Abstract
Background
Patients with glioblastoma (GBM) have a dismal prognosis. Nearly all will relapse with no clear standard of care for recurrent disease (rGBM). Approximately 50% of patients have tumors harboring epidermal growth factor receptor (EGFR) amplification. The antibody–drug conjugate depatuxizumab mafodotin (depatux-m) binds cells with EGFR amplification, is internalized, and releases a microtubule toxin, killing the cell. Here we report efficacy, safety and pharmacokinetics (PK) of depatux-m + temozolomide (TMZ) in patients with EGFR-amplified rGBM.
Methods
M12-356 (NCT01800695) was an open-label study encompassing patients with newly diagnosed or rGBM across 3 treatment arms. Results are reported for adults with EGFR-amplified, measurable rGBM who received depatux-m (0.5–1.5 mg/kg) on days 1 and 15, and TMZ (150–200 mg/m2) on days 1–5 in a 28-day cycle. Patients were bevacizumab and nitrosourea naïve.
Results
There were 60 patients, median age 56 years (range, 20–79). Fifty-nine patients previously received TMZ. Common adverse events (AEs) were blurred vision (63%), fatigue (38%), and photophobia (35%). Grades 3/4 AEs were split between ocular and non-ocular AEs, occurring in 22% of patients each. Systemic PK exposure of depatux-m was dose proportional. The objective response rate was 14.3%, the 6-month progression-free survival rate was 25.2%, and the 6-month overall survival rate was 69.1%.
Conclusions
Depatux-m + TMZ displayed an AE profile similar to what was described previously. Antitumor activity in this TMZ-refractory population was encouraging. Continued study of depatux-m in patients with EGFR-amplified, newly diagnosed, or recurrent GBM is ongoing in 2 global, randomized trials (NCT02573324, NCT02343406).
Keywords: ABT-414, antibody–drug conjugate, depatux-m, EGFR amplification, rGBM
Importance of the Study
Glioblastoma (GBM) is the most aggressive type of primary brain tumor, with extremely poor prognosis and high unmet need for novel therapies. Approximately 50% of patients have tumors with EGFR amplification, presenting an attractive therapeutic target. Depatuxizumab mafodotin (depatux-m) is an antibody–drug conjugate that specifically targets tumor cells expressing EGFR, and has demonstrated efficacy in recurrent GBM. Here we present further data showing efficacy of depatux-m in combination with temozolomide in recurrent GBM. These findings indicate that further study of depatux-m in glioblastoma is justified. Global, randomized trials for EGFR-amplified newly diagnosed and recurrent GBM are ongoing.
Glioblastoma (GBM) is the most frequent malignant brain tumor in adults. Prognosis is poor, with a median overall survival (OS) ranging 14–16 months, and a 24-month survival of approximately 30%.1,2 Few patients survive 5 years from diagnosis.3 Relapse after initial therapy almost always occurs, and there is currently no clear standard of care for recurrent GBM (rGBM). Lomustine monotherapy is a commonly used approach,4 and temozolomide (TMZ) rechallenge can be used to manage rGBM, at times with an alternative dosing schedule employed, which is thought to overcome resistance.5 Bevacizumab is also routinely used at recurrence, but no studies have definitively shown a benefit in OS in rGBM.6 Currently, there is no treatment that impacts OS in rGBM, and this continues to be an active area of investigation in clinical trials.
Amplification of the Epidermal Growth Factor Receptor (EGFR) gene is observed in approximately 50% of GBMs7–10 and presents an attractive tumor-specific target. About 50% of EGFR-amplified tumors also harbor the variant III mutation (EGFRvIII),11 a deletion of EGFR exons 2–7 that has a distinct conformation and is tumor specific and constitutively active. Approximately 80% of cases with EGFR amplification at diagnosis retain amplification at recurrence.12–14 Approaches employing inhibitors of EGFR signaling, such as the receptor tyrosine kinase inhibitors gefitinib and erlotinib, have been disappointing.15–20 Similarly, naked EGFR-directed antibodies such as cetuximab have also failed to improve survival in the GBM population.21
Depatuxizumab mafodotin (depatux-m, formerly ABT-414) is an antibody–drug conjugate (ADC) that uses EGFR as an entry point to deliver a toxic payload directly to tumor cells. Dysregulated EGFR activation in tumor cells leads to a unique conformation of EGFR that allows binding by the EGFR-specific, monoclonal antibody depatuxizumab (depatux, formerly ABT-806). Efficacy of depatux treatment in GBM is limited.22 However, conjugation of receptor-directed antibodies to toxins in order to form ADCs have been successful approaches in other cancers.23,24Thus, cysteine (cys) residues on depatux were conjugated to the anti-microtubule agent monomethyl auristatin F (MMAF), a potent toxin, via a noncleavable linker25,26 to generate the ADC depatux-m. Depatux-m binds the EGFR epitope on the surface of the cell exposed in the active receptor conformation, either wild-type or EGFRvIII mutant. Depatux-m is then internalized and releases the toxic payload cys-mcMMAF (cys-mafodotin), which in turn binds to the microtubule network, arresting proliferation and killing the cell. The dose-limiting toxicities of depatux-m are distinct from those typically associated with EGFR receptor tyrosine kinase inhibitors. In addition, depatux-m has very limited binding of EGFR in normal tissues.27 Finally, preclinical studies demonstrated antitumor activity with and without TMZ in GBM cell lines and mouse xenograft models,26 leading to interest in strategies such as the trial described here.
Previously, we reported tolerable safety data and the pharmacokinetics (PK) profile of depatux-m in newly diagnosed GBM when combined with radiotherapy and TMZ, and rGBM as monotherapy.28,29 Furthermore, we have shown encouraging efficacy in patients with EGFR-amplified, rGBM treated with depatux-m monotherapy, and that EGFR amplification enriches for response.30 Therefore, we now present efficacy, safety, and PK data in patients with EGFR-amplified rGBM treated with depatux-m in combination with TMZ in a multicenter, international phase I clinical trial.
Materials and Methods
Study M12-356 (NCT01800695) was a multicenter, phase I, open-label study composed of 3 treatment arms designed to evaluate the safety, preliminary efficacy, and PK of depatux-m alone or in combination with other treatments in patients with GBM.28 In the arm presented here, we treated patients with rGBM with depatux-m in combination with TMZ in a dose escalation cohort, and then a dose expansion cohort at the recommended phase II dose (RP2D). Results from the other arms of the trial have been published previously.28–30 This study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. All patients or appropriate surrogates provided written informed consent prior to enrollment according to national regulation; the study design was approved by the institutional review board/ethics committee of each participating institution.
Patients
Trial eligibility criteria were described previously,28,29 comprising, in brief, adults with recurrent/progressive GBM defined by Response Assessment in Neuro-Oncology (RANO) criteria,31 who were bevacizumab naïve, with Karnofsky performance status (KPS) of at least 70, and normal end-organ function. We previously demonstrated that responses to depatux-m, either alone or with TMZ, occurred exclusively in EGFR-amplified disease.29,30 Therefore, the trial was amended during accrual to require centrally confirmed EGFR amplification as an eligibility criterion for the dose expansion cohort; accordingly, the current analysis describes results among patients with centrally confirmed EGFR-amplified rGBM treated at any dose of depatux-m in combination with TMZ pooled from the dose expansion and dose escalation cohorts (results with depatux-m monotherapy in EGFR-amplified rGBM were reported separately30). Among this cohort of 60 patients, 9 were treated in the dose escalation cohort (out of a total of 29, the remainder of whom did not have EGFR-amplified disease, as previously reported29) and 51 in the expansion cohort (46 of whom were enrolled under an amendment that required progressive disease [PD] <3 mo after the most recent dose of TMZ). Of note, eligibility criteria for the expansion cohort were more restrictive than the escalation cohort, requiring primary “de novo” rather than secondary GBM (clinical criteria32) and measurable disease, and prohibiting prior bevacizumab, nitrosourea, and EGFR-directed therapy (such as rindopepimut).
Treatment Regimen
All patients received depatux-m via intravenous (i.v.) infusion over 30–40 minutes on days 1 and 15, and 150–200 mg/m2 TMZ on days 1–5 of a 28-day cycle. Among the 9 patients accrued to the dose escalation cohort, the depatux-m dose was 0.5 mg/kg (n = 1), 1.0 mg/kg (n = 3), 1.25 mg/kg (n = 2), or 1.5 mg/kg (n = 3). All 51 patients in the expansion cohort received the RP2D of 1.25 mg/kg.29 Radiographic assessment of disease progression was performed before every other cycle. Treatment was intended to continue until either intolerable toxicity or disease progression as assessed locally by the investigator using RANO criteria.31 Central imaging review was not performed, as efficacy was not the primary endpoint of this phase I trial.
Pharmacokinetics
To evaluate the effect of depatux-m on TMZ PK in the arm B dose escalation cohort, depatux-m infusion in cycle 1 was administered on day 2 instead of day 1. Plasma concentrations of TMZ were collected prior to TMZ dosing (0 hour) and at 0.5, 1, 2, 4, and 6 hours after TMZ dose administration under fasting conditions on day 1 of cycles 1 and 2. Serum samples for determination of depatux-m and total depatux concentrations and plasma samples for determination of cys-mafodotin concentrations were collected before and at multiple timepoints after depatux-m administration in cycles 1 and 2. In the arm B expansion cohort, PK samples were collected before and/or immediately after depatux-m administration in cycles 1 and 2.
Serum samples for determination of antidrug antibody (ADA) were collected once every 2 weeks before each depatux-m infusion up to day 1 of cycle 3, and once every 4 weeks before depatux-m infusion in subsequent cycles. When possible, ADA samples were collected approximately 35 days after the last depatux-m infusion. At each timepoint that ADA was determined, PK samples for depatux-m, total depatux, and cys-mafodotin were collected.
Depatux-m serum concentrations and ADA titers were determined using validated electrochemiluminescence immunoassays.28 Cys-mafodotin and TMZ plasma concentrations were determined by validated liquid chromatography methods with tandem mass spectrometric detection. PK parameters, including peak concentration (Cmax), terminal elimination half-life (t1/2), area under the serum concentration-time curve (AUC), and clearance (CL, if applicable) were determined using noncompartmental methods.
Tumor Molecular Characterization
Molecular characterization, including tests to determine EGFR expression, amplification, and EGFRvIII mutation status, was performed on pretreatment, archival tumor tissue. EGFR amplification testing was performed retrospectively28 on patients in the escalation cohort, and performed prospectively (as it was required for eligibility) in the expansion cohort. O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation was assessed retrospectively when sufficient tissue was available (Supplementary Table S1), but results did not affect eligibility, and correlations with other biomarkers or efficacy were not explored. Isocitrate dehydrogenase 1 (IDH1) mutation status was not evaluated, as IDH1 mutation and EGFR amplification have been reported as mutually exclusive.33
Efficacy Analyses and Statistical Methods
We determined the objective response rate (ORR = partial response [PR] + complete response [CR]) among patients with RANO-defined measurable disease at baseline (required for eligibility in the expansion cohort). We also estimated the 6-month progression-free survival rate (PFS6), with PFS (defined as the time from the first dose of depatux-m to RANO-defined disease progression or date of death from any cause), OS (determined from the time of first dose of depatux-m to death due to any cause), safety, and tolerability of depatux-m. The 95% CI was constructed for the estimated ORR (determined from the exact binomial distribution), PFS, and OS. For PFS and OS, Greenwood’s formula was used to calculate confidence limits for the quartiles of survival distribution. Descriptive statistics were provided for patient demographic variables. Safety/toxicity summaries were provided for all patients who received at least one dose of depatux-m. Frequencies of adverse events (AEs) were tabulated by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 4.1) and listed by MedDRA (version 19) system organ class and preferred term. The data cutoff was March 15, 2017.
Results
Patient Characteristics
There were 60 patients accrued from April 2013 to March 2016 with EGFR-amplified rGBM. Forty-two percent were women and the median age was 56 years (Table 1). Fifty-eight (97%) demonstrated EGFR overexpression as assayed by mRNA levels. Twenty-eight (47%) patients were found to have tumors with an EGFRvIII mutation by PCR (Table 1). All had prior radiation therapy (RT; Supplementary Table S2). Forty-six patients were considered TMZ refractory per eligibility criteria of later amendments (PD within 3 months of the last TMZ treatment). Nine patients enrolled under earlier amendments were also refractory by this definition. Of the remaining 5 patients, 3 were not refractory, 1 was TMZ naïve, and 1 was unknown.
Table 1.
Patient demographics
| Characteristics |
N = 60 n (%) |
|---|---|
| Sex | |
| Female | 25 (42) |
| Male | 35 (58) |
| Median age, y (range) | 56 (20–79) |
| KPS, baseline | |
| 100 | 10 (17) |
| 90 | 23 (38) |
| 80 | 21 (35) |
| 70 | 6 (10) |
| Prior surgeries | |
| 1 | 39 (65) |
| 2 | 20 (33) |
| 3 | 0 |
| 4 | 1 (2) |
| Prior therapies | |
| TMZ | 59 (98) |
| Radiation therapy | 60 (100) |
| Rindopepimut | 4 (7) |
| SAHA (suberoylanilide hydroxamic acid) | 1 (2) |
| Nivolumab | 1 (2) |
| PLX3397 | 1 (2) |
| EGFRvIII mutation status (central analysis) | |
| Positive | 28 (47) |
| Negative | 31 (52) |
| Insufficient tissue | 1 (2) |
Safety
All patients who received at least one dose of depatux-m and TMZ were included in the analysis. The most common AEs (Table 2 and Supplementary Table S3) were ocular, and were observed in 87% of patients. The most frequent included blurred vision (63%) and photophobia (35%). The most common non-ocular AE was decreased platelet count/thrombocytopenia (45%, attributable to TMZ34 but potentially exacerbated by concurrent depatux-m), followed by fatigue (38%).
Table 2.
All adverse events
| Adverse Events | N = 60 |
|---|---|
| n (%) | |
| All AEs (≥20% of patients) | 60 (100) |
| Ocular | 52 (87) |
| Vision blurred | 38 (63) |
| Photophobia | 21 (35) |
| Eye pain | 12 (20) |
| Foreign body sensation in eye | 12 (20) |
| Keratitis | 12 (20) |
| Non-ocular | |
| Platelet count decreased/thrombocytopenia | 27 (45) |
| Fatigue | 23 (38) |
| Headache | 19 (32) |
| Constipation | 17 (28) |
| Nausea | 17 (28) |
| ALT increased | 13 (22) |
| AST increased | 12 (20) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Forty percent of patients had a grade 3/4 AE considered to have a reasonable possibility of being depatux-m related, with both ocular and non-ocular grade 3/4 AEs occurring in 22% of patients each (Table 3). Ocular AEs were attributed to generalized microcystic keratopathy and included keratitis (13%), blurred vision (5%), and photophobia (3%). The majority of ocular AEs (65%) were grade 1/2, and the only grade 4 events were keratitis in 4 patients (7%; Supplementary Table S3). Serious AEs35 that were possibly attributable by the treating investigator to depatux-m treatment were observed in 5 patients (8%: 2 keratitis, 1 fatigue, 1 headache, 1 seizure). A separate analysis of AEs in patients with rGBM enrolled in the dose escalation cohort29 was consistent with the broader cohort presented here.
Table 3.
Grade 3/4 AEsa having a reasonable possibility of being depatux-m related
| Grade 3/4 AEs | N = 60 |
|---|---|
| n (%) | |
| All Grade 3/4 AEs | 24 (40) |
| Ocular | 13 (22) |
| Keratitis | 8 (13) |
| Vision blurred | 3 (5) |
| Photophobia | 2 (3) |
| Corneal deposits | 1 (2) |
| Foreign body sensation in eye | 1 (2) |
| Lacrimation disorder | 1 (2) |
| Ulcerative keratitis | 1 (2) |
| Non-ocular | 13 (22) |
| Platelet count decreased/thrombocytopenia | 7 (12) |
| GGT increased | 4 (7) |
| AST increased | 3 (5) |
| ALT increased | 2 (3) |
aPer investigator assessment.
GGT, gamma-glutamyltransferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Discontinuation for treatment-related AEs was rare, occurring in only 6 patients (10%): 3 for thrombocytopenia, 1 stroke (not drug related), 1 herpes zoster (TMZ related), and 1 secondary malignancy (acute promyelocytic leukemia, TMZ and depatux-m related). No patient discontinued therapy for ocular side effects. Dose delays occurred in 35 patients (58%), most commonly because of ocular side effects in 14 (23% overall). Dose reductions occurred in 7 patients (12%), all due to ocular side effects.
Resolution of Ocular Side Effects
The median time to onset of any type or grade of ocular AE was 4 weeks (95% CI = 3.1, 4.3). Among 14 patients with a grade 3/4 ocular AE, 11 had improved to grade 2 or better at last analysis. As discussed previously,30,36 the ability to determine median time to resolution of ocular side effects was severely limited by a high censoring rate, in part because follow-up beyond 35 days after last dose was not required. Based on limited data available, median time to resolution was 15.4 weeks (95% CI = 15.4, 22.6).
Pharmacokinetics
PK parameters of TMZ were comparable in the absence (day 1 of cycle 1) and presence (day 1 of cycle 2) of depatux-m coadministration (Supplementary Table S4). The 90% CI of the ratios of the geometric means of dose-normalized Cmax and AUC of TMZ between the 2 dosing conditions were within 0.80–1.25, suggesting that depatux-m has no significant effect on TMZ PK.
Systemic PK exposures (Cmax and AUC) of depatux-m, total depatux, and cys-mafodotin after i.v. infusion of depatux-m were approximately dose proportional over the dose range studied (0.5–1.5 mg/kg).29 The PK of depatux-m in the expansion cohort was consistent with that in the dose escalation cohorts.
Low-titer positive ADA was detected in only 2 samples at baseline and 1 sample after depatux-m treatment in cycle 1 among all samples from the 60 patients. The patient who had a positive ADA sample after depatux-m treatment also showed positive ADA at baseline. For the patients able to return to the clinic for a 35-day follow-up visit (n = 14), no positive ADA was detected from any sample. The data suggest that depatux-m does not cause resistance by generating circulating antidrug antibodies.
Efficacy of Depatux-m
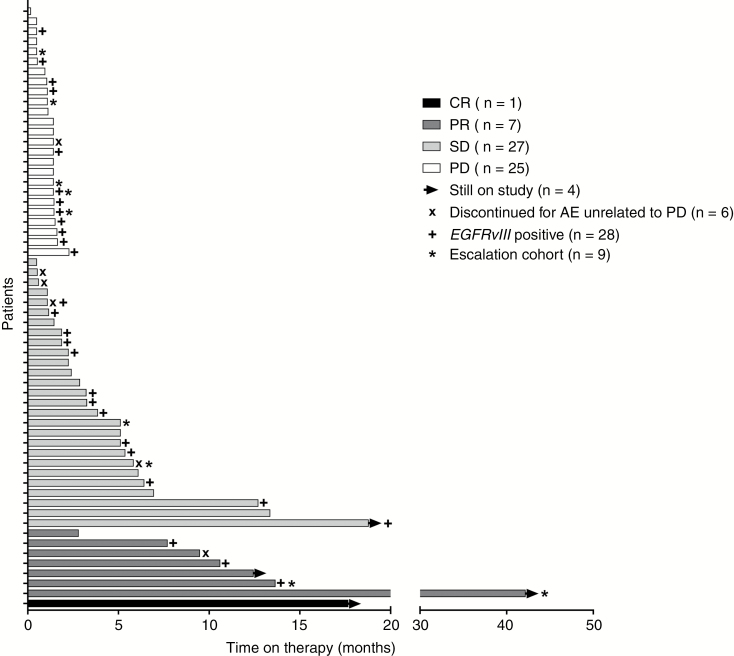
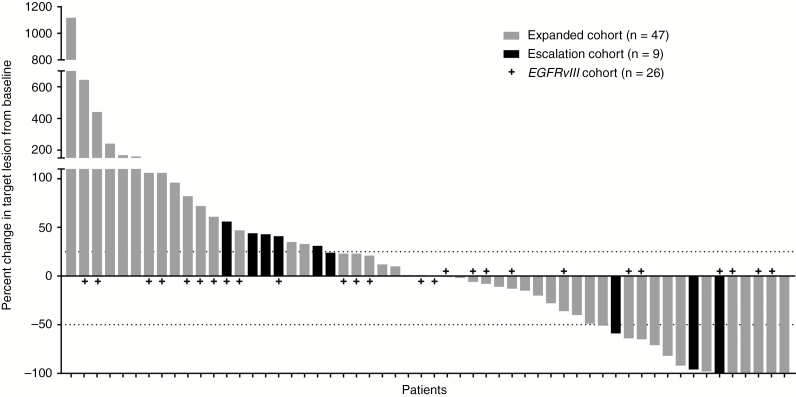
Of 60 patients, 58 had RANO-defined measurable disease at baseline evaluable for radiographic response.31 Among these, the ORR was 13.8% (1/58 CR, 7/58 PR, 95% CI = 6.2%, 25.4%), 26 patients had stable disease (SD), and 24 patients had PD (per local investigator). The median duration of response was 5.6 months (95% CI = 1.5, 9.7). Time on study for all 60 patients is shown in Figure 1. Notably, one patient had a durable response for >40 months, and the patient is now 5 years post-study enrollment and remains without any evidence of progressive disease. Although not meeting the threshold for RANO-defined PR (requiring more than 50% improvement durable for at least 4 weeks), there was a notable reduction in tumor size of up to 25% in 9 patients and 25%–50% in 4 patients (Figure 2); median time to progression among this group was 3.7 months (95% CI = 1.1, 4.8).
Fig. 1.
Best response and time on therapy. The best responses as determined by the investigator using RANO criteria and time on depatux-m therapy are shown for 60/60 patients (analysis included 1 patient each with SD and PD without measurable disease at baseline).
Fig. 2.
Best percentage change in tumor size. The percent change in target lesion from baseline are shown for 56/58 patients who had measurable disease at baseline and at least one post-baseline measurement. Best tumor percent change is defined as the maximum reduction/minimum increase from baseline in tumor size. Values were determined per investigator measurements.
Among all 60 patients, the PFS6 was 25.2% (95% CI = 14.9%, 36.9%) and the median PFS was 2.1 months (95% CI = 1.1, 3.4). The OS6 was 69.1% (95% CI = 55.5%, 79.3%) and the median OS was 7.4 months (95% CI = 6.5, 9.6). Seven patients were censored for PFS and 14 for OS at the time of analysis.
Importantly, 5 patients (8%) underwent resection for presumed PD. However, histological evaluation found almost entirely necrotic tissue per local pathologist assessment. All of these surgeries were performed ≥8 months from most recent RT, and one had disease recurrence histologically confirmed prior to enrollment. These 5 patients were conservatively classified as having SD and were censored for PFS.
There was no correlation between EGFRvIII mutation, PFS, or OS, although such a correlation was hypothesized because the mutation is recognized by the antibody and induces a conformational change, exposing the antigenic epitope of EGFR to depatux-m binding. Correlations with MGMT methylation were not assessed, as it was not required for eligibility and limited patient data were available.
Discussion
In this study of EGFR-amplified, mainly TMZ-refractory rGBM, no new safety events were observed from combined depatux-m and TMZ compared with other arms of the study (arm A, concurrent RT, TMZ, and depatux-m; arm C, depatux-m monotherapy). Depatux-m did not appear to significantly worsen the common side effects that would be expected from TMZ, with the possible rare exception of thrombocytopenia. Ocular side effects were the most common AEs, as seen in other depatux-m arms,30,36 but did not cause any patients to permanently discontinue study treatment and always improved with dose delays or reductions. Nine patients remained on treatment for more than 9 months (Figure 1), suggesting that depatux-m treatment was tolerated for an extended time period, and ocular side effects did not preclude prolonged therapy. Of note, the frequency of ocular side effects observed here was similar to that observed in other cohorts treated at lower doses.29,37 Reductions in tumor size were observed in 27 patients (Figure 2).
The patient with long-standing, durable response provided an opportunity for extensive longitudinal monitoring of ocular side effects and their resolution. This patient developed primarily grade 3 ocular AEs (photophobia, blurred, vision, foreign body sensation in the eye, with the exception of grade 4 keratitis) after 15 months of treatment, which recurred despite subsequent dose delays and dose reduction. Treatment was interrupted again after 23 months on study due to recurrence of grade 3 ocular side effects. Ocular side effects improved to grade 1 by month 25 on treatment but a decision was made not to rechallenge with drug at that time due to ongoing near complete response. All ocular side effects had fully resolved by month 27 of study enrollment, which was 4 months after permanent cessation of depatux-m. The patient remains without evidence of residual ocular symptoms.
An ORR of 13.8% and a 45% SD rate per RANO criteria were observed with depatux-m + TMZ treatment. The ORR and PFS6 in the current analysis are comparable to those observed in the depatux-m monotherapy arm.30 Of note, among 15 patients with a ≥50% decrease in tumor size of any duration (8 of whom were formal RANO-defined responses durable for at least 4 weeks; Figure 2), 12 were TMZ refractory, including 5/6 with a 100% decrease in tumor size, and 2 were from the escalation cohort treated at less than 1.25 mg/kg depatux-m.
As above, histology of tissue resected in 5 patients for presumed PD demonstrated necrosis, raising the possibility that the reported ORR, PFS6 rate, and median PFS were underestimated. This observation also suggests that depatux-m may contribute to pseudoprogression, and as a consequence in ongoing trials, patients with ambiguous imaging findings are permitted to remain on therapy at the discretion of the treating investigator. To further elucidate this phenomenon, a study investigating the correlation between histological and radiographic evidence of PD after treatment in patients is ongoing.
Limitations of the study include its small size and overall design, which is not typical of a phase Ib study, but was intentional in order to more easily develop a new drug for treatment in a population with high unmet need. Furthermore, as the study lacked an active comparator, outcomes were difficult to interpret. In some cases, a lack of archival tumor tissue also prevented further analyses with regard to biomarker status and how that may correlate with outcome. However, we observed in this international, multicenter study that depatux-m in combination with TMZ administered in patients with EGFR-amplified rGBM showed encouraging efficacy and manageable side effects, supporting further trials. An open-label, phase I/II study in Japan (M13-714, INTELLANCE J, NCT02590263) of patients with newly diagnosed or rGBM is currently ongoing. The phase III INTELLANCE 1 (NCT02573324) study in newly diagnosed GBM is also ongoing.
Funding
AbbVie provided financial support for this study (NCT01800695) and facilitated the design, study conduct, analysis, and interpretation of the data, as well as the writing, review, and approval of the manuscript. AL was supported in part by 5P30CA013696-43 and 5UG1CA189960-04 (unrelated to the current study).
Conflict of interest statement. Andrew B. Lassman: In the last 2 years, received: honoraria from prIME Oncology, WebMD, Italian Association for Cancer Research, American Academy of Neurology, American Society of Clinical Oncology; consulting/advisory role for Bioclinica, Celgene, Sapience, AbbVie, Cortice, Kadmon, Novocure, AstraZeneca, Genentech; research support (to institution) from AbbVie.
Martin J. van den Bent: Received honoraria from Roche, AbbVie, Celldex, Merck Ag, Cavion, Actelion, BMS, Blue Earth Diagnostics and Novartis; received research funding from AbbVie
Hui K. Gan: Consulting/advisory role for AbbVie; speakers’ bureau for Ignyta, Bristol-Myers Squibb; research funding from AbbVie; travel, accommodations, expenses from Ignyta; affiliated with the Ludwig Institute for Cancer Research
David A. Reardon: Received honoraria from and has a consulting or advisory role with Abbvie, Bristol Myers Squibb, Cavion, Celldex, Inovio, Merck, Novartis, Roche/Genentech, Amgen, Novocure, Oxigene, Regeneron and Stemline Therapeutics; is involved in speakers’ bureaus with Roche and Merck; received research funding from Incyte, Midatech and Celldex
Priya Kumthekar: Advisory fees from AbbVie, Angiochem, Orbus Therapeutics; investigator for AbbVie
Nicholas Butowski: Received honoraria from and has a consulting or advisory role with Roche/Genentech, Medicenna, VBL Theraputics, Omniox; is involved in speakers’ bureaus with Roche and Merck; received research funding from Insys
Zarnie Lwin: Has served on AbbVie Scientific Advisory Board and received honoraria
Louis B. Nabors: Serves on a Scientific Advisory Board for Cavion, Merck, and BMS; investigator for AbbVie
Kyriakos P. Papadopoulos: Received research funding from AbbVie, MedImmune, Daiichi Sankyo, GlaxoSmithKline, Onyx, Sanofi, Novartis
John Simes: Received research funding for an investigator-initiated trial from AbbVie
Helen Wheeler: Investigator for AbbVie
Tobias Walbert: Serves on a Scientific Advisory Board for AbbVie and Tocagen
Andrew M. Scott: Received research funding and travel support from AbbVie; received research funding from Daiichi-Sankyo; consultant for and has stock in Life Science Pharmaceuticals; affiliated with the Ludwig Institute for Cancer Research
Erica Gomez, Ho-Jin Lee, Lisa Roberts-Rapp, Hao Xiong, Peter J. Ansell, Earle Bain, Kyle D. Holen, David Maag: Employees of AbbVie and may own stock or options
Ryan Merrell: Serves on a Scientific Advisory Board for AbbVie
Supplementary Material
Acknowledgments
AbbVie and the authors thank patients and their families/ caregivers; study investigators and staff; and Mrinal Y. Shah, PhD, of AbbVie, for medical writing support. All authors were involved in the data gathering, analysis, review, interpretation, and manuscript preparation and approval.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 3. Franceschi E, Minichillo S, Brandes AA. Pharmacotherapy of glioblastoma: established treatments and emerging concepts. CNS Drugs. 2017;31(8):675–684. [DOI] [PubMed] [Google Scholar]
- 4. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 5. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma—an update. Crit Rev Oncol Hematol. 2016;99:389–408. [DOI] [PubMed] [Google Scholar]
- 6. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 7. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280(21):5350–5370. [DOI] [PubMed] [Google Scholar]
- 9. Yoshimoto K, Dang J, Zhu S, et al. Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res. 2008;14(2):488–493. [DOI] [PubMed] [Google Scholar]
- 10. van den Bent MJ, Roberts-Rapp LA, Ansell P, et al. Epidermal growth factor receptor (EGFR) amplification rates observed in screening patients for randomized clinical trials in glioblastoma. Ann Oncol. 2017; 28(suppl_5). doi:10.1093/annonc/mdx366.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padfield E, Ellis HP, Kurian KM. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol. 2015;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weller M, Hentschel B, Kaulich K, et al. EGFR gene amplification and variant III (EGFRvIII) mutation in primary and recurrent glioblastoma. J Clin Oncol. 2016; 34:2042. [Google Scholar]
- 13. van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015;17(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lassman A, Dimino C, Mansukhani M, et al. PATH-35. Maintenance of EGFR abnormalities over time and comparison of molecular methodologies in glioblastoma (GBM). Neuro Oncol. 2017;19:vi178. [Google Scholar]
- 15. van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92(1):99–105. [DOI] [PubMed] [Google Scholar]
- 17. Raizer JJ, Abrey LE, Lassman AB, et al. ; North American Brain Tumor Consortium A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98(1):93–99. [DOI] [PubMed] [Google Scholar]
- 19. Raizer JJ, Giglio P, Hu J, et al. ; Brain Tumor Trials Collaborative A phase II study of bevacizumab and erlotinib after radiation and temozolomide in MGMT unmethylated GBM patients. J Neurooncol. 2016;126(1):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakravarti A, Wang M, Robins HI, et al. RTOG 0211: a phase ½ study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys. 2013;85(5):1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. [DOI] [PubMed] [Google Scholar]
- 22. Cleary JM, Reardon DA, Azad N, et al. A phase 1 study of ABT-806 in subjects with advanced solid tumors. Invest New Drugs. 2015;33(3):671–678. [DOI] [PubMed] [Google Scholar]
- 23. Krop IE, Kim SB, González-Martín A, et al. ; TH3RESA study collaborators Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. [DOI] [PubMed] [Google Scholar]
- 24. Moskowitz CH, Nademanee A, Masszi T, et al. ; AETHERA Study Group Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. [DOI] [PubMed] [Google Scholar]
- 25. Reilly EB, Phillips AC, Buchanan FG, et al. Characterization of ABT-806, a humanized tumor-specific anti-EGFR monoclonal antibody. Mol Cancer Ther. 2015;14(5):1141–1151. [DOI] [PubMed] [Google Scholar]
- 26. Phillips AC, Boghaert ER, Vaidya KS, et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR epitope. Mol Cancer Ther. 2016;15(4):661–669. [DOI] [PubMed] [Google Scholar]
- 27. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55(4):657–670. [DOI] [PubMed] [Google Scholar]
- 28. Reardon DA, Lassman AB, van den Bent M, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2016;17(7):965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gan HK, Reardon DA, Lassman AB, et al. Safety, pharmacokinetics and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol. 2017;20(6):838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Bent M, Gan HK, Lassman AB, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80(6):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment In Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 32. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. [DOI] [PubMed] [Google Scholar]
- 33. Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013;13(5):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol. 2007;9(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Food and Drug Administration. Safety Reporting Requirements for INDs and BA/BE Studies. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2012. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm227351.pdf. Accessed June 27, 2018. [Google Scholar]
- 36. Lassman AB, van den Bent MJ, Gan HK, et al. Efficacy analysis of ABT-414 with or without temozolomide (TMZ) in patients (pts) with EGFR-amplified, recurrent glioblastoma (rGBM) from a multicenter, international phase I clinical trial. J Clin Oncol. 2017;35:2003. [Google Scholar]
- 37. van den Bent M, Eoli M, Sepulveda JM, et al. First results of the randomized phase II study on depatux-m alone, depatux-m in combination with temozolomide and either temozolomide or lomustine in recurrent EGFR amplified glioblastoma: first report from INTELLANCE 2/EORTC trial 1410. Neuro Oncol. 2017;19:vi316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.