ABSTRACT
Bone and joint infections include septic arthritis, prosthetic joint infections, osteomyelitis, spinal infections (discitis, vertebral osteomyelitis and epidural abscess) and diabetic foot osteomyelitis. All of these may present through the acute medical take. This article discusses the pathogenesis of infection and highlights the importance of taking a careful history and fully examining the patient. It also emphasises the importance of early surgical intervention in many cases. Consideration of alternative diagnoses, appropriate imaging and high-quality microbiological sampling is important to allow appropriate and targeted antimicrobial therapy. This article makes some suggestions as to empiric antibiotic choice; however, therapy should be guided by local antimicrobial policies and infection specialists. Involvement of a multidisciplinary team is essential for optimal outcomes.
KEYWORDS: bone and joint infection, osteomyelitis, septic arthritis, prosthetic joint infection, biofilm, diabetic foot infection, discitis, vertebral osteomyelitis
Key points
In suspected bone and joint infections quality microbiological sampling is important. In those with features of sepsis or acute skin and soft tissue infection (SSTI), blood cultures and, where possible, aspirates should be taken immediately followed by prompt empiric antibiotic therapy. In other cases antibiotic therapy should wait until intraoperative samples are taken. In all acute bone and joint infections, orthopaedic surgeons, infection specialists and radiologists should be involved early
Acute septic arthritis should be managed with diagnostic aspiration followed by prompt arthroscopy or arthrotomy and washout in conjunction with antibiotics. In some cases, where surgery may not be possible, serial closed joint aspirations might be an alternative option
Acute haematogenous osteomyelitis needs prompt surgical drainage if there is a purulent collection. Other cases can be managed by antibiotics alone but with repeat imaging if there is failure to settle
Patients with possible spinal infection need blood cultures, prompt MRI and spinal surgery review. In stable patients not requiring surgical intervention, a radiological biopsy should be considered to direct antimicrobial therapy. Tuberculosis, Brucella, Nocardia and/or fungal cultures should be requested in patients with appropriate risk factors
In diabetic foot infections it is important to recognise severe limb-threatening infections that require urgent surgical management. Signs of this are systemic sepsis, poor glycemic control, gas in soft tissues, abscess and infection from an ulcer tracking deeply through the foot to another site (eg plantar ulcer to dorsum of foot)
Introduction
Bone and joint infections cause serious morbidity and pose significant management challenges. They may cause acute sepsis with bone and joint destruction, chronic pain, discharging wounds and permanent disability. With expanding populations and increasing age, bone and joint infections, especially those involving devices, will have a growing impact on healthcare resources. For effective management, well-coordinated multidisciplinary working is important.
General considerations
Pathogens can gain access into bone and joints through the blood stream (haematogenous route) or via direct inoculation from a contiguous focus of infection. Acute haematogenous infections are most common in children and the elderly.
The presence of foreign material such as implanted devices or dead bone significantly reduces the number of organisms required to cause infection.1 Foreign or non-viable material also allows normal skin commensals, such as coagulase-negative Staphylococcus spp, to become significant pathogens. Micro-organisms adhere to the inert surface where they are relatively protected from the blood supply, immune processes and antibiotics. Organisms, such as Staphylococcus spp can produce extracellular polymeric substance (EPS). Micro-organisms embedded in EPS create a biofilm on the inert surfaces. By communicating with each other they are able to up and downregulate gene expression enabling regulation of growth and adaptation to the environment. Biofilm formation is an important mechanism for bacterial survival in chronic bone and joint infection.2 Chronically established bone and joint infection can be persistent, evolve or relapse, even in the face of prolonged antimicrobial therapy. Biofilm has important implications for diagnostics, as well as surgical and antibiotic management.
The ‘hot’ joint
An inflamed ‘hot’ joint has a wide differential including inflammatory causes (septic arthritis, reactive, rheumatoid arthritis, spondyloarthropathies, SLE, gout or pseudo-gout) and non-inflammatory causes (degenerative joint diseases, trauma, avascular necrosis and Charcot's arthropathy).3,4 Septic arthritis is important to exclude, as delayed or inadequate treatment can lead onto cartilage and then joint destruction.
Acute septic arthritis
Acute septic arthritis can affect any joint. It most commonly affects the knee but may also involve wrists, ankles, hips and the symphysis pubis. Polyarticular septic arthritis is more common in patients with inflammatory joint disease or overwhelming sepsis.5 Injecting drug use is a risk factor for septic arthritis of the sternoclavicular, sternomanubral or sacroiliac joints, often also associated with endocarditis.6
The presentation can be similar to other inflammatory causes, such as crystal arthropathy, with an acutely painful, swollen, warm, red joint and a reduced range of movement. Features suggesting septic arthritis include fever and/or chills and absence of prior history or risk factors for gout, but dual pathology can occur. Risk factors for septic arthritis include extremes of age, bacteraemia, inflammatory joint disease, diabetes, intravenous drug use, alcoholism, immunosuppression, malignancy, recent trauma, intra-articular injections, arthroscopy or a prosthetic joint. The clinical history should include a sexual history (for gonococcal arthritis). A history of tick bite in an endemic area may raise the possibility of Lyme arthritis.
Blood cultures may be positive in up to 50% of cases but are usually negative in gonococcal septic arthritis.7 Plain X-rays should be done to exclude other causes of hot joint, but are usually normal in early septic arthritis. Ultrasound is sensitive for detecting joint effusions and synovitis. Synovial fluid should be aspirated and examined for leucocytes, urate and pyrophosphate crystals and by Gram stain and culture. A semiquantitative synovial leucocyte count can differentiate inflammatory from non-inflammatory causes but cannot differentiate infection from other inflammatory causes.4 Gram stain has low sensitivity (<75%) especially in gonococcal arthritis (<25%).4 Cultures are also often negative in the latter and, if suspected, molecular diagnostics (16S polymerase chain reaction) on synovial fluid may be considered and a urethral swab or urine should be sent for culture / nucleic acid amplification testing. Rectal and pharyngeal swabs may be indicated. If Lyme arthritis is suspected diagnosis is by serology.
Diagnostic joint aspiration should be performed before antibiotics are given, except when the patient is acutely septic and aspiration delayed. Table 1 highlights the common bacterial causes and example antibiotic choices. All cases of suspected acute septic arthritis should be referred urgently to orthopaedics. Prompt arthroscopic or open washout is generally recommended despite the absence of good quality clinical trials. Observational studies show that in patients where surgery may be high risk, a more conservative approach of repeated aspirations may be effective.8
Table 1.
Suggested empiric antibiotics for native joint septic arthritis in adults (but consult local guidelines). Modify, with microbiological advice, when culture results are available
| Suggested empiric antibiotic choice (after blood cultures and joint aspirate) | ||||
|---|---|---|---|---|
| Patient group | Possible organisms | No known drug allergies | Penicillin allergy (non-severe eg rash) | Penicillin allergy (severe eg anaphylaxis) |
| No specific risk factors | Staphylococcus spp, beta-haemolytic streptococci | IV flucloxacillin | IV anti-staphylococcal cephalosporin (eg cefuroxime) | Clindamycin |
| Frail, recurrent UTIs, end-stage renal failure, recent abdominal surgery? | Aerobic Gram-negative rods | IV co-amoxiclav | IV 3rd generation cephalosporin (eg ceftriaxone) | Clindamycin plus ciprofloxacin |
| MRSA risk | Meticillin-resistant S aureus | Add IV glycopeptidea | Add IV glycopeptidea | Add IV glycopeptidea |
| Suspected gonococcal septic arthritis 18 ,b | Neisseria gonorrhoeae | IV 3rd generation cephalosporin (eg ceftriaxone) | IV 3rd generation cephalosporin (eg ceftriaxone) | Clindamycin plus ciprofloxacin (stop clindamycin if proven Neisseria infection) |
| Intravenous drug usage | S aureus. Less likely Pseudomonas aeruginosa, Fungal | IV flucloxacillin | IV anti-staphylococcal cephalosporin (eg cefuroxime) | Clindamycin |
| Known colonised with multidrug resistant organism | ||||
| MRSA, ESBL, CPE etc | Discuss with microbiology | |||
Table adapted from 3,4
aGlycopeptides include vancomycin and teicoplanin. These should be used at high doses in bone and joint infection ie vancomycin at 10–12 mg/kg and teicoplanin at 10 mg/kg. Modifications to dosing will need to be made in the setting of low body weight and/or impaired renal function.
bConsult local infectious diseases / micro or genitourinary medicine physicians
ESBL = extended-spectrum beta-lactamases; CPE = carbapenemase-producing enterobacteriaceae; IV = intravenous; MRSA = Meticillin-resistant Staphylococcus aureus
There is no good evidence to guide duration of antibiotic therapy for septic arthritis in adults. If synovial fluid Gram stain and cultures (taken before antibiotics) are negative, gonococcal arthritis is not suspected and surgical findings are inconclusive then antibiotics should be reviewed, and stopped if there is a likely alternative diagnosis (eg crystal arthropathy). In proven septic arthritis, antibiotics are typically given for 2–4 weeks. The longer (4-week) course may be indicated with difficult organisms, such as Staphylococcus aureus or Pseudomonas aeruginosa infections. Failure to settle or relapse may mean a repeat washout is required; however, inflammatory changes often persist for several weeks after presentation. There is no evidence as to when antibiotics can be changed to the oral route. This would depend on clinical progress, extent of infection, bioavailability of oral agents and likely patient compliance. In likely gonococcal arthritis, the patient should also be referred to a genitourinary clinic for a full sexually transmitted infection screen.
Infections involving prosthetic joints (PJI) should always be referred back to orthopaedics, who should also involve infection specialists. Acute PJI occurs either postoperatively (up to 3 months after the initial arthroplasty) or through haematogenous spread after a period in which the prosthesis has been sound. For acute PJIs, blood cultures, plain X-ray, ultrasound and aspiration of the joint are the initial diagnostic modalities.9 The prosthesis at this stage is usually sound (not loose) and the most appropriate management is a DAIR procedure (a radical open Debridement with exchange of modular components but Retention of the Implant followed by Antibiotics).10 This needs to be done by an orthopaedic surgeon experienced in managing PJIs. Joint aspiration / arthroscopic washout may be required as an interim measure to drain pus if the relevant expertise is not immediately available. More chronic infections (usually presenting as increasing pain, loose prosthesis or a discharging sinus) may be managed by revision of the prosthesis in one or two stages or excision arthroplasty. When the prosthesis is sound, a DAIR may be considered. Expert management of the soft tissues and dead space is important and may require plastic surgery input eg a muscle flap. When surgery is performed for chronically infected prosthetic joints, this should be done off antibiotics and multiple samples taken using separate instruments for separate sites, for microbiology and histology. Postoperative antibiotics need to be managed by infection specialists and may be prolonged.11
Osteomyelitis
In children the most common site for acute haematogenous osteomyelitis is the growing end of long bones. In adults, it is the spine. The most common organism is S aureus but other pathogens such as beta-haemolytic Streptococcus spp, Haemophilus influenzae, Kingella kingae or Mycobacterium tuberculosis are possible. In patients with sickle cell disease, osteomyelitis is commonly due to Salmonella spp. Osteomyelitis in children may relapse decades later in adulthood. Osteomyelitis may also occur in relation to infected fracture fixation devices. Infection may then contribute to delayed or non-union of the fracture. Figure 1 demonstrates the pathogenesis of osteomyelitis.
Fig 1.
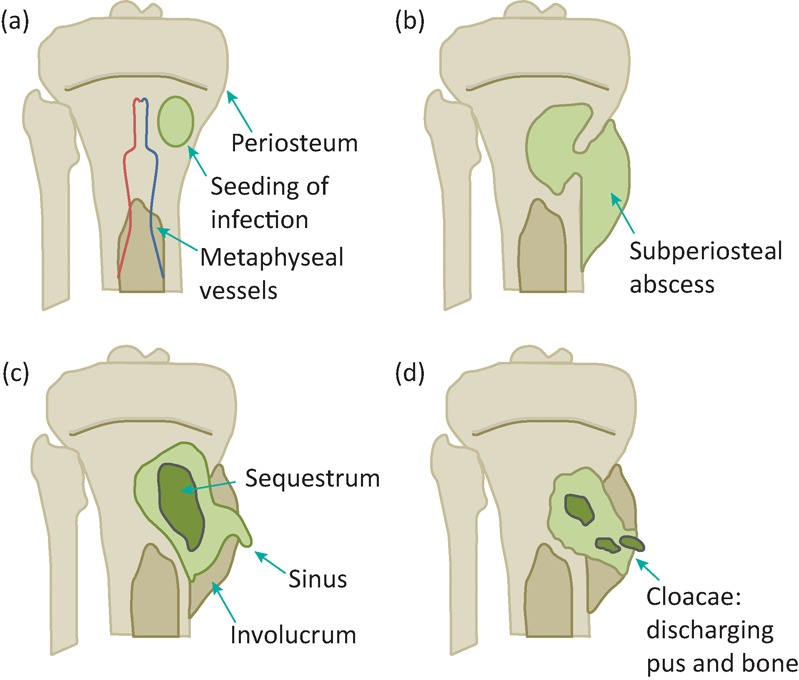
The pathogenesis of osteomyelitis. (a) Haematogenous bone infection results in medullary pus formation, acute inflammation, systemic illness and often, secondary bacteraemia. Pus may then track into joints or through the cortex. (b) In cases that progress to chronicity, a subperiosteal abscess may form leading to periosteal stripping and devitalisation of bone. Viable bone is resorbed leading to lucency. (c) Bacteria on the surface of dead bone persist leading to chronic suppuration, tissue destruction, sinus formation and often further bone death. Dead bone form sequestra. Involucrum is new bone formation outside existing bone as a result of periosteal stripping and then new bone growth from the periosteum. (d) This may be breached by cloacae, through which pus and fragments of dead bone escape.
Acute osteomyelitis usually presents with fever and pain at the site of infection. Other skeletal sites should be examined as multifocal osteomyelitis can occur. Blood cultures and plain films should be performed. The purpose of plain films is to look for other causes of pain (eg fracture) and evidence of periosteal reaction or lucency. The imaging modality of choice for osteomyelitis, however, is MRI, which may show bone oedema, abscess formation, and periosteal reaction. If infection is chronic there may also be evidence of a sinus, cloacae, periostitis, sequestrum and/or involucrum (Fig 2). Occasionally, pathological fractures occur. If there are soft tissue collections, prompt aspiration for microbiology is appropriate. In acute osteomyelitis, many patients will respond to antibiotic treatment but those with evidence of pus on imaging require urgent surgical drainage / decompression. When osteomyelitis is chronic and/or related to a fracture fixation device it needs management by a multidisciplinary team experienced in the management of bone infections. Ideal management is with a combination of surgical resection with meticulous intraoperative sampling with/without stabilisation, with/without reconstruction, soft tissue management by plastic surgeons and appropriately managed postoperative antibiotics.
Fig 2.
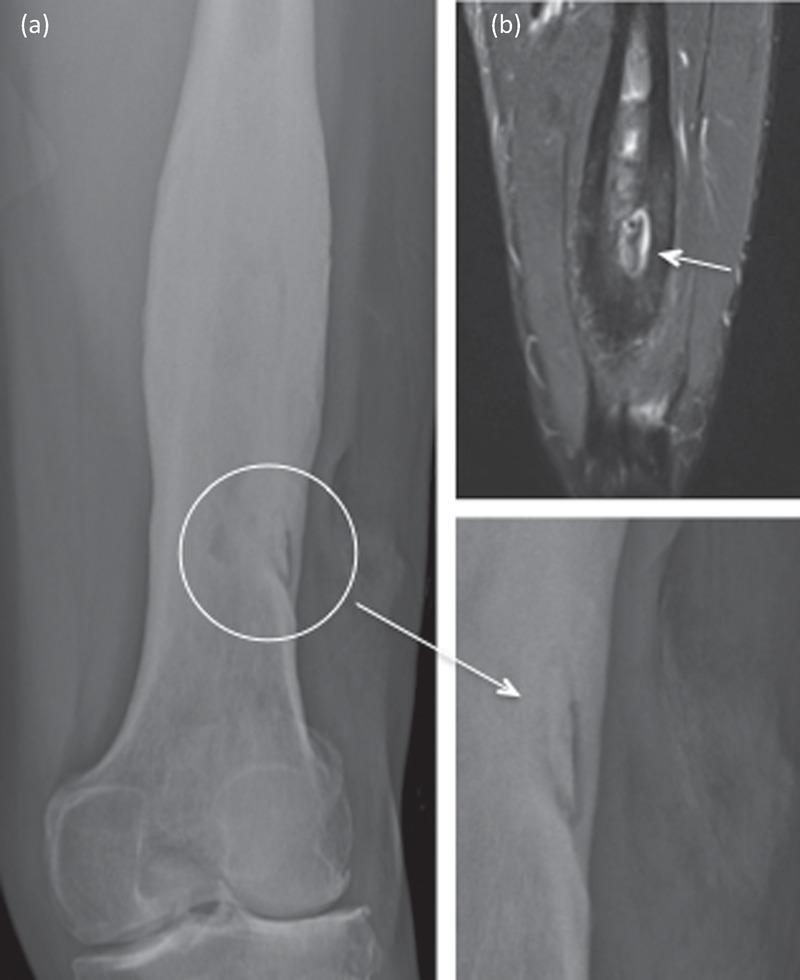
X-ray (a) and MRI (b) of the left femur, showing an area of osteomyelitis, with a central sequestrum (dead bone) and involucrum (new bone formation) around this – marked with white arrows. Chronic disease is noted through the shaft of the femur, as well as visible overlying soft tissue deformity.
Spinal infections (vertebral osteomyelitis, discitis, epidural abscess)
Spinal infections are most commonly either haematogenous or postsurgical. Haematogenous infections are most commonly due to S aureus. Streptococcus spp, aerobic Gram-negative bacilli and M tuberculosis should also be considered as should Brucella spp in endemic areas and fungi in immunocompromised patients.12 All patients with known fever and weight loss and/or bacteraemia and/or endocarditis should have prompt spinal imaging if they have new or worsening back pain. Imaging, usually by MRI (or computed tomography if MRI is contraindicated), should look for evidence of epidural abscess, discitis, vertebral osteomyelitis and, critically, for evidence of cord / cauda equina compression or vertebral instability requiring urgent surgical intervention.13 Epidural collections usually require surgical drainage.14 Unless blood cultures have already revealed a causative organism, deep microbiological samples should be obtained. This can be with intraoperative samples if surgery is indicated, or by radiological biopsy in other cases. This should be done urgently and, where possible, antibiotics withheld until after the biopsy has been taken (antibiotic therapy should not be delayed however in septic/unstable patients). Cases should be discussed with microbiology to ensure the relevant tests are done in the laboratory.
Native vertebral osteomyelitis should be managed with 6 weeks of appropriately targeted therapy (possibly longer in complicated cases or where organisms such as Brucella spp are identified).15 Duration of therapy for epidural abscesses depends upon whether it was surgical drained and clinical/radiological response.
Diabetic foot osteomyelitis
Diabetic foot infections usually occur following skin ulceration in patients with neuropathy and/or vascular insufficiency.16 Infections can go on to cause adjacent osteomyelitis. In severe infections this can rapidly become limb and life threatening. It is essential for all patients, especially diabetics, presenting though acute medical services to have a full foot examination including the removal of dressings. An audit (England and Wales) in 2015 showed that two-thirds of diabetic inpatients did not have a specific diabetic foot risk examination while an inpatient.17
Urgent surgical intervention may be required in those patients with abscesses, necrotising soft tissue infections and/or uncontrolled sepsis. Clinical evidence of pus tracking from one area (eg ulcer) to another may be indicative of deep spreading infection. Less urgent surgery may be required for those with substantial non-viable tissue or extensive bone or joint involvement. An early vascular assessment and involvement of a vascular surgeon to consider revascularisation in appropriate cases, especially those with critical ischaemia, is essential. Table 2 gives a guide to empiric antibiotic management based upon the severity of disease. Tissue sampling can be helpful for antimicrobial management. Superficial swabs often represent colonisation only, whereas deep tissue curettings / bone sampling can allow for appropriately targeted treatment.
Table 2.
Diabetic foot infections: pathogens and suggested empiric antimicrobial therapy (but consult local guidelines)
| Severitya | Usual pathogens | Treatment | ||
|---|---|---|---|---|
| No known drug allergy | Penicillin allergy (non-severe eg rash) | Penicillin allergy (severe eg anaphylaxis) | ||
| Uninfected | ||||
| Mild (usually treated with oral agents) | Meticillin-sensitive Staphylococcus aureus (MSSA); Streptococcus spp | Flucloxacillin or co-amoxiclav | Doxycycline, clindamycin or trimethoprim/sulfamethoxazole | |
| Meticillin-resistant S aureus (MRSA) | A glycopeptide | A glycopeptide | ||
| Moderate (may be treated with oral or initial parenteral agents) | MSSA; Streptococcus spp; Enterobacteriaceae; anaerobes | Co-amoxiclav | 3rd generation cephalosporin eg ceftriaxone plus metronidazole | Clindamycin and ciprofloxacin |
| Risk of Pseudomonas aeruginosa | Anti-pseudomonal beta-lactam eg piperacillin- tazobactam or ceftazidime plus metronidazole | Anti-pseudomonal beta-lactam eg ceftazidime plus metronidazole | ||
| MRSA | If high risk of MRSA, add a glycopeptide | |||
| Severe (usually treated with parenteral agents) | MRSA; Enterobacteriacae; Pseudomonas; anaerobes | Antipseudomonal beta-lactam eg ceftazidime and metronidazole If high risk of MRSA, add a glycopeptide | Clindamycin and ciprofloxacin | |
aSeverity based on the Infectious Diseases Society of America severity score16 as follows:
Uninfected: No symptoms or signs of infection present (symptoms/signs defined as at least two of local swelling or induration, erythema, local tenderness or pain, local warmth, purulent discharge)
Mild: Local infection involving only the skin and subcutaneous tissue. If erythema, must be >0.5–≤2 cm around the ulcer. Exclude other causes of inflammatory response of the skin
Moderate: Local infection (as above) with erythema >2 cm, or involving structures deeper than skin and subcutaneous tissue, and no systemic inflammatory response signs
Severe: Local infection with signs of systemic inflammatory response with two or more of temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or PaCO2 <32 mmHg, white blood cell count >12,000 or <4000 cells/μL or ≥10% immature (band) forms
Conclusions
Bone and joint infections can present through the acute medical take. It is important to take a careful history and remove any dressings during initial assessment and to recognise when urgent surgical intervention is required. Microbiological sampling should be done in all cases, to allow for targeted antimicrobial management. Comorbidities must be adequately managed. Involvement of the multidisciplinary team is essential.
References
- 1.Elek SD. Conen PE. The virulence of Staphylococcus pyogenes for man. A study of the problems of wound infection. Br J Exp Pathol. 1957;38:573–86. [PMC free article] [PubMed] [Google Scholar]
- 2.Flemming HC. Wingender J. Szewzyk U, et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2006;14:563–75. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 3.Coakley G. Mathews C. Field M, et al. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford) 2006;45:1039–41. doi: 10.1093/rheumatology/kel163a. [DOI] [PubMed] [Google Scholar]
- 4.Atkins BL. Bowler IC. The diagnosis of large joint sepsis. J Hosp Infect. 1998;40:263–74. doi: 10.1016/s0195-6701(98)90302-4. [DOI] [PubMed] [Google Scholar]
- 5.Dubost JJ. Fis I. Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore) 1993;72:296–310. doi: 10.1097/00005792-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Kak V. Chandrasekar PH. Bone and joint infections in injection drug users. Infect Dis Clin North Am. 2002;16:681–95. doi: 10.1016/s0891-5520(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 7.Rice PA. Gonococcal arthritis (disseminated gonococcal infection) Infect Dis Clin North Am. 2005;19:853–61. doi: 10.1016/j.idc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ravindran V. Logan I. Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology (Oxford) 2009;48:1320–2. doi: 10.1093/rheumatology/kep220. [DOI] [PubMed] [Google Scholar]
- 9.Osmon DR. Berbari EF. Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 10.Grammatopoulos G. Kendrick B. McNally M, et al. Outcome following Debridement, Antibiotics and Implant Retention (DAIR) in hip peri-prosthetic joint infection – An 18-year experience. J Arthroplasty. 2017;32:2248–55. doi: 10.1016/j.arth.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Moran E. Byren I. Atkins BL. The diagnosis and management of prosthetic joint infections. J Antimicrob Chemother. 2010;65:45–54. doi: 10.1093/jac/dkq305. [DOI] [PubMed] [Google Scholar]
- 12.Cornett CA. Vincent SA. Crow J. Hewlett A. Bacterial spine infections in adults: evaluation and management. J Am Acad Orthop Surg. 2016;24:11–8. doi: 10.5435/JAAOS-D-13-00102. [DOI] [PubMed] [Google Scholar]
- 13.Nickerson EK. Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117:121–38. doi: 10.1093/bmb/ldw003. [DOI] [PubMed] [Google Scholar]
- 14.Suppiah S. Meng Y. Fehlings MG, et al. How best to manage the spinal epidural abscess? A current systematic review. World Neurosurg. 2016;93:20–8. doi: 10.1016/j.wneu.2016.05.074. [DOI] [PubMed] [Google Scholar]
- 15.Berbari EF. Kanj SS. Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA. Berendt AR. Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132–73. doi: 10.1093/cid/cis346. [DOI] [PubMed] [Google Scholar]
- 17.Health and Social Care Information Centre National Diabetes Inpatient Audit (NaDIA) – 2015. London:: NHS Digital; 2016. http://digital.nhs.uk/catalogue/PUB20206. [Accessed 12 June 2017] [Google Scholar]
- 18.Bignell C. FitzGerald M. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS. 2011;22:541–7. doi: 10.1258/ijsa.2011.011267. [DOI] [PubMed] [Google Scholar]


