Abstract
Glioblastoma (GBM) is a highly malignant CNS tumor with very poor survival despite intervention with conventional therapeutic strategies. Although the CNS is separated from the immune system by the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier, emerging evidence of immune surveillance and the selective infiltration of GBMs by immune suppressive cells indicates that there is breakdown or compromise of these physical barriers. This in turn offers hope that immunotherapy can be applied to specifically target and reduce tumor burden. One of the major setbacks in translating immunotherapy strategies is the hostile microenvironment of the tumor that inhibits trafficking of effector immune cells such as cytotoxic T lymphocytes into the CNS. Incorporating important findings from autoimmune disorders such as multiple sclerosis to understand and thereby enhance cytotoxic lymphocyte infiltration into GBM could augment immunotherapy strategies to treat this disease. However, although these therapies are designed to evoke a potent immune response, limited space in the brain and cranial vault reduces tolerance for immune therapy–induced inflammation and resultant brain edema. Therefore, successful immunotherapy requires that a delicate balance be maintained between activating and retaining lasting antitumor immunity.
Keywords: glioblastoma, immunotherapy, lymphocyte migration, tumor microenvironment, blood-brain barrier
Glioblastoma (GBM) is a highly aggressive and invasive cancer, considered to be one of the most lethal malignancies in humans. In adults the median survival is typically less than 18 months despite chemotherapy and radiation therapy, and the average 5-year survival is merely 10%.1 These dismal statistics are primarily due to the ability of the tumor to invade surrounding tissue and its resistance to conventional therapeutic strategies.
The current standard of care for patients with GBM is surgery followed by a combination of radiation and chemotherapy.2 Unfortunately, these aggressive therapeutic strategies have achieved only limited success. Invasion of these tumors into healthy surrounding brain tissue makes it virtually impossible to surgically resect the tumor completely. Subsequent treatment with temozolomide also has limited effect, since tumor cells can acquire resistance by inducing the DNA damage repair complex O6-methylguanine DNA methyl transferase or by using base excision and mismatch repair pathways.2 Accompanying radiation therapy also brings challenges, including resistance and lethal side effects.3
Given these circumstances, immunotherapy may be a promising therapeutic approach. Currently, several types of immunotherapy are being investigated, including modulation of cytokines, tumor specific vaccines, oncolytic viruses, adoptive transfer of engineered immune cells and checkpoint blockade, including cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1).4,5 In selected reports, these treatment modules have shown great success in rodent models. However, these preclinical models may not adequately reflect therapeutic challenges that exist in human disease. For example, an effective immune therapy strategy requires not only that immune cells be cytotoxic to tumor cells, but that they be also able to traffic to the site of the tumor and survive the hostile and immune suppressive tumor microenvironment. Therefore, trafficking the appropriate kind of immune cells from the periphery into the brain remains one of the key challenges to delivering effective immune therapy irrespective of the strategy employed. In this review we provide an overview of immune cell trafficking to the brain in GBM, incorporating findings from autoimmune disorders of the CNS and the impact of current immunotherapies on immune cell trafficking to the brain.
Principles of Leukocyte Trafficking into the CNS
Leukocytes are the primary immune cells involved in protection against infectious diseases and comprise the myeloid and lymphoid lineages. Leukocytes develop in primary lymphoid organs such as the bone marrow and thymus, following which they are released into the blood stream. Whereas monocytes migrate into tissues and mature in response to environmental cues, T lymphocytes are usually activated in secondary lymphoid organs, such as the regional lymph nodes, where they first contact processed antigenic stimuli presented by antigen presenting cells (APCs). Activated leukocytes extravasate from the circulation across the endothelium and enter their target organ, following a gradient of chemoattractant cytokines. Thus, in order to respond to a cellular aberration, immune cells must migrate to the appropriate destination.
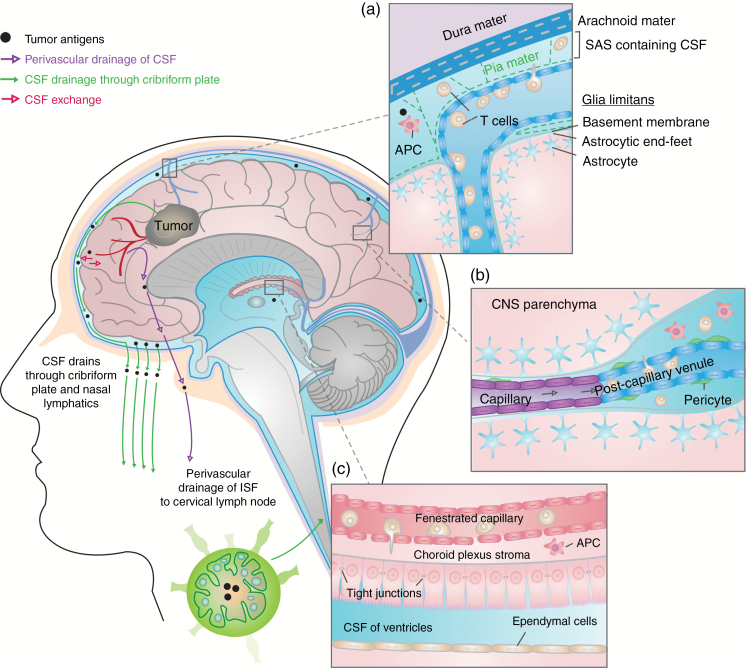
Leukocytes can enter the CNS through 3 main routes: (i) through the choroid plexus, located in each of the four ventricles, into the CSF, (ii) across superficial leptomeningeal vessels into the subarachnoid space (SAS), or (iii) into the perivascular space (also known as a Virchow–Robin space) through postcapillary venules in the brain parenchyma (Fig. 1).6,7 Movement of immune cells into the brain directly through brain vasculature may be partially prevented by the blood–brain barrier (BBB). Endothelial cells of the BBB lack fenestrations and are held together by tight junctions to prevent free passage of cells, antibodies, and secretory molecules to and from the brain.6,7 Pericytes wrap around the abluminal surface of the vasculature in the CNS and contribute to blood vessel stability and BBB integrity.6–8 In contrast, blood vessels of the choroid plexus are composed of fenestrated endothelia; however a blood–CSF barrier (BCSFB) is formed by a layer of specialized epithelial cells characterized by the presence of adherence junctions that separate the vascular compartment from the CSF-filled ventricles.6,7,9 Together, these provide region-specific barriers between blood and CNS tissue.
Fig. 1.
Routes for leukocyte trafficking in the CNS. Three main routes have been described for leukocyte trafficking from the blood vasculature to the brain. A parallel lymphatic system allows for drainage of CSF (carrying cells and tumor antigens) and interstitial fluid (carrying tumor antigens, but not cells, along arteries, retrograde to blood flow) to the cervical lymph node. T cells engage antigen:MHC complexes presented by peripheral APCs in the lymph node, and activated lymphocytes can thereby enter the brain through the routes thus described. (a) The subarachnoid space (SAS) through leptomeningeal vessels. The leptomeninges are formed by the arachnoid mater, the pia mater, and the CSF in the SAS. Lymphocytes and APCs in the SAS present antigen and may engage T cells. (b) The perivascular space through postcapillary venules. Endothelial cells in postcapillary venules within the parenchyma are joined by tight junctions. T cells that traverse this layer enter the perivascular space within the brain parenchyma. (c) The CSF through the choroid plexus. Blood leukocytes cross the fenestrated endothelial layer to enter the choroid plexus stroma. The choroid plexus epithelial basement membrane and tight junctions between epithelial cells form the BCSFB. T cells that cross the BCSFB enter the CSF within the ventricles.
Lymphocyte entry into the brain parenchyma is restricted by the glia limitans, composed of astrocytic foot processes, and the basement membrane.6,7 Under normal conditions, leukocytes are not found in the brain parenchyma. However, small numbers of immune cells, including T cells and major histocompatibility complex (MHC) II–expressing APCs, are found in the choroid plexus stroma, in CSF within ventricles, in the SAS, and in perivascular spaces. These areas interface between the CNS and circulating blood leukocytes.10 Optimal T-cell activation occurs in the peripheral lymph nodes, where CSF, carrying CNS proteins, drains from the SAS.11,12 Recent studies have further described the presence of meningeal lymphatics that drain CSF and interstitial fluid from the capillary endings and venules in the brain parenchyma into deep-seated cervical lymph nodes (Fig. 1).13 Together, the structure of the CNS vasculature and lymphatics provides a means for peripheral surveillance of CNS antigens under steady-state conditions.
Mechanisms of Leukocyte Trafficking to the CNS During Tumor Development and Progression
Under inflammatory conditions such as tumor development, the integrity of the BBB and BCSFB is compromised.14 Decreased expression of tight junction molecules, discontinuous glia limitans, and sparse distribution of pericytes surrounding endothelial cells enable breaching of these physical barriers.7 Recruitment of activated leukocytes to the CNS involves a sequence of steps beginning with unconstrained, transient interaction of leukocytes with vascular endothelial cells.8 Integrins such as α4β1 integrin and lymphocyte associated antigen 1 (LFA-1) on the T-cell surface bind and interact with vascular cell adhesion molecule (VCAM) and intracellular cell adhesion molecule 1 (ICAM-1), glycoproteins expressed on endothelial cells.8,9 P-selectin mediated rolling may occur on endothelial cells of the choroid plexus, although this is normally absent in BBB endothelia.8,9 Similarly BBB endothelia exclusively express activated leukocyte cell adhesion molecule 1, which interacts with lymphocyte function-associated antigen and cluster of differentiation 6 (CD6) on lymphocytes, especially CD4+ T helper cells type 1 (Th1) to facilitate transmigration.15 Rolling is followed by the activation of G protein coupled receptors on the T lymphocytes, leading to a conformational change of the surface integrins that increases the affinity and avidity of the interaction with the endothelial surface.8,9 The lymphocytes can then gradually crawl toward regions where the endothelial cells are most permissible to diapedesis. Ultimately, these activated T cells transmigrate through the vascular lumen.11 After migrating through the BBB and entering the perivascular space, T cells must traverse the glia limitans before entering the brain parenchyma. Since part of the glia limitans consists of dystroglycans, matrix metalloproteases (MMPs) secreted from these activated T cells help disrupt this layer.7 Astrocytes within the glia limitans secrete cytokines like tumor necrosis factor alpha (TNFα), interleukin (IL)-12, transforming growth factor beta (TGFβ), or IL-6 under different circumstances to regulate entry of (allow or block) specific types of immune cells.7
Chemokines direct trafficking of leukocytes into the CNS by providing molecular cues that attract circulating immune cells to the site of aberrant cellular activity. Immune cells express a host of chemokine G protein coupled receptors that mediate diverse biological activities (Table 1).16 Not only do chemokines enable migration of lymphocytes into the CNS, they also influence directional movement within the brain parenchyma. Tumor cells evade immunity by selectively expressing chemokines that recruit immune suppressive cells and suppressing those that attract cytotoxic lymphocytes.17 For example, isocitrate dehydrogenase 1 (IDH1)–mutated tumors are characterized by DNA hypermethylation resulting in decreased expression of chemotactic factors such as C-X-C motif chemokine ligand (CXCL) 9, CXCL10, chemokine C-C ligand (CCL) 2, and CXCL12, which promote immune infiltration.18,19 CXCL9 and 10 have been found to be upregulated in late stage astrocytomas and signal via C-X-C chemokine receptor (CXCR) 3 on leukocytes such as T cells, natural killer (NK) cells, natural killer T (NKT) cells, macrophages, and microglia.20 CXCR3 expression may negatively correlate with survival in certain cancers, including GBM, whereas its expression is associated with a favorable prognosis in other cancers.21,22 Possibly, this varied prognosis that underscores the various activities of CXCR3 relates to its 3 spliced isoforms that mediate opposing actions on migration, proliferation, and survival.23 Furthermore, enzymes in the tumor microenvironment may alter the biologic activity of chemokines, preventing lymphocyte response although the chemokine is present.24
Table 1.
Prominent chemokines secreted by GBMs and their role in leukocyte migration
| Chemokine | Receptors | Affected Cell Types | Role in Leukocyte Trafficking to the CNS | References |
|---|---|---|---|---|
| CXCL9, CXCL10, CXCL11 | CXCR3 | Tumor cells, macrophages/ microglia, CD4+ Th1 cells, Tregs, NK cells, NKT cells | • Promotes tumor cell proliferation and migration. • Inhibits antitumor immune responses by attracting suppressive immune cells. • Promotes antitumor immune response by attracting cytotoxic T and NK cells |
21–23 |
| CCL17, CCL22 | CCR4 | Tregs | • Promotes binding of Tregs to BBB endothelia | 25 |
| CCL20 | CCR6 | Macrophages/microglia, DCs, T cells, B cells | • Negatively impacts recruitment of antitumor immune cells. • Facilitates a Th17 response. • High expression of CCL20 and CCR6 correlates with poor survival. |
26 |
| CCL2 (MCP-1) | CCR2 | Macrophage/microglia, CD8+ T cells | • Attracts macrophages and monocytes. • Facilitates recruitment of CD8+ T cells and adoptively transferred T cells. |
6,27,32 |
| CCL19, CCL21 |
CCR7 | Naïve T cells, BBB endothelia | • Homing naïve T cells for activation to lymphoid organs. • Promotes adhesion of lymphocytes to ICAM-1. • Promotes α4β7 integrin adhesion to MadCAM-1. • Maintain lymphoid homeostasis and recruitment of APCs. |
7,30,31 |
| CCL5 | CCR1, CCR5 | Macrophages, microglia, Th1-T cells | • Chemoattraction of inflammatory Th1 type cells | 33,65,66 |
| May be IL-6 (not well known) | Ninjurin-1 | Macrophages/ microglia | • Promotes macrophage/microglia endothelial transmigration. | 34 |
Other chemokines upregulated in GBMs include CCL17 and CCL22. These chemokines selectively enable adhesion of chemokine C-C receptor–positive (CCR4+) T regulatory cells (Tregs) to endothelial cells of the BBB, thereby contributing to the inhibition of a local immune response.25 Also, overexpression of both CCL20 and CCR6 correlated with decreased overall survival of patients suffering with advanced glioma.26 Contrastingly, gliomas expressing elevated levels of macrophage chemoattractant protein 1 (MCP-1; also known as CCL2) attract CD8+ T cells from circulation, and this has been utilized effectively for adoptive T-cell strategies.17,27
T cells (mostly memory CD4+), dendritic cells (DCs), and B cells are present in the perivascular space; however, not all immune cells have equal access to the CNS. CD4+ T cells acquire Ninjurin-1 and very late antigen 4 expression in the lung, licensing their entry into the brain.28 Similarly, Th1 polarized CD4+ T cells can recruit CD8+ T cells to the brain.29
Antigen-specific activation of lymphocytes occurs in secondary lymphoid organs. Chemokines such as CCL19, CCL21, and CXCL13 play a crucial role in the homing of naïve T cells into the lymph nodes and their subsequent exposure to stimuli.7 Both CCL19 and CCL21 promote adhesion of lymphocytes to ICAM-1.30 However, it is the ability of CCL21 to induce adhesion of α4β7 integrin to mucosal vascular addressin cell adhesion molecule 1 (MadCAM-1) that channels the lymphocytes into the secondary lymphoid organs.30 CCL21 and CXCL13 expression transiently decreases after a wave of T-cell activation to create a competition for space and interaction with APCs.31 During chronic inflammation, such as in multiple sclerosis (MS), there is enhanced expression of these cytokines for sustained autoimmunity.30 In GBM, elevated and continuous expression of CCL21 and CXCL13 may well affect this homeostatic balance with potential to skew lymphocyte trafficking.
Microglia are true parenchymal macrophages that are normally dormant but when activated express CXCR3 and migrate toward interferon gamma (IFN-γ)–induced chemokines CXCL9, 10, and 11 in search of cellular aberration.20 In addition to resident microglia, GBMs recruit macrophages via expression of colony stimulating factor 1 (CSF-1) and MCP-1 (CCL2).6,32 Recruited macrophages also express CXCR3 and use this signal to cross the BBB into the brain. Murine and human GBM cell lines express high levels of CCL5, a strong chemoattractant for macrophages that can signal through both CCR1 and CCR5.33 Knocking down either receptor alone is insufficient to mitigate CCL5-mediated recruitment of these myeloid cells; however, a CCR1/CCR5 dual antagonist like Met-CCL5 efficiently abrogates infiltration of macrophages into the CNS.33 More recently, using experimental autoimmune encephalitis (EAE) models, Ninjurin-1 has been found to mediate adherence of macrophages to the BBB endothelia, promoting their transmigration.34 Together microglia and macrophages play an important role in determining the success of immunotherapy, as they significantly contribute to the immune suppressive and inflammatory microenvironment within GBM tumors. However, a generalized approach to target these myeloid cells may prove inefficient, since they comprise several distinct subsets, some of which can be antitumorigenic. By interrogating individual cells with single cell RNA sequencing and flow cytometry, distinct signatures were recently identified that distinguished blood-derived tumor associated macrophages and microglia based on location and phenotype.35
Taken together, the ability of immune cells to traffic into the CNS in response to chemokine gradients presents as an integral step to ensure the success of any immunotherapy strategy. These chemokines and associated trafficking molecules may also serve as attractive targets for combinatorial therapies.
Therapeutic Strategies to Improve Lymphocyte Trafficking to the Brain
Immunotherapy as a strategy to treat cancer has been studied for decades. Much effort has gone into understanding the dynamic yet nuanced relationship between an evolving tumor and the immune system. The theory of immune surveillance and immune editing postulates that the immune system recognizes and eliminates tumor cells.36 However, “immune edited” tumor cells devise mechanisms to commandeer the immune system to promote tumor growth. The principle of immunotherapy therefore is to reverse this inhibition and re-invigorate immune cells to fight malignancy. In the case of CNS tumors, it has been challenging not only to use immunotherapy to release the tumor’s chokehold on the immune system but to also ensure that effector cells successfully cross the BBB and/or BCSFB.
Engineered T-Cell Therapy
Multiple approaches have been devised to target tumor antigens using patient-specific T cells. These T cells likely utilize common mechanisms to traffic into the CNS. One method is to engineer T-cell receptors (TCRs) against tumor-specific antigens. Recent preclinical studies have identified that transducing CD8+ T cells with a retroviral vector encoding for H3.3K27 mutation-specific TCRs can effectively target glioma cells, resulting in increased secretion of IFN-γ and diminished tumor size.37
Chimeric antigen receptor (CAR) T-cell therapy has been successful in the treatment of malignancies like acute lymphoblastic leukemia and chronic myeloid leukemia.38 One advantage afforded by CAR T cells is the ability to mount an antigen-specific immune response in an MHC-independent fashion, enabling T cells to bypass the barrier of reduced MHC expression in GBMs while maintaining tumor specificity.38,39 However, CAR T-cell therapy targets cell surface neoantigens, which may be limited in the case of GBM. Although relatively non-immunogenic tumors, antigens like epidermal growth factor receptor variant III (EGFRvIII), human epidermal growth factor receptor 2/cytomegalovirus (CMV), and IL-13 receptor subunit alpha-2 (IL-13Rα2) are frequently detected in GBM.5,40 EGFRvIII and IL-13Rα2 targeting CAR T cells successfully traffic into the brain parenchyma, although the exact mechanism of such accurate trafficking remains unknown. It is possible that these CAR T cells utilize preexisting chemokine gradients to migrate to the site of the tumor.17
In a recent case report, IL-13Rα2 CAR T-cell therapy resulted in a clinical response lasting 7.5 months in a patient with recurrent multifocal GBM.41 CAR T cells were initially provided through intracavitary infusions. However, intraventricular infusions were employed when new, distant lesions appeared. Dramatic reductions in size were subsequently observed in distant lesions, suggesting that CAR T cells trafficked through the CSF. Because the primary tumors did not uniformly express the target protein, infiltrating endogenous immune cells likely contributed to the observed response. Cytokines, including TFNα, IL-2, IFN-γ, CXCL9, and CXCL10 were 10-fold higher than pre-infusion levels, suggesting that CAR therapy may also mount an endogenous immune response.42 Effectively, endogenous leukocytes were detected in the CSF following CAR infusion. Similarly, in a study using EGFRvIII CAR T cells infused intravenously, a marked increase was seen both in GBM tumor-infiltrating lymphocyte (TIL) number and clonotypic diversity.43
In addition to inflammatory cytokines and cells, increases in inhibitory molecules and cells were observed following CAR T-cell infusion, such as indoleamine 2,3-dioxygenase 1, IL-10, and/or TGFβ in case of EGFRvIII CAR therapy, and IL-6, IL-8, IL-10, and CCL2 following IL-13Rα2 CAR treatment.43,44 These compensatory mechanisms likely contribute to immune resistance and GBM recurrence. Although CAR T-cell therapy has a very limited toxicity profile, it has a short life in patient CSF.42 Engineered CAR T cells targeting multiple antigens may be more effective in a shorter time span.
Recently, interest has developed in designing CARs that target extracellular components to alter the tumor vasculature and allow enhanced trafficking of endogenous T lymphocytes, including targeting αvβ3 integrin and vascular endothelial growth factor (VEGF) receptor 2.17 Other efforts to improve CAR T-cell trafficking involve combining this treatment with other modules such as IFN-γ treatment and PD ligand 1 (PD-L1) blockade, which aid in enhancing chemokine-directed migration of the T cells into the tumor.25 Treatment with checkpoint inhibitors following CAR infusions may also be useful in increasing the duration and efficacy of engineered T-cell therapy.
Vaccine-Based Therapy
Eliciting an endogenous immune response through a vaccine-based approach using tumor cell lysate, synthetic peptides, or even fragments of DNA as immunogens to boost and/or elicit long-term immune responses has gained much attention as an immunotherapeutic strategy.45 Unfortunately, in GBM, even though unique targets may be indentified, very few remain therapeutically viable. Nevertheless, results from early studies involving DC vaccines and IDH-mutant targeting peptides have shown encouraging results.
Dendritic cell vaccines
DCs are central orchestrators of T-cell functionality, and T cells capable of expressing more than one cytotoxic effector molecule are recognized as being essential for a successful antitumor immune response. A recent study demonstrated that administration of CMV-expressing DCs enhanced the polyfunctionality of adoptively transferred T cells in patients with newly diagnosed GBM.46 Adoptively transferred DCs must be directed to a lymph node to successfully activate T cells, yet their homing is inefficient. However, preconditioning adoptively transferred DCs can target them to lymph nodes in a CCL3-dependent manner.47 DC vaccines may therefore hold promise as a therapeutic strategy to improve efficiency of T-cell mediated responses.
IDH-mutant specific vaccines
IDH1 mutations, seen in a vast majority of low-grade glioma patients serve as glioma-specific neoantigens and are hence valuable targets for vaccination-based therapeutic strategies.19 Studies in preclinical, orthotopic models have shown that inhibitors to this mutant not only reverses the mutation-specific inhibition in signal transducer and activator of transcription (STAT) 1 expression and thereby IFN-γ induced CXCL10 expression, but also enhances the efficacy of other peptide vaccines, providing a survival advantage in mice.48 Further, studies using MHC humanized animal models showed that synthetic peptides to IDH(R132H) can successfully generate Th1-specific CD4+ T-cell responses.19 The peptides preferentially bind to/present on MHC-II molecules and result in a robust IFN-γ response.19 Thus, targeting IDH1 mutations in low-grade glioma patients could provide positive outcomes.
Checkpoint Inhibition
Checkpoint molecules regulate T cell activation following disease or infection. Tumors co-opt these checkpoints to elude immune cytotoxicity, thereby making them suitable targets for immunotherapy.49 Glioblastoma TILs express multiple checkpoint molecules, including lymphocyte-activation gene 3, T-cell immunoglobulin and mucin-domain containing 3, and the more commonly targeted, CTLA-4 and PD-1.50 PD-L1, the ligand to PD-1, may show positive expression on the surface of tumor cells in both patients with new diagnoses and recurrent GBM patients.51 PD-L1 expression within tumor tissue makes targeting PD-L1 or its receptor PD-1 suitable for immunotherapy. However, no correlation has been seen between PD-L1 expression and survival.51 Antibody blockade of CTLA-4 has been shown to be efficacious in preclinical studies using orthotopic glioma tumors, and CTLA-4 blockade ± PD-1 inhibition was well tolerated in patients with newly diagnosed GBM.52–54 Blockade of both CTLA-4 and PD-1 can increase trafficking of activated T cells into solid tumors, accompanied by an increase in cytokines like IFN-γ, IL-2, perforin, and granzyme, indicating the activated state of these TILs.52,53,55 Interestingly, increased trafficking due to PD-1 blockade was specific to TILs and not to adoptively transferred T cells that trafficked to organs such as the spleen.55 Further, CXCL10 expression was elevated in the CD11b+ monocytic population of tumors treated with anti–PD-1, possibly a result of increased IFN-γ secretion from the transferred T cells in a feedback signaling loop.55 Therefore, checkpoint blockade may be beneficial in mounting a lasting antitumor effect. However, to be an effective strategy, the tumor must be immunogenic. In hypermutative tumors, such as those found in patients with biallelic mismatch repair deficiency, several neoantigens are expressed that make lucrative targets for T-cell recognition. Under these circumstances, treatment with checkpoint blockade allows T cells to effectively respond to the repertoire of novel tumor antigens.56
For most GBM subtypes (except possibly the mesenchymal type) that are relatively immunologically inactive, treatment with checkpoint inhibitors alone may not be effective. Therefore, there are ongoing efforts to combine vaccination strategies with checkpoint inhibition to prolong the immune response.40 It is important, however, to consider that “releasing the brakes” on immune cells via checkpoint blockade while also increasing available antigens may drive T cells toward a terminally differentiated, exhausted state, prompt expression of alternative checkpoint molecules, or downregulate co-stimulatory molecules.57 This in turn could curtail the benefits of checkpoint blockade. Furthermore, prolonging immune response also implies the possibility of sustained inflammation and autoimmunity.58 Establishment of effective anti-inflammatory agents are likely needed to minimize this side effect.58
Interferon Treatment
The interferon family of cytokines comprises type I (IFN-α and β), type II (IFN-γ), and type III interferons (IFN-λ), all of which signal through distinct yet specific IFN receptors activating the Janus kinase (JAK)–STAT pathway.59 Studies in GBM have shown all 3 classes of interferons play an important role in immune regulation, making them targets for immunotherapy treatment.60–62
Extensive studies using EAE models have shown that IFN-β is immunosuppressive. Treatment of MS patients with recombinant IFN-β1 decreases T-cell infiltration into the CNS, reduces the ability of APCs to present antigen, and hence reduces lesion size.63 One of the primary functions of IFN-β is to counteract the pro-inflammatory signature of IFN-γ. IFN-β also prevents T-cell trafficking into the CNS by decreasing the expression of VCAM-1 and, to smaller extent, ICAM-1; molecules used for T-cell tethering and rolling on endothelial vessels.63,64 It is speculated that IFN-β negatively affects the CCR5 signaling axis involving IFN-γ–secreting Th1 type T cells that respond to ligands macrophage inflammatory protein 1 alpha and CCL5 on macrophages, microglia, and astrocytes to promote immune cell recruitment to the brain.65,66 Furthermore, IFN-β inhibits T-cell secretion of MMP-9, which degrades extracellular matrix and facilitates T-cell movement from the glia limitans into the brain parenchyma.66 Hence, while in the case of MS, treatment with recombinant IFN-β may be useful to contain autoimmunity, for GBM where IFN-β is known to be upregulated, treatment with IFN-β inhibitors may be useful to increase T-cell trafficking and activation in the CNS.
In contrast to IFN-β, both IFN-α and IFN-γ promote immune surveillance. Rodent models involving deletion of the IFN receptor α-1 have shown that in the absence of type I IFN signaling, Tregs and myeloid-derived suppressor cells permeate into the site of tumor development through upregulation of CCL22, whereas cytotoxic T lymphocytes are inhibited by CXCL10 downregulation.62 Most resident T cells in normal choroid plexus are CD4+ T memory cell populations that can secrete IFN-γ and IL-4, effectively modulating T-cell trafficking into the choroid plexus during injury, infection, and disease.67 Exposure of choroid plexus epithelial cells to IFN-γ increased expression of an array of trafficking molecules, including ICAM-1 and VCAM-1, as well as T-cell chemoattractants CCL5, CXCL9, and CXCL10.67 Monocyte recruitment factors such as CSF-1 was also upregulated, suggesting that IFN-γ mediates movement of both innate and adaptive immune cells into the CSF via the choroid plexus.67 Interestingly, these studies also found a reciprocal and synergistic relationship between TNFα and IFN-γ signaling that furthers the ability of leukocytes to move through the choroid plexus.67
Early strategies to deliver IFN-γ included intracranial dosing with recombinant protein, but despite promising preclinical studies, this did not significantly increase patient survival, possibly due to suboptimal dosing.68 Accordingly, investigators genetically engineered T cells to constitutively express IFN-γ61 However, this genetic manipulation of the T cells altered their natural gene expression, resulting in an increased expression of apoptotic factors like Fas L.61 Subsequent vaccination strategies involving granulocyte-macrophage colony-stimulating factor (GM-CSF) and IFN-γ expressing glioma cells transplanted into mice have produced promising results.61 Success has also been achieved in animal models intracranially injecting replication-deficient adenoviral vectors that expressed either IFN-γ or TNFα into syngeneic mice gliomas.69 Together, these treatments increased CD4+ and CD8+ TIL density, decreased tumor burden, and increased survival. However, one major caveat to IFN-γ as an immunotherapy agent is its ability to induce synthesis of nitric oxide (NO) by inducible nitric oxide synthetase (iNOS), which is an immune suppressant at elevated levels.61 NO can limit T-cell trafficking into the brain by repressing the nuclear factor-kappaB (NF-κB) pathway in the choroid plexus epithelium.70 Thus, the efficacy of treating GBM either with IFN-γ alone or in combination with other treatment strategies may be limited by NO production, and concomitant treatment with iNOS inhibitors could circumvent this complication.
Balancing Immune Activation and Excessive Inflammation: Lessons from Autoimmunity
One important consideration for GBM immunotherapy is the determination of when to cease therapy once an immune response is achieved. Prolonged activation of the immune system using any or all of the strategies discussed above may lead to uncontrolled inflammation.4 The resulting edema and inflammatory mass in the brain can result in damage to healthy neuronal tissue, encephalitis, or herniation which can be fatal.71 The primary approach to treating edema is corticosteroids. Dexamethasone is preferentially used for patients with CNS tumors due to minimal mineralocorticoid effects and enhanced half-life.71 At the molecular level, dexamethasone transcriptionally inhibits synthesis of key immune modulatory cytokines like IL-1β, IL-4, IL-5, IL-8, and GM-CSF.71 It can negatively regulate NF-κB, JAK-STAT, and other signaling pathways which could decrease immune cell activation and infiltration into the CNS.71 Additional studies are needed to determine the impact of dexamethasone on immunotherapeutic treatment strategies.
A second strategy to treat vasogenic cerebral edema is the use of anti-angiogenic factors to normalize or repair vasculature thereby reducing their leakiness.72 VEGF is a pro-angiogenic factor that is upregulated in GBM and acts through VEGF receptor 1 and 2 to promote endothelial growth and sprouting.72 In the brain expression of VEGF also causes breakdown of the BBB by promoting formation of intra-endothelial gaps and endothelial fenestrations.72 As a consequence, the vasculature becomes abnormal, predisposing tumor tissue to hypoxia and facilitating vasogenic edema.72 Anti-VEGF drugs like bevacizumab (Avastin) could help temporarily normalize the vasculature.72 Normalized tumor vasculature also increases oxygenation within the tumor, resulting in significant improvement in response to radiation therapy that relies on the ability to generate reactive oxygen species.73 Treatment of patients with bevacizumab in combination with radiation therapy and/or IFN-β increases their cytotoxicity, making it an important therapeutic agent not just to reduce edema but also to improve delivery of therapeutic agents, albeit the effect may be transient.74
In response to immunotherapy, patients may develop immune-related adverse events (irAEs).75 IrAEs are most commonly reported during checkpoint blockade, as this therapy can cause a widespread activation of immunity, including targeting of normal organs (autoimmunity). Therefore, the systemic release of checkpoint restriction of the immune system can permit activation of self-reactive lymphocytes, resulting in damage to healthy tissue alongside exacerbation of neurological symptoms.76 Some common manifestations of irAEs include vitiligo, mucosal dehydration, colitis, cytopenias, fever, headache, confusion, seizures, and hallucinations. In some cases, treatment with immunotherapy must be discontinued due to severity of the side effects. To treat irAEs in GBM patients, it may be helpful to evaluate the therapeutic armamentarium used for autoimmune disorders such as MS, rheumatoid arthritis, and inflammatory bowel disease. Some agents used include abatacept, tocilizumab, tofacitinib, and infliximab. These target various stages of immune activation or the pathways and products of excessive immune activation to mitigate the symptoms of irAEs. Currently, administration of these agents is limited to physician experience rather than preestablished regimens. Systematic evaluation of the optimal implementation of these immune modulating agents is urgently needed so that uncontrolled immune responses, particularly in the central nervous system, can be reliably controlled.77
Conclusions
Glioblastoma is an aggressive and complex tumor, characterized by enormous molecular and genetic heterogeneity, and its location in the brain makes it hard to access. Based on responses noted in a variety of other previously refractory cancers such as metastatic melanoma, immunotherapy provides the possibility of prolonged response and improved survival. One of the biggest challenges in translating promising preclinical results to clinical efficacy is enhancing the trafficking of the immune cells into the CNS. The presence of the BBB and BCSFB alongside the immune suppressive tumor microenvironment hinders the free movement of immune cells to and from the brain. Establishment of a chemoattractant gradient to facilitate movement of effector cells, both endogenous and adoptively transferred, is a pivotal factor affecting immune trafficking. As detailed in this review, immune cells express chemokine receptors which interact with their corresponding ligands on endothelial cells of the BBB and BCSFB as well as epithelial cells of the choroid plexus in order to transmigrate into the site of tumor development. Key components include chemokines such as CXCL9, CXCL10, CCL2, CCL5, as well as cell adhesion molecules like ICAM-1 and VCAM. Most of these chemokines can be induced on endothelial and epithelial cells by IFN-γ, making it an alluring therapeutic target. Commonly studied and proposed strategies for GBM immunotherapy, including CAR T-cell therapy, checkpoint blockade, and vaccination strategies, aim to enrich for cytotoxic T cells that express elevated levels of IFN-γ that could potentiate movement of immune cells into the CNS. However, immunotherapy may exacerbate cerebral edema and create autoimmune toxicities (irAEs) that can be life-threatening. Striking a balance between immune overactivation and maintaining lasting antitumor immunity has been a challenge in the clinic. Use of immune suppressants, most commonly corticosteroids such as dexamethasone, while successful in relieving intracranial pressure and resolving edema, may hinder immunotherapy strategies if provided early in treatment. Treatment of irAEs will likely require development of paradigms that incorporated a variety of immune modulatory agents prescribed for autoimmune disorders like multiple sclerosis and rheumatoid arthritis. However, systematic evaluation of these immune modulatory agents for CNS immune-related toxicities has not been performed, but this will be necessary to be able to fully evaluate future immunotherapy regimens that are likely to evoke vigorous immune responses and local inflammation. The future success for brain tumor immunotherapy will require more advanced mechanisms to monitor immune response both locally and systemically, distinguishing tumor progression from inflammatory response and creating effective methods to control damaging immune responses.
Funding
This research was supported in part by the Intramural Research Program of the NIH, NCI.
Conflict of interest statement
The authors of this paper do not have any conflicts of interest to disclose.
Acknowledgments
We would like to acknowledge Kristin Odom for the creation of Fig. 1 and Brett Theeler, MD for insightful review of the manuscript.
References
- 1. American Brain Tumor Association. Brain Tumor Statistics (2017). http://www.abta.org/about-us/news/brain-tumor-statistics. Accessed December 14, 2017. [Google Scholar]
- 2. Agnihotri S, Burrell KE, Wolf A, et al. . Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch Immunol Ther Exp (Warsz). 2013;61(1):25–41. [DOI] [PubMed] [Google Scholar]
- 3. Gzell C, Back M, Wheeler H, Bailey D, Foote M. Radiotherapy in glioblastoma: the past, the present and the future. Clin Oncol. 2017;29(1):15–25. [DOI] [PubMed] [Google Scholar]
- 4. Weathers SP, Gilbert MR. Current challenges in designing GBM trials for immunotherapy. J Neurooncol. 2015;123(3):331–337. [DOI] [PubMed] [Google Scholar]
- 5. Polivka , Jiri JR, Jiri P, Holubec L, et al. . Advances in experimental targeted therapy and immunotherapy for patients with glioblastoma multiforme. Anticancer Res. 2017;37(1):21–33. [DOI] [PubMed] [Google Scholar]
- 6. Jack AS, Lu J, Lu J. Immune cell infiltrates in the central nervous system tumors. Austin Neurosurgery. 2015;2(1):1–12. [Google Scholar]
- 7. Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120(5):1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248(1):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engelhardt B. T cell migration into the central nervous system during health and disease: different molecular keys allow access to different central nervous system compartments. Clin Exp Neuroimmunol. 2010;1(2):79–93. doi: 10.1111/j.1759-1961.2010.009.x. [DOI] [Google Scholar]
- 10. Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev Brain Res. 1999;117(2):145–152. [DOI] [PubMed] [Google Scholar]
- 11. van Zwam M, Huizinga R, Melief MJ, et al. . Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med (Berl). 2009;87(3):273–286. [DOI] [PubMed] [Google Scholar]
- 12. Harris MG, Hulseberg P, Ling C, et al. . Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci Rep. 2014;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louveau A, Smirnov I, Keyes TJ, et al. . Structural; and functional features of central nervous system lymphatics. Nature. 2016;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P. Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 2012;7(1):74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyck R, Lécuyer MA, Abadier M, et al. . ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood-brain barrier. J Cereb Blood Flow Metab. 2017;37(8):2894–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wikipedia. Chemokines. https://en.wikipedia.org/wiki/Chemokine. Accessed December 14, 2017. [Google Scholar]
- 17. Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–7174. [DOI] [PubMed] [Google Scholar]
- 18. Amankulor N, Kim Y, Arora S, et al. . Mutant IDH1 regulates tumor-associated immune system in gliomas. Genes Dev. 2017;31(1):774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schumacher T, Bunse L, Pusch S, et al. . A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. [DOI] [PubMed] [Google Scholar]
- 20. Liu C, Luo D, Reynolds BA, et al. . Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis. 2011;32(2):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klatte T, Seligson DB, Leppert JT, et al. . The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. 2008;179(1):61–66. [DOI] [PubMed] [Google Scholar]
- 22. Chen F, Yin S, Niu L, et al. . Expression of the chemokine receptor CXCR3 correlates with dendritic cell recruitment and prognosis in gastric cancer. Genet Test Mol Biomarkers. 2018;22(1):35–42. [DOI] [PubMed] [Google Scholar]
- 23. Boyé K, Pujol N, D Alves I, et al. . The role of CXCR3/LRP1 cross-talk in the invasion of primary brain tumors. Nat Commun. 2017;8(1):1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barreira Da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol. 2015;16(8):850–858. [DOI] [PubMed] [Google Scholar]
- 25. Oelkrug C, Ramage JM. Enhancement of T cell recruitment and infiltration into tumours. Clin Exp Immunol. 2014;178(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Qin H, Li L, et al. . Overexpression of CCL20 and its receptor CCR6 predicts poor clinical prognosis in human gliomas. Med Oncol. 2012;29(5):3491–3497. [DOI] [PubMed] [Google Scholar]
- 27. Brown CE, Vishwanath RP, Aguilar B, et al. . Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179(5):3332–3341. [DOI] [PubMed] [Google Scholar]
- 28. Sampson JH, Maus MV, June CH. Immunotherapy for brain tumors. JCO. 2017; 35(21):2450–2456. [DOI] [PubMed] [Google Scholar]
- 29. Hoepner S, Loh JM, Riccadonna C, et al. . Synergy between CD8 T cells and Th1 or Th2 polarised CD4 T cells for adoptive immunotherapy of brain tumours. PLoS One. 2013;8(5):e63933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Columba-Cabezas S, Elena BS, Aloisi AF. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2006;13(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mueller SN, Hosiawa-Meagher KA, Konieczny BT, et al. . Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–674. [DOI] [PubMed] [Google Scholar]
- 32. Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblastoma. Front Surg. 2016;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pham K, Luo D, Liu C, Harrison JK. CCL5, CCR1 and CCR5 in murine glioblastoma: immune cell infiltration and survival rates are not dependent on individual expression of either CCR1 or CCR5. J Neuroimmunol. 2012;246(1–2):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ifergan I, Kebir H, Terouz S, et al. . Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70(5):751–763. [DOI] [PubMed] [Google Scholar]
- 35. Müller S, Kohanbash G, Liu SJ, et al. . Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. [DOI] [PubMed] [Google Scholar]
- 37. Hou Y, Kohanbash G, Okada K, et al. . Novel and shared neoantigen for glioma T cell therapy derived from histone 3 variant H3.3 K27M mutation. J Immunother Cancer. 2015;3(Suppl 2):P445. [Google Scholar]
- 38. Rodriguez A, Brown C, Badie B. Chimeric antigen receptor T-cell therapy for glioblastoma. Transl Res. 2017;187:93–102. [DOI] [PubMed] [Google Scholar]
- 39. Miao H, Choi BD, Suryadevara CM, et al. . EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One. 2014;9(4):e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown CE, Alizadeh D, Starr R, et al. . Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sridhar P, Petrocca F. Regional delivery of chimeric antigen receptor (CAR) T-cells for cancer therapy. Cancers (Basel). 2017;9(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Rourke DM, Nasrallah MP, Desai A, et al. . A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399). doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown CE, Alizadeh D, Starr R, et al. . Regression of glioblastoma after chimeric antigen receptor T-Cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swartz AM, Batich KA, Fecci PE, Sampson JH. Peptide vaccines for the treatment of glioblastoma. J Neurooncol. 2015;123(3):433–440. [DOI] [PubMed] [Google Scholar]
- 46. Reap E, Suryadevara CM, Batich KA, et al. . Dendritic cells enhance polyfunctionality of adoptively transferred T cells which target cytomegalovirus in glioblastoma. Cancer Res. 2018;78(1):256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitchell DA, Batich KA, Gunn MD, et al. . Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kohanbash G, Carrera DA, Shrivastav S, et al. . Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127(4):1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Preusser M, Lim M, Reardon DA, Sampson JH. Prospects of immunecheckpoint modulators in treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berghoff AS, Kiesel B, Widhalm G, et al. . Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11(8):1809–1819. [DOI] [PubMed] [Google Scholar]
- 53. Huang RR, Jalil J, Economou JS, et al. . CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17(12):4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Omuro A, Vlahovic G, Lim M, et al. . Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase 1 cohorts of CheckMate 143. Neuro Oncol. 2018;20(5)674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng W, Liu C, Xu C, et al. . PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72(20):5209–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bouffet E, Larouche V, Campbell BB, et al. . Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 57. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang J, Liu F, Liu Z, et al. . Immune checkpoint in glioblastoma: promising and challenging. Front Pharmacol. 2017;8(242):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Weerd NA, Nguyen T. The interferons and their receptors—distribution and regulation. Immunol Cell Biol. 2012;90(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30(8):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kane A, Yang I. Interferon-gamma in brain tumor immunotherapy. Neurosurg Clin N Am. 2010;21(1):77–86. [DOI] [PubMed] [Google Scholar]
- 62. Fujita M, Scheurer ME, Decker SA, et al. . Role of type 1 IFNs in antiglioma immunosurveillance—using mouse studies to guide examination of novel prognostic markers in humans. Clin Cancer Res. 2010;16(13):3409–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yong VW, Chabot S, Stuve O, Williams G. Interferon beta in the treatment of multiple sclerosis: mechanisms of action. Neurology. 1998;51(3):682–689. [DOI] [PubMed] [Google Scholar]
- 64. Hartrich L, Weinstock-Guttman B, Hall D, et al. . Dynamics of immune cell trafficking in interferon-beta treated multiple sclerosis patients. J Neuroimmunol. 2003;139(1-2):84–92. [DOI] [PubMed] [Google Scholar]
- 65. Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96(12):6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zang YC, Halder JB, Samanta AK, Hong J, Rivera VM, Zhang JZ. Regulation of chemokine receptor CCR5 and production of RANTES and MIP-1alpha by interferon-beta. J Neuroimmunol. 2001;112(1-2):174–180. [DOI] [PubMed] [Google Scholar]
- 67. Kunis G, Baruch K, Rosenzweig N, et al. . IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain. 2013;136(11):3427–3440. [DOI] [PubMed] [Google Scholar]
- 68. Färkkilä M, Jääskeläinen J, Kallio M, et al. . Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br J Cancer. 1994;70(1):138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ehtesham M, Samoto K, Kabos P, et al. . Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9(11):925–934. [DOI] [PubMed] [Google Scholar]
- 70. Baruch K, Kertser A, Porat Z, Schwartz M. Cerebral nitric oxide represses choroid plexus NFκB-dependent gateway activity for leukocyte trafficking. EMBO J. 2015;34(13):1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gerstner ER, Duda DJ, di Tamaso E, et al. . VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol. 2009; 6(4):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McGee MC, Hamner JB, Williams RF, et al. . Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;76(5):1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang N, Chen J, Ferraro GB, et al. . Anti-VEGF treatment improves neurological function in tumors of the nervous system. Exp Neurol. 2018;299(Pt B):326–333. [DOI] [PubMed] [Google Scholar]
- 75. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017;13(12):755–763. [DOI] [PubMed] [Google Scholar]
- 77. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Corrigendum: current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:311. [DOI] [PMC free article] [PubMed] [Google Scholar]