The recently published Annual Report to the Nation on the Status of Cancer revealed that cancer mortality decreased between 1999 and 2015 by an average of 1.8% per year for men and 1.4% for women. This equates to a decrease in cancer mortality by more than 20% since the turn of the century.1 Simultaneously, in the United States, spending on cancer drugs has nearly doubled to $45 billion just over the last 5 years.2 While at first glance, these two facts are clearly related—better treatment options translate to increased survival—this is not the reality. Several new drugs are approved at a high cost but with marginal effect over less expensive options,3 while numerous off-patent drugs remain at brand-name prices.4
What plays into the price of drugs is multifactorial and complex. There are manufacturing and distribution costs of the drug itself, the costs of large and often negative clinical trials, cost of Food and Drug Administration (FDA) and other governmental policy regulations, and cost-sharing of the drug itself across the targeted patient population, to name a few.5 While a drug is still on patent, companies have total control over the price to recover the cost of the drug’s development. Then, after 20 years, the drug comes off-patent. Does the high-cost issue go away?
The answer is no. The cycle starts when profits from off-patent drugs, particularly those for limited indication, start to fall over a few years. The large pharmaceutical company starts looking to offload the drug. It gets sold to a smaller company that increases the cost to recoup the expenditure involved in buying the drug. Maybe this happens a few times over until the cost is significantly higher than the original generic. In drugs with high cost-sharing, meaning a large target population, this may encourage competition for other companies to start manufacturing the generic drug; or if there are several comparable options on the market (think statins), then the cost is kept low through negotiations with insurance companies.5
At times, drug price hikes make national news headlines such as Mylan’s EpiPen and Turing Pharmaceutical’s Daraprim (pyrimethamine) scandals. However, drugs for orphan diseases have smaller cost-sharing, allowing supply and demand to drive prices up in vulnerable patient populations without a lot of other choices.5 Other companies are not incentivized to go through the process of FDA licensing and start manufacturing because there is limited profit. Aside from driving up the cost, this also puts these patients at risk for significant drug shortage if there is an issue with manufacturing. A smaller patient population means less public outcry, and hence these hikes may continue for years, flying under the radar.
The field of neuro-oncology is in the midst of such a crisis with lomustine. Lomustine is an oral alkylating nitrosourea chemotherapy used frequently in neuro-oncology with numerous indications. In glioblastoma it is used for recurrent disease,6 and recent data suggest benefit in combination with temozolomide for newly diagnosed O6-methylguanine-DNA methyltransferase promoter methylation.7 It is used in combination with procarbazine and vincristine for anaplastic gliomas8,9 and low-grade gliomas10 and with vincristine and cyclophosphamide or cisplatin for medulloblastoma.11 Several of these regimens are included in the National Comprehensive Cancer Network guidelines12 and are part of clinical trials (NCT02796261). Its only non–brain tumor indication is Hodgkin lymphoma, where it is rarely used given the number of alternatives.
Lomustine was marketed by Bristol-Myers Squibb as CeeNU until 2013. At that time it was sold to NextSource Biotechology, a leading specialty pharmaceutical manufacturer and was re-branded as Gleostine. This is currently the only company producing lomustine in the United States. Since 2013, we have seen the cost of lomustine increase by >1500% from $50 to $768 per capsule.13
Because primary brain tumors, particularly diffuse gliomas, are rare, there has been little public outrage. In 2017, the Central Brain Tumor Registry of the United States reported an estimated 56000 (14.9%) glioblastomas; 20000 (5.4%) “other astrocytomas”; 5000 (1.4%) oligodendrogliomas; and 3000 (0.8%) oligoastrocytomas, for a total of 84000 people with diffuse glioma from 2010–2014.14 This, of course, pales in comparison to cancers with higher incidence, such as lung cancer, and translates into a very limited cost-sharing.
There are few alternatives to lomustine for neuro-oncology patients. Another nitrosourea is 1,3 bis(2 chloroethyl)-1 nitrosourea (BCNU), which is administered intravenously and has more pulmonary toxicity than lomustine. The data for its use come predominantly from the time before temozolomide was incorporated into standard of care for newly diagnosed glioblastoma, and therefore its efficacy and toxicity are not well delineated in the modern era.
To better understand the impact of this price hike on neuro-oncology patients and the neuro-oncology community, the Society for Neuro-Oncology (SNO) sent a survey to its membership in January 2018. The full results can be seen in the SNO Newsletter, Volume 9, Issue 1.
A total of 480 SNO community members responded to the survey, with 88% of responders involved in direct patient care. The majority, 62%, were adult neuro- or medical oncologists and 14% other allied health professions, including pediatric neuro-oncologists and nurses. Roughly two-thirds (62%) of the responders were from the United States. Lomustine was prescribed weekly by 23% of responders and at least twice per month in 31%. Indications for use were highly variable with 76% using it for glioblastoma; 64% for anaplastic oligodendrogliomas; 55% for anaplastic astrocytoma, and numerous providers using it for medulloblastoma. It was used as part of the PCV (procarbazine, CCNU, vincristine) regimen in 67% of responders followed by single agent use in 63%.
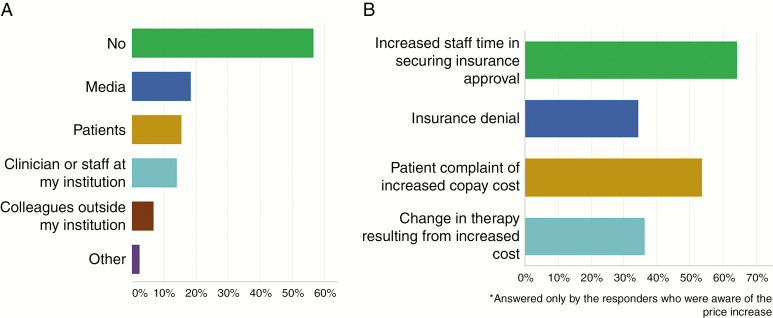
A total of 56% of responders were NOT aware of the increased price before receiving the survey (Figure 1A). Of those who were aware, 18% were informed from media; 15% from patients; and 14% from clinician or staff at other institutions. Several commented that though they were aware of the cost increase, they did not realize the extent.
Fig. 1.
(A) Results of survey question: “I was aware of the cost increase in LOMUSTINE (GLEOSTINE) prior to this email (check all that apply),” n = 370. In response, 56% answered NO. Of responders who answered YES and were aware, 18% were from media; 15% from patient; and 14% from clinician or staff at their institution; and 7% from colleagues outside their institution. (B) Results of survey question: “Which of the following issues have you personally encountered in prescribing lomustine (GLEOSTINE) to your patients (check all that apply),” n = 213. In response, 64% reported increased staff time in securing insurance approval, 34% insurance denial, 34% patient complaint of increased copay costs, and 36% change in therapy resulting from increased cost.
Of the responders who were aware of the price increase, 64% reported more time spent securing insurance approval, and 53% of patients complained about the increased cost of copay. Insurance denials were noted by 34%, and increased cost translated into a need to change therapy in over one-third of cases (36%) (Figure 1B). Challenges reported by individual practitioners included having to import lomustine from a different country, experiencing significant delays in starting treatment, changing to BCNU because of reimbursement, and excluding patients from clinical trial participation.
Overall, the survey indicated broad impact on the SNO community and especially our patients. Moving forward, SNO will work with patient advocacy groups and others to explore ways to ensure a secure supply of lomustine at a reasonable price, including advocating for policy changes at the congressional level. Predatory price increases are clearly harmful to our patients, and strategies to prevent such market dominance and control are necessary. We welcome any suggestions and advice from the neuro-oncology community about strategies for coping and for accelerating meaningful reform.
Conflict of interest statement.
None declared.
References
- 1. Cronin KA, Lake AJ, Scott S, et al. . Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Informatics IIfH. Medicines Use and Spending in the US, IQVIA Institute for Human Data Science, 2018. [Google Scholar]
- 3. Bekelman JE, Joffe S. Three steps toward a more sustainable path for targeted cancer drugs. JAMA. 2018;319(21):2167–2168. [DOI] [PubMed] [Google Scholar]
- 4. Tallapragada NP. Off-patent drugs at brand-name prices: a puzzle for policymakers. J Law Biosci. 2016;3(1):238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolchinsky P. Protecting Off-Patent Sole-Source Drugs from Price-Jacking. December 27, 2017. Available from: https://medium.com/the-biotech-social-contract/kolchinsky-tbsc-3-2efffcb7038f [Google Scholar]
- 6. Wick W, Gorlia T, Bendszus M, et al. . Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–1963. [DOI] [PubMed] [Google Scholar]
- 7. Herrlinger U, Tzaridis T,Mack F, et al. ACTR-58. Phase III trial of CCNU/temozolomide (TMZ) combination therapy vs standard TMZ therapy for newly diagnosed mgmt-methylated glioblastoma patients: the CETEG/NOA-09 trial. Neuro Oncol. 2017;19:vi13–vi14. [Google Scholar]
- 8. Cairncross G, Wang M, Shaw E, et al. . Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. . Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344–350. [DOI] [PubMed] [Google Scholar]
- 10. Buckner JC, Shaw EG, Pugh SL, et al. . Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Packer RJ, Gajjar A, Vezina G, et al. . Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 12. NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers. Version 1.2018-March 20, 2018. NCCN.org. [Google Scholar]
- 13. Loftus P. Cancer drug price rises 1400% with no generic to challenge it. Wall Street Journal. December 25, 2017. [Google Scholar]
- 14. Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]