While immune checkpoint inhibitors (ICIs) provide significant clinical benefits in cancer patients, their use is associated with a broad spectrum of immune-related adverse events (irAEs).1
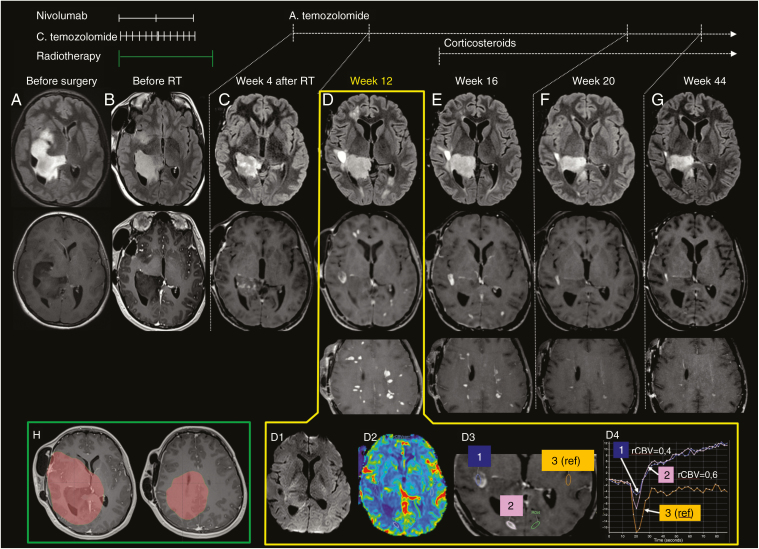
We report on a 27-year-old immunocompetent woman with a diagnosis of IDH1 R132G-mutant and O6-methylguanine-DNA methyltransferase (MGMT) methylated glioblastoma (GBM) in the right temporal lobe. After a partial resection, she received concurrent radiotherapy with temozolomide (RT-TMZ) associated with nivolumab (3 mg/kg every 15 days) in the setting of the phase III study CA 209–548 (NCT02667587). Four weeks after the initiation of RT-TMZ and after the third nivolumab infusion, she presented with grade 4 thrombopenia and neutropenia requiring TMZ and nivolumab discontinuation without interruption of RT. Nivolumab was unblinded after this serious AE. Adjuvant TMZ was started one month after the end of RT. A brain MRI performed 3 months later (week 12; Fig. 1D), namely 14 weeks after the last nivolumab administration, revealed the appearance of multifocal, bilateral, and asymmetrical supratentorial lesions throughout the white matter in T2 fluid attenuated inversion recovery (FLAIR) with contrast enhancement and no increased relative cerebral blood volume. Of note, some of these lesions appeared outside of the RT field (Fig. 1H). The patient experienced marked fatigue grade 2 and transient blurred vision over a period of a few days; clinical and ophthalmologic examinations were normal. At the time of these findings, oral corticosteroids were tapered from the end of RT and she was taking a daily dose of 4 mg of prednisone. Spinal cord MRI was normal. Cerebrospinal fluid (CSF) analysis disclosed elevated protein levels (1 g/L), lymphocytic pleocytosis (14 cells/mm3, 95% lymphocytes), no malignant cells, and no oligoclonal bands. Extensive screening—including lactate, PCR for herpesviruses, HIV, B and C hepatitis, treponema pallidum hemagglutination/Venereal Disease Research Laboratory, Lyme disease, angiotensin-converting enzyme, anti-nuclear antibodies, anti-extractable nuclear antigens antibodies, anti-neutrophil cytoplasmic antibodies, anti-aquaporin 4, anti-myelin oligodendrocyte glycoprotein, and onconeural antibodies—excluded all alternative metabolic, infectious, and autoimmune etiologies.
Fig. 1.
Brain MRI with T2 FLAIR (upper row), post-gadolinium T1 (middle row) before surgery (A), after surgery and before radiotherapy (B), and after treatments (C–G) displayed a progressive decrease of the non-enhancing temporo-occipital tumor. At week 12 (D), several new high signal intensity T2-FLAIR lesions, with low T1 signal and enhancement after gadolinium injection appeared laterally to the main lesion, in the left occipital and right frontal lobes. Numerous other supratentorial lesions were visible on the other slices. Diffusion MRI (D1) showed no high signal intensity in any of the lesions, and apparent diffusion coefficient (ADC) was high. Dynamic susceptibility contrast MR perfusion displayed no relative cerebral blood volume (rCBV) increase in any of the lesions (D2). Regions of interest analysis of 2 representative new enhancing lesions (D3–D4) confirm a decrease in leakage-corrected rCBV and an increased permeability. No increased rCBV in the new enhancing lesions and the multifocal appearance favored the diagnosis of a monophasic inflammatory disorder over distant tumor progression. Follow-up after corticosteroid treatment (E–G) demonstrated a progressive decrease in lesion size and number. (H) Planning target volume (60 Gy). The timeline of treatments at the different stages is reported in the upper part; C. temozolomide = concomitant TMZ; A. temozolomide = adjuvant TMZ.
She was diagnosed with an inflammatory CNS disorder and intravenous methylprednisolone infusions (1 g/day for 3 days) were started, followed by prednisone (1 mg/kg/day). Follow-up imaging showed progressive resolution of the previously described MRI abnormalities 4 weeks later (Fig. 1E) to 8 months later (Fig. 1G). Repeated CSF analysis (3 wk after the event) showed elevated protein levels (0.98 g/L), <5 cells, no oligoclonal bands, and no malignant cells.
Adjuvant TMZ was restarted after temporary discontinuation and is still ongoing; the patient is currently asymptomatic without evidence of tumor progression or new relapsing inflammatory lesions.
In this report we describe a subacute monophasic multifocal inflammatory CNS disorder occurring after standard RT-TMZ plus nivolumab in a young GBM patient.
Neurological irAEs are increasingly recognized but still rare2; encephalitis is the best-characterized event together with relapses in multiple sclerosis patients.3 Our case does not fulfill diagnostic criteria for multiple sclerosis or acute demyelinating encephalo-myelopathy.
Although occurring 3 months later, we concluded that there is a potential link between the subacute monophasic multifocal CNS inflammatory disorder and the RT-TMZ-nivolumab regimen because of (i) no tumor progression within the follow up period, (ii) clinical and radiological recovery with steroids alone, (iii) no infectious disease, and (iv) no previous history of CNS inflammatory disease.
The role of ICI has to be considered with caution. RT-induced multifocal inflammation has been exclusively reported in patients with preexisting multiple sclerosis or with evidence of previous inflammatory disorders4,5; however, observations remain limited in order to rule out the role of RT and/or RT-TMZ. Interestingly, this event was associated with a tumor “minor” response,6 and the patient is currently doing well 8 months after the adverse event.
Considering the potential severity of their neurologic sequelae, de novo CNS inflammatory disorders occurring in cancer patients treated with chemotherapy, radiotherapy, and ICI require early recognition and treatment.
Funding
Authors received no specific funding for this work.
Conflict of interest statement. Authors declare no conflict of interest.
Authorship statement. A. L. Di Stefano: study concept and design, acquisition and interpretation of data, drafting manuscript, responsibility for the integrity of the study. J. Savatovsky, A. Idbaih, and D. Psimaras: study concept and design, acquisition and interpretation of data, drafting manuscript, study supervision. L. Feuvret, C. Villa, V. Reina, M. Pha, C. Houillier, G. Berzero: acquisition and interpretation of data, critical revision of manuscript for intellectual content. All read and approved the manuscript.
References
- 1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 2. Touat M, Talmasov D, Ricard D, Psimaras D. Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol. 2017;30(6):659–668. [DOI] [PubMed] [Google Scholar]
- 3. Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler. 2015;21(5):670. [DOI] [PubMed] [Google Scholar]
- 4. Milic M, Rees JH. Acute demyelination following radiotherapy for glioma: a cautionary tale. Pract Neurol. 2017;17(1):35–38. [DOI] [PubMed] [Google Scholar]
- 5. Miller RC, Lachance DH, Lucchinetti CF, et al. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66(4):1178–1186. [DOI] [PubMed] [Google Scholar]
- 6. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]