponding to our fast-sideward-diffusion state with about every 5–7th step being a sideward step. If the tail was bound, sideward stepping may be constrained, corresponding to our slow-sideward-diffusion state. If the tail is bound across a microtubule seam and the second microtubule binding domain is also not able to cross the seam, the motor might not be able to make sideward steps anymore corresponding to our exclusive-forward-motion state. There are many more possibilities. For example, the tail domain might be bound ahead of Kip3, establishing an obstacle and/or obstacle sensor for the motor itself. In this manner, the second microtubule binding domain could induce sideward stepping.
At reduced ATP concentration, the number of sideward steps per forward steps decreased significantly and was comparable to our slow-sideward-diffusion state (Fig. 3). The reason for this decrease might be that at a lower stepping rate, the leading head of Kip3 has more time to “choose” the next binding site on the microtubule. Because the closest binding site, presumably corresponding to the lowest energy state, is the binding site in front, forward steps might be preferred under these conditions. The decrease in sideward stepping with decreasing ATP concentration might also be due to a change in the waiting state from a two-head-bound state at high ATP to a low ATP state, in which one head is strongly and the other weakly bound, occasionally truly detaching from the microtubule (6, 38). This change in the waiting state has also been proposed to prevent torque generation of kinesin-1 (6). In contrast to our results, multimotor gliding assays with Kip3 at 10 μM ATP showed a reduced rotational pitch, implying that sideward steps become more frequent with respect to forward steps under these conditions (39). However, in gliding assays, kinesins that simultaneously interact with a microtubule might apply loads on each other, affecting the net sideward stepping rate, which is biased by force (21). Thus, multimotored gliding assays are difficult to compare with single-motor optical tweezers stepping assays.
In conclusion, we found that Kip3 switched protofilaments in a diffusive manner with discrete, random sideward steps. The sideward-stepping rate depended on the ATP concentration and on one of the three states the motor was in. Our 3D assay could be useful to investigate other kinesins or motor proteins like myosins and dyneins. The assay is of special interest for motor proteins that have already been shown to switch protofilaments but were only investigated with video tracking like kinesin-5 or single-headed kinesin-1 (40), cytoplasmatic and axonemal dynein (41, 42), or myosin-V (43). For Kip3, protofilament switching might play an important role for its biological function because random switching would be the most efficient way of bypassing obstacles and defects on the microtubule lattice to reach the microtubule’s plus end—an essential requirement for Kip3’s length control mechanism.
Note added in proof
In a recent study (44), the 3D motion of single Kip3 motors, attached to quantum dots via a multifunctional GFP tag (Kip3-mfGFP), was tracked on similarly suspended microtubules. Although the authors measured a speed, Michaelis-Menten constant, and sideward-stepping rate consistent with our study, they found a net leftward bias. To study the origin of this discrepancy, we recorded 41 traces of single Kip3-mfGFP as used in (44) on 25 different microtubules in our assay geometry. We indeed found one trace with three continuous turns—more compared to any trace recorded with our construct. The angular motion of all other traces was within ±180° but overall exhibited a more pronounced protofilament tracking behavior than found in our results, interspersed with frequent periods of biased left- or rightward movement and less of our slow diffusive state. Apart from the different experimental approach—we may have had a residual net force of <0.1 pN acting on the motors—differences include a different microtubule stabilization; a different tag on Kip3; coupling of the motor either directly or via a flexible linker to a small or large sphere, respectively; and how the central axis of the angular motion was determined during the data analysis. Together with the authors of (44) (S. Diez, personal communication), we believe that one of these factors or a combination thereof causes the different sidestepping behavior. Further experiments are necessary to fully reconcile our findings.
Author Contributions
M.B. performed measurements. M.B. and E.S. designed the research, analyzed the data, and wrote the manuscript.
Acknowledgments
We thank Salvatore Girardo (Microstructure facility, CRTD, TU Dresden) for the micropatterns, Claudia Schirmer and Marco Storch (MPI-CBG, Dresden) for the initial help with total internal reflection fluorescence assays, and Aniruddha Mitra and Stefan Diez (TU Dresden) for discussions on 3D stepping assays.
This work was supported by the Deutsche Forschungsgemeinschaft (Emmy Noether Program), European Research Council (ERC Starting Grant 2010, Nanomech 260875), the Rosa Luxemburg Foundation, the Technische Universität Dresden, and the Universität Tübingen.
Editor: Steven Rosenfeld.
Footnotes
Supporting Materials and Methods and five figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)31107-X.
Supporting Citations
Reference (45) appears in the Supporting Material.
Supporting Material
References
- 1.Howard J. Sinauer Associates; Sunderland, MA: 2001. Motor Proteins and the Cytoskeleton. [Google Scholar]
- 2.Svoboda K., Schmidt C.F., Block S.M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993;365:721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- 3.Asbury C.L., Fehr A.N., Block S.M. Kinesin moves by an asymmetric hand-over-hand mechanism. Science. 2003;302:2130–2134. doi: 10.1126/science.1092985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildiz A., Tomishige M., Selvin P.R. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 5.Schief W.R., Clark R.H., Howard J. Inhibition of kinesin motility by ADP and phosphate supports a hand-over-hand mechanism. Proc. Natl. Acad. Sci. USA. 2004;101:1183–1188. doi: 10.1073/pnas.0304369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramaiya A., Roy B., Schäffer E. Kinesin rotates unidirectionally and generates torque while walking on microtubules. Proc. Natl. Acad. Sci. USA. 2017;114:10894–10899. doi: 10.1073/pnas.1706985114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rischitor P.E., Konzack S., Fischer R. The Kip3-like kinesin KipB moves along microtubules and determines spindle position during synchronized mitoses in Aspergillus nidulans hyphae. Eukaryot. Cell. 2004;3:632–645. doi: 10.1128/EC.3.3.632-645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga V., Helenius J., Howard J. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat. Cell Biol. 2006;8:957–962. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M.L., Jr., Carvalho P., Pellman D. Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat. Cell Biol. 2006;8:913–923. doi: 10.1038/ncb1457. [DOI] [PubMed] [Google Scholar]
- 10.Varga V., Leduc C., Howard J. Kinesin-8 motors act cooperatively to mediate length-dependent microtubule depolymerization. Cell. 2009;138:1174–1183. doi: 10.1016/j.cell.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Du Y., English C.A., Ohi R. The kinesin-8 Kif18A dampens microtubule plus-end dynamics. Curr. Biol. 2010;20:374–380. doi: 10.1016/j.cub.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Glunčić M., Maghelli N., Tolić I.M. Kinesin-8 motors improve nuclear centering by promoting microtubule catastrophe. Phys. Rev. Lett. 2015;114:078103. doi: 10.1103/PhysRevLett.114.078103. [DOI] [PubMed] [Google Scholar]
- 13.Su X., Qiu W., Pellman D. Mechanisms underlying the dual-mode regulation of microtubule dynamics by Kip3/kinesin-8. Mol. Cell. 2011;43:751–763. doi: 10.1016/j.molcel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su X., Arellano-Santoyo H., Pellman D. Microtubule-sliding activity of a kinesin-8 promotes spindle assembly and spindle-length control. Nat. Cell Biol. 2013;15:948–957. doi: 10.1038/ncb2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C., Zhu C., Lu S.H. Kif18A is involved in human breast carcinogenesis. Carcinogenesis. 2010;31:1676–1684. doi: 10.1093/carcin/bgq134. [DOI] [PubMed] [Google Scholar]
- 16.Nagahara M., Nishida N., Mori M. Kinesin 18A expression: clinical relevance to colorectal cancer progression. Int. J. Cancer. 2011;129:2543–2552. doi: 10.1002/ijc.25916. [DOI] [PubMed] [Google Scholar]
- 17.Braun J., Möckel M.M., Mayer T.U. Synthesis and biological evaluation of optimized inhibitors of the mitotic kinesin Kif18A. ACS Chem. Biol. 2015;10:554–560. doi: 10.1021/cb500789h. [DOI] [PubMed] [Google Scholar]
- 18.Locke J., Joseph A.P., Moores C.A. Structural basis of human kinesin-8 function and inhibition. Proc. Natl. Acad. Sci. USA. 2017;114:E9539–E9548. doi: 10.1073/pnas.1712169114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jannasch A., Bormuth V., Schäffer E. Kinesin-8 is a low-force motor protein with a weakly bound slip state. Biophys. J. 2013;104:2456–2464. doi: 10.1016/j.bpj.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bormuth V., Nitzsche B., Diez S. The highly processive kinesin-8, Kip3, switches microtubule protofilaments with a bias toward the left. Biophys. J. 2012;103:L4–L6. doi: 10.1016/j.bpj.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bugiel M., Böhl E., Schäffer E. The Kinesin-8 Kip3 switches protofilaments in a sideward random walk asymmetrically biased by force. Biophys. J. 2015;108:2019–2027. doi: 10.1016/j.bpj.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahamdeh M., Schäffer E. Optical tweezers with millikelvin precision of temperature-controlled objectives and base-pair resolution. Opt. Express. 2009;17:17190–17199. doi: 10.1364/OE.17.017190. [DOI] [PubMed] [Google Scholar]
- 23.Mahamdeh M., Campos C.P., Schäffer E. Under-filling trapping objectives optimizes the use of the available laser power in optical tweezers. Opt. Express. 2011;19:11759–11768. doi: 10.1364/OE.19.011759. [DOI] [PubMed] [Google Scholar]
- 24.Bugiel M., Jannasch A., Schäffer E. Implementation and tuning of an optical tweezers force-clamp feedback system. In: Gennerich A., editor. Chapter 5. Humana Press; 2016. pp. 109–136. (Optical Tweezers: Methods and Protocols). [DOI] [PubMed] [Google Scholar]
- 25.Bugiel M., Mitra A., Schäffer E. Measuring microtubule supertwist and defects by three-dimensional-force-clamp tracking of single kinesin-1 motors. Nano Lett. 2018;18:1290–1295. doi: 10.1021/acs.nanolett.7b04971. [DOI] [PubMed] [Google Scholar]
- 26.Bormuth V., Howard J., Schäffer E. LED illumination for video-enhanced DIC imaging of single microtubules. J. Microsc. 2007;226:1–5. doi: 10.1111/j.1365-2818.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 27.Bugiel M., Fantana H., Jannasch A. Versatile microsphere attachment of GFP-labeled motors and other tagged proteins with preserved functionality. JBM. 2015;2:e30. [Google Scholar]
- 28.Rogers K.R., Weiss S., Cross R. KIF1D is a fast non-processive kinesin that demonstrates novel K-loop-dependent mechanochemistry. EMBO J. 2001;20:5101–5113. doi: 10.1093/emboj/20.18.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block S.M., Goldstein L.S., Schnapp B.J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 30.Bormuth V., Varga V., Schäffer E. Protein friction limits diffusive and directed movements of kinesin motors on microtubules. Science. 2009;325:870–873. doi: 10.1126/science.1174923. [DOI] [PubMed] [Google Scholar]
- 31.Schäffer E., Nørrelykke S.F., Howard J. Surface forces and drag coefficients of microspheres near a plane surface measured with optical tweezers. Langmuir. 2007;23:3654–3665. doi: 10.1021/la0622368. [DOI] [PubMed] [Google Scholar]
- 32.Tolić-Nørrelykke S.F., Schäffer E., Flyvbjerg H. Calibration of optical tweezers with positional detection in the back focal plane. Rev. Sci. Instrum. 2006;77:103101. [Google Scholar]
- 33.Helenius J., Brouhard G., Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- 34.Ray S., Meyhöfer E., Howard J. Kinesin follows the microtubule’s protofilament axis. J. Cell Biol. 1993;121:1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrétien D., Fuller S.D. Microtubules switch occasionally into unfavorable configurations during elongation. J. Mol. Biol. 2000;298:663–676. doi: 10.1006/jmbi.2000.3696. [DOI] [PubMed] [Google Scholar]
- 36.Kikkawa M., Ishikawa T., Hirokawa N. Direct visualization of the microtubule lattice seam both in vitro and in vivo. J. Cell Biol. 1994;127:1965–1971. doi: 10.1083/jcb.127.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandblad L., Busch K.E., Hoenger A. The Schizosaccharomyces pombe EB1 homolog Mal3p binds and stabilizes the microtubule lattice seam. Cell. 2006;127:1415–1424. doi: 10.1016/j.cell.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Clancy B.E., Behnke-Parks W.M., Block S.M. A universal pathway for kinesin stepping. Nat. Struct. Mol. Biol. 2011;18:1020–1027. doi: 10.1038/nsmb.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bormuth V. Technische Universität Dresden; 2009. Optimized optical tweezers to study the mechanics of kinesin-8: stepping, slipping, protein friction. PhD thesis. [Google Scholar]
- 40.Yajima J., Mizutani K., Nishizaka T. A torque component present in mitotic kinesin Eg5 revealed by three-dimensional tracking. Nat. Struct. Mol. Biol. 2008;15:1119–1121. doi: 10.1038/nsmb.1491. [DOI] [PubMed] [Google Scholar]
- 41.Can S., Dewitt M.A., Yildiz A. Bidirectional helical motility of cytoplasmic dynein around microtubules. eLife. 2014;3:e03205. doi: 10.7554/eLife.03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi S., Saito K., Yajima J. Torque generation by axonemal outer-arm dynein. Biophys. J. 2015;108:872–879. doi: 10.1016/j.bpj.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y., Sato O., Goldman Y.E. Single-molecule stepping and structural dynamics of myosin X. Nat. Struct. Mol. Biol. 2010;17:485–491. doi: 10.1038/nsmb.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitra A., Ruhnow F., Diez S. Directionally biased sidestepping of Kip3/kinesin-8 is regulated by ATP waiting time and motor-microtubule interaction strength. Proc. Natl. Acad. Sci. USA. 2018;115:E7950–E7959. doi: 10.1073/pnas.1801820115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalet X. Mean square displacement analysis of single-particle trajectories with localization error: Brownian motion in an isotropic medium. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2010;82:041914. doi: 10.1103/PhysRevE.82.041914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.