Abstract
A phase I trial of an engineered poliovirus for the treatment of recurrent glioblastoma (GBM) has attracted attention due to 8 survivors reaching the 24-month and 5 reaching the 36-month survival landmarks.1 Genetically engineered viruses (oncolytic viruses) have been in trials for GBM for almost two decades.2 These replication-competent (tumor-selective, oncolytic, replication-conditional) viruses or replication-defective viral vectors (gene therapy) deliver cytotoxic payloads to tumors, leading to immunogenic death and intratumoral inflammatory responses. This transforms the tumor microenvironment from immunologically naïve (“cold”) to inflamed (“hot”), increasing immune cell recognition of tumor antigens and the durable responses observed in virotherapy.3,4 Several current and past virotherapy trials have reported a “tail” of apparent responders at the 24-month landmark. Other modalities have also reported a “tail” of seemingly long-term survivors. These trials seem to show that these responder “tails” characterize a defined subset of GBM patients.
Keywords: virotherapy, oncolytic virus, clinical trials, review, GBM
Malignant gliomas (MGs) (glioblastoma [GBM], anaplastic astrocytoma [AA], and anaplastic oligodendroglioma [AO]) are universally fatal. For GBM, median overall survival is 12–16 months, with most deaths occurring before 24 months.5 Better prognosis and increased durability of “response” to standard of care are associated with biologic and demographic features, like mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2),6 hypermethylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter,7 deletions of 1p/19q chromosomal arms,8 absence of telomerase reverse transcriptase (TERT) mutations,9 young age,10 extent of surgical resection,11 and good performance score.12 Even “favorable” MGs recur eventually, resulting in fatality. Upon recurrence, treatment options are additional surgery, coupled with FDA-approved therapies not employed upon the tumor’s first presentation, like bevacizumab,13–19 lomustine, or CCNU20; carmustine wafers (Gliadel); and/or tumor-treating fields (TTFs) (Optune).21,22 “Off-label” FDA-approved chemotherapies such as irinotecan23 or targeted therapies24 based on rare “actionable” mutation are less commonly used. Survival from the time of recurrence is less than 12 months, with survival greater than 24 months judged as unusual. Extending survival beyond 24 months garners considerable excitement.
Investigational therapies have been numerous for recurrent (r)GBM. Most are phase 0, I, or II trials: phase III trials are rare. Although the primary objective of phase I trials is to establish treatment tolerability, there is an ever-increasing demand to discover a positive signal of effectiveness. This demand arises from the need to justify the resources required to test efficacy in a late stage randomized trial. A positive signal is based on a radiologic response by MRI and/or clinical responses such as survival time from the treatment and/or progression-free survival time from the treatment because there is no accepted molecularly based biomarker of response. Most clinical trials in rGBM have not shown substantial efficacy for the therapies under investigation. However, recent clinical trials that have provoked interest include those that have used viruses, engineered to replicate and be toxic to tumor cells, and/or to deliver an anticancer gene to cells in the tumor microenvironment.2,3 This therapy (virotherapy) elicits a cellular immune response that ultimately leads to rejection of GBM cells in the central nervous system.2 Virotherapy is being recognized as a special type of immunotherapy, administered intravenously or in situ (peritumorally after resection of the rGBM or intratumorally via a stereotactic-guided catheter or via convection-enhanced delivery [CED]). Two types of viral vectors are utilized: the first uses viral vectors that infect and do not replicate but still deliver an anticancer gene (Fig. 1), while the second uses replication-competent viruses that infect and replicate (Fig. 2). The first (“gene therapy”) has found several FDA-approved applications for noncancer human diseases, while the second has been exclusively used for cancer. Both types of virotherapy have been tested in early and late phase clinical trials for GBM, with reports of durable responses and survival greater than 24 months after recurrence. Here, we will discuss some of the more salient findings from virotherapy trials in the context of the broader landscape of trials of other modalities for rGBM.
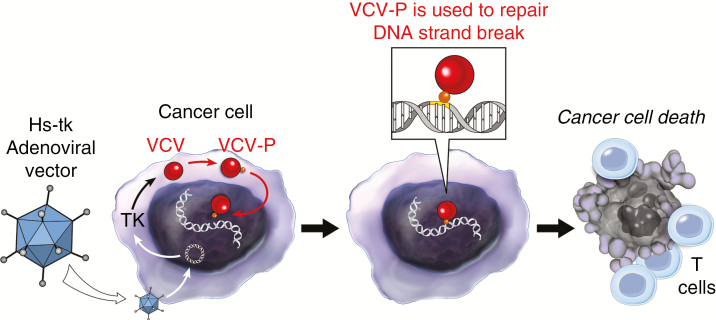
Fig. 1.
Replication-defective viral vectors infect cancer cells and deliver an anticancer gene but do not replicate. Adenoviral vectors have been engineered to deliver the herpes simplex thymidine kinase gene (Hs-tk). Once inside the cell, the adenoviral DNA exists as an extrachromosomal element in the nucleus and transcribes/translates the tk gene. Thymidine kinase phosphorylates nucleoside analogs, such as valacyclovir (VCV). The phosphorylated VCV (VCP-P) molecules are then used by the cancer cell DNA polymerases to repair DNA strand break (caused by radiation), leading to DNA replication arrest and immunogenic cell death. This leads to increased recognition of tumor antigens by immune effector cells.
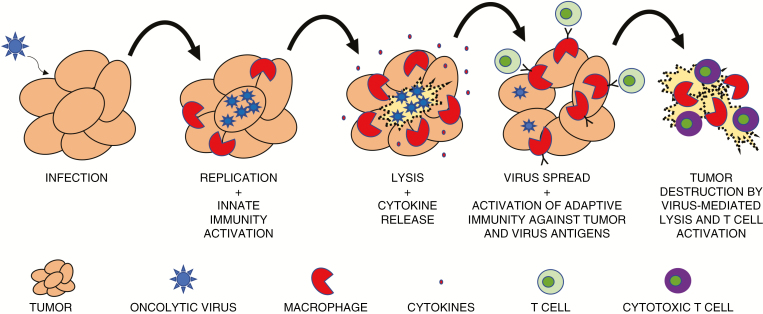
Fig. 2.
Replication-competent viruses infect tumor cells and replicate. As they lead to tumor cell cytotoxicity and spread an infection to surrounding tumor cells, innate immune cells, such as macrophages, are activated and release cytokines, inflaming the tumor microenvironment. Lysis of tumor cells is thought to improve antigen presentation and infiltration of activated effector T cells against tumor and viral antigens. This leads to durable antitumor responses.
History of Virotherapy Clinical Trials
GBM virotherapy clinical trials started in the 1990s: the first application with tremendous interest consisted of a replication-defective mouse retroviral vector to deliver the herpes simplex virus type 1 (HSV1) thymidine kinase (tk) gene that endowed infected tumor cells with novel chemosensitivity to antivirals, such as acyclovir, ganciclovir, and valacyclovir (Supplementary Table 1).25,26 The first clinical foray of this technology stereotactically implanted mouse fibroblasts that secreted these vectors into GBMs.27 Although clinical data showed that retroviral vectors generated by these fibroblasts were not effective in transducing GBMs in vivo,28 a large multicenter randomized phase III trial of this technology in newly diagnosed GBMs was pursued without evidence of efficacy.29
Two advances in vector technology were made to improve the problem of vector infection and biodistribution. The first consisted of utilizing adenoviral vectors, shown in early phase clinical trials to infect and express a gene in GBMs more effectively than the retroviral vector technology.30,31 The second consisted of maintaining the capability of the virus to replicate: by successive cycles of infection and replication, the virus biodistributed better throughout the injected tumor than replication-defective viral vectors.32 The first genetically engineered replication-selective virus was based on HSV1.33
Both advances were tested in clinical trials for GBM. Adenoviral vectors that delivered HSV1-tk were shown to promote an inflammatory response and cavitation necrosis in rGBM patients after stereotactic injection, and for the first time an actual maximum tolerated dose was established for the adenoviral vector approach.34 Adenoviral vectors were also utilized to deliver p5335 and IFN-β.36 Evidence for biologic effects in treated patients was observed in posttreatment biopsies. For replication-competent viruses, a phase I clinical trial showed that stereotactic injection of an engineered HSV1 (G207) in rGBM patients was well tolerated and showed viral DNA in post-injected tumors and changes consistent with viral-induced tumor necrosis and inflammation.37 In fact, 4/21 patients were alive an average of 12.8 months from injection and 1 showed an impressive radiographic response. The overall median survival in this trial was 15.9 months for 13 GBM patients and 40.5 months for the 4 AA patients. A different type of replication-competent HSV1 (1716) was tested in a phase I trial in 9 rGBM patients. The authors reported that 4 patients were alive 24, 19, 17, and 14 months after injection.38 A replication-competent adenovirus (ONYX-015) was tested by peritumoral injection in rGBM patients in a phase I multi-institutional study.39 In this study, there were durable responses in 3/24 patients at the time of last published follow-up (about 27 mo after injection): 3 of these “responders” were diagnosed as AA (N = 2) or AO (n = 1). Two AA patients remain alive and tumor free more than 17 years after undergoing treatment (Stephen B. Tatter, MD, Wake Forest, personal communication). MRIs showed tumor regression in some and posttreatment samples showing extensive lymphoid and plasma cell infiltrates in injected tumors. Therefore, most phase I clinical trials performed before 2010 with different types of replication-competent viruses or with replication-defective adenoviral vectors showed a subset of recurrent MG patients with a response (measured by MRI, by posttreatment sampling, and/or by survival responses) that seemed atypical compared with those of the general rGBM population.
Durability of Responses in Recent Virotherapy Clinical Trials
Replication-Defective Adenoviral Vectors
Moolten’s original approach of gene delivery of HSV1-tk40 to endow tumor cell chemosensitivity continues to be pursued using replication-defective adenoviral vectors (Table 1, Fig. 1). The approach was utilized upfront, coupled with standard of care surgical resection and chemoradiotherapy.41 Importantly, there was a one-week period of concurrent radiation and valacyclovir in treated patients, to take advantage of the synergy between radiation-induced DNA damage and the tk-mediated phosphorylated valacyclovir, acting as a “false” nucleotide that halted the DNA damage repair process. Further, there was an immunotherapy effect related to the expression of tk, acting as a “super-antigen,” and to the immunogenic cell death caused by the gene therapy.42 In a phase I41 and multi-institutional, matched-cohort phase II trial,43 there was evidence of a survival effect. In subjects with a gross total resection and treated with peritumoral injection of the gene therapy (n = 18) the median overall survival was 25 months compared with 16.9 months for a prospectively collected matched cohort group (n = 44). One-, 2-, and 3-year survivals of 90%, 53%, and 32% compared with 64%, 28%, and 6%, respectively (hazard ratio = 0.5; range, 0.29–0.86). Therefore, the approach of replication-defective adenoviral vectors to deliver HSV1-tk and endow upfront GBMs with valacyclovir chemosensitivity to synergize with radiation shows a subset of patients who exhibit a durable long-term response.
Table 1.
Recent GBM virotherapy trials (2010–present)
| Virus | IndicationA | Administration Route | Number of Patients | Survivors at 24 Months | Survivors at 36 Months | Favorable Variables in Survivors |
|---|---|---|---|---|---|---|
| Replication-defective adenovirus expressing tk41,43 | Newly diagnosed AA or GBM (phase II) | PeritumoralB | 48 | 17c | 9C | Gross total resection |
| Replication-defective adenovirus expressing tk (ASPECT)44 |
Newly diagnosed AA or GBM (phase III) | PeritumoralB | 119 | 30D | 6 | NAE |
| Replication-competent HSV1 (G207)45 | rGBM | IntratumoralF followed by resection and peritumoralB re-injection | 6 | 0 | 0 | NAE |
| Replication-competent HSV1 (G207) with radiation47 | rGBMJ | IntratumoralF followed 24 hours later by a single 5 Gy dose of radiation | 9 | 0 | 0 | NAE |
| Replication-competent retrovirus expressing CD (Toca511)54,55 | rGBMK | IntratumoralF followed by resection and peritumoralB re-injection | 56 | 12 | 5 | Neural transcriptome profile, low mutation burden, age less than 45, smaller tumors |
| Replication-competent reovirus56 | rGBML | IntratumoralF | 15 | 2G | 0 | NAE |
| Replication-competent parvovirus (ParvOryx01)58 | rGBM | IntratumoralF or intravenous followed by resection and peritumoralB re-injection | 18 | 3 | 1 | Small tumors less than 300 mm2 (n = 3), KPS = 100 (n = 3) |
| Replication-competent adenovirus (DNX-2401)61 | rGBMM | Arm A: IntratumoralF Arm B: IntratumoralF followed by resection and peritumoralB re-injection |
Arm A: 25 Arm B: 12 |
Arm A: 5 Arm B: 2 |
Arm A: 5 Arm B: 0 |
IDHmut (n = 1) |
| Replication-competent poliovirus (PVSRIPO)1 | rGBM | IntratumoralF | 61H | 8I | 5L | Age less than 45 (n = 3), IDH1mut (n = 1); MGMT promoter methylation (n = 5) |
AUnless explicitly stated, all trials are phase 0/I. BPeritumoral indicates free-hand injection in resected tumor cavity. CThree patients from this group remain alive more than 8 years after treatment. DNot significantly different than randomized control group. E Not available. FIntratumoral is by sterotaxy and/or by CED. GBoth ultimately perished to recurrent disease. HThere were a total of 35 patients who had been treated at the 24-month landmark. ISix additional patients were alive but had been censored before reaching the 24-month landmark. JFour patients with anaplastic astrocytoma. KTen patients with anaplastic astrocytoma. LThree patients with anaplastic astrocytoma. MTwo patients with anaplastic astrocytoma.
However, these results are in contrast to a phase III randomized open-label study (ASPECT) of a similar approach (but different vector manufacturer) that did not show benefit in terms of median overall survival.44 Both arms of this trial showed a “tail” of approximately 15% survivors past 3 years. Analysis of the ASPECT trial was complicated by the uneven use of temozolomide between the groups and lack of concomitant radiation during the actual administration of gene therapy. The lag time between the gene therapy and radiotherapy would limit treatment effect (Figure 1), and this difference could be a reasonable biological explanation for the increased survivors seen in the Wheeler et al study43 compared with the ASPECT trial.
Replication-Competent Viruses: Herpes Simplex Virus Type 1
The original genetically engineered herpes simplex virus type 1 (HSV1) (G207) was tested in a phase I trial for rGBM with posttreatment evidence of inflammatory responses45 and in one patient evidence for unusual survival past 6 years.46 The addition of radiation to G207 was reported to show 3/9 radiographic responses.47 Another genetically engineered HSV1 (Imlygic, Amgen) was FDA approved for in situ injection in melanoma based on a randomized phase III trial.48,49 Imlygic stimulated cytotoxic CD8+ T-cell infiltration and upregulation of immune checkpoint signaling, providing a rationale for combining with pembrolizumab.4 There are currently other types of HSV1 in clinical trials for rGBM in the USA (M032, NCT02062827 and rQNestin34.5v.2, NCT03152318; Supplementary Table 2) and Japan (T. Todo, personal communication).
Replication-Competent Viruses: Retroviruses (Toca511)
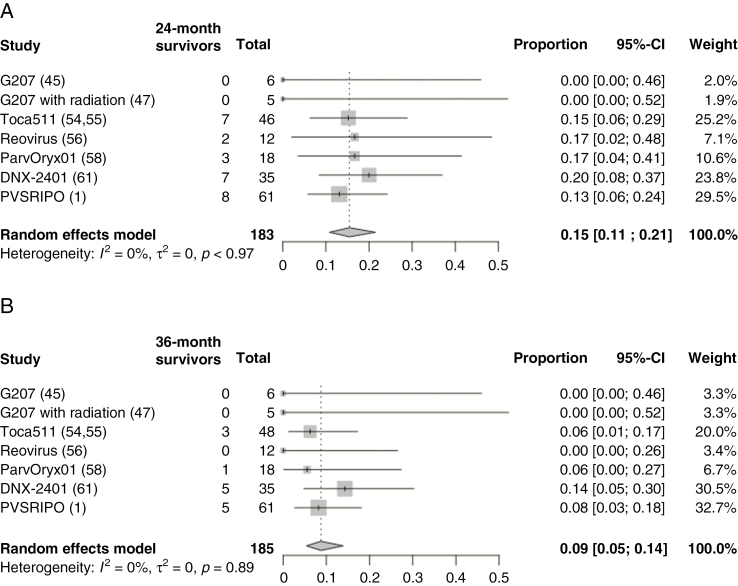
Toca511 (Tocagen) expresses the yeast cytosine deaminase gene that endows Toca511-tranduced cells with chemosensitivity to 5-fluorocytosine (5-FC), after conversion to bioactive, 5-fluorouracil.50,51 Preclinically, Toca511 efficacy was due to an immune response with focal inflammation at the site of injection.52,53 A phase I clinical trial in patients involved tumor resection of rGBM followed by peritumoral injection of Toca511 and then administration of 5-FC.54 Overall survival was 13.6 months. Importantly, 12/43 with treated MGs were alive at the 24-month landmark. Additional data were published for a total of 53 treated patients.55 Six patients (3 GBMs and 3 AAs) achieved a complete response and were alive 33.9 to 52.2 months after treatment. Two of the AAs were IDH mutated. Four patients were less than 45 years of age. Tumor burden at the time of 5-FC administration was mostly nonmeasurable, because of the previous resection. Responding patients had low genomic mutational burden in tumors compared with patients who did not respond. This contrasts with the concept that immunotherapy works best with high mutational burden cancers and thus Toca511 may break immune tolerance of less immunogenic tumors.
Replication-Competent Viruses: Reovirus
Reovirus is a small RNA virus tested for several cancers, including rGBM. In a phase I clinical trial in rGBM, biopsy-proven GBMs were infused with reovirus by CED.56 Two of 15 patients survived 2 years. In a second trial, reovirus was administered intravenously in 6 patients with high-grade glioma and 3 with brain metastases.57 Tumors were resected 3–17 days after. Samples showed low-level reovirus proteins and transcripts in tumors, suggesting blood–brain barrier passage. There were also immune cell infiltrates in tumors. No efficacy or survival data were provided. Reovirus thus seems to benefit a small subset of GBM patients, perhaps by immune-mediated mechanisms.
Replication-Competent Viruses: Parvovirus (ParvOryx01)
H-1 parvovirus is a small, non-enveloped, single stranded DNA virus whose natural host is the rat. A phase I clinical trial in 18 rGBM subjects consisted of some tumors injected stereotactically while others were treated intravenously: 10 days later, resection of the GBM was followed by peritumoral injection.58 Three of 18 patients survived 2 years or more with MRI evidence of responses. These 3 patients did not have the usual favorable variables for outcome, except for KPS = 100 and small tumors. They also reported that the virus crossed the blood–brain barrier. There were prominent immune cell infiltrates consisting of activated CD8+ and CD4+ T cells. Peripheral blood mononuclear cells (PBMCs) of 9 subjects mounted significant antiviral cell responses to parvoviral antigens. PBMCs of 3/6 patients showed reactivity against a panel of GBM antigens. Therefore, a subset of patients had a clinical response with intratumoral evidence of viral gene products and immunological evidence of peripheral reactivity against viral and tumor antigens.
Replication-Competent Viruses: Adenovirus (DNX-2401)
DNX-2401 was engineered with a 24 base pair deletion of the E1A gene, restricting viral replication to cells with a dysfunctional retinoblastoma pathway.59,60 The virus possesses an arginine/glycine/aspartic acid motif in its fiber that targets integrins, enriched in GBM, to enter cells. DNX-401 was tested in a phase I trial in a 2-arm scheme.61 Arm A consisted of 25 patients who underwent tumor biopsy followed by intratumoral injection of the agent, while arm B consisted of 12 patients whose rGBM was injected with the agent, followed 14 days later by en bloc resection to obtain posttreatment specimens and peritumoral injection of the agent. In arm A, there was tumor reduction in 18/25 patients, with 5/25 surviving more than 3 years. In arm B, 2/12 patients survived for 2 years. In arm A, 3 complete responders (GBMs, IDH wildtype) exhibited initial contrast enhancement that then progressively decreased, consistent with inflammatory pseudoprogression. A re-resected rGBM showed enhancing areas that were necrotic with inflammatory cells. A patient developed a recurrence at a second site, shown to be inflammatory. Tumors in arm B exhibited CD8+ T cells expressing T-bet and increases in CD4+ T cells. GBM cells cultured from the specimens also showed evidence of immunogenic cell death markers. Although viral proteins were seen at the 14-day timepoint, the authors report no viral proteins past 30 days, suggesting initial viral replication that then stops. This would be consistent with a subsequent longer lasting immune effect against the cancer, evoked by DNX-2401 injection.
Replication-Competent Viruses: Poliovirus (PVSRIPO)
This is engineered with a foreign (rhinovirus) ribosome entry site, ablating neurovirulence. Tropism is mediated by entry via the CD155 receptor, highly expressed in tumors.62,63 In a phase I trial, 61 patients with biopsy-proven rMG were treated with an intratumoral infusion of PVSRIPO.1 Eight of 61 patients (or 8/35 if one uses only the subgroup of patients treated for at least 24 mo) had a durable radiographic response, with survival greater than 2 years, and another 6 patients were alive but were followed for less than 24 months (censored data). The median cross-sectional area of treated tumors was 873 mm2 (a little less than 3 cm in diameter). The durable response group consisted primarily of patients whose tumors were smaller than this value. This suggests that a relatively small lesion may be an important prognostic factor for this therapy. Most of the durable response tumors possessed at least one of the variables known to favor improved survival. Only 1/8 survivors at the 24-month landmark had no favorable variable (a 59-y-old with IDH wildtype and unmethylated MGMT). Several patients were treated relatively recently (before 24 mo); several data points for this trial are still maturing and additional follow-up results are needed to fully interpret the trial.
Additional Considerations
Several virotherapy trials have progressed to later stages and are actively accruing. Several other virotherapy technologies are being tested in phase I trials (Supplementary Table 2).
Durability of Responses in Non-Virotherapy Trials for rGBM
Surgical Clinical Trials
Surgical trials have tested carmustine wafers, dendritic cell vaccinations and other immunotherapies, and thermal therapies (ie, NeuroBlate; Supplementary Tables 3–5). Notably, a phase III randomized prospective trial compared carmustine wafers (n = 183) with CED of cintredekin besudotox (n = 93), a recombinant chimeric cytotoxin composed of human interleukin-13 fused to a mutated motif of pseudomonas aeruginosa exotoxin A.64 Patients randomized to carmustine wafer underwent maximal resection followed by implantation of carmustine wafer along the surgical cavity. Those randomized to cintredekin besudotox underwent resection followed by delayed catheter placement and peritumoral infusion. Fourteen of 93 patients treated with carmustine wafers and 27/183 patients treated with cintredekin besudotox were alive 24 months after treatment, with a fair proportion remaining alive at the 36-month landmark.
Two trials have evaluated thermal therapy strategies in rGBM (Supplementary Table 5). The first was a phase I trial assessing laser interstitial therapy (NeuroBlate) in 10 patients with rGBM.65 All underwent stereotactic biopsy followed by laser therapy. Three patients survived 12 months and 1 (age, 34 y; KPS = 90) remained alive at 24 months. The second trial was a phase II trial assessing intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy.66 Fifty-nine patients underwent intratumoral injection of magnetic fluid followed by thermotherapy and adjunct stereotactic beam radiation. Nine of 59 patients survived 24 months and some extended up to 36 months. Smaller tumor volume was associated with improved survival.
IDH mutation and MGMT promoter methylation status were not reported, since these trials were completed before these markers were clinically available. This limits the modern interpretation of survivors and responders.
Nonsurgical Clinical Trials
Anti-angiogenic therapies, tyrosine kinase inhibitors, epigenetic modifiers, and other targeted inhibitors have been studied in the setting of rGBM. Many are phase II with few phase III trials. Patients enrolling in nonsurgical clinical trials may represent a population different from those entering surgical clinical trials. These trials can still be informative in assessing the rate of durable response for patients who did not get a biopsy or repeat resection at recurrence.
The rates of 24-month survival in trials assessing anti-angiogenic therapies vary from 0 to 33% (Supplementary Table 6 and Fig. 3). Most use anti-angiogenic therapies in combination with other therapies (alkylating agents or lomustine). The most noteworthy was a phase II trial of 30 rGBM patients receiving biweekly bevacizumab in combination with temozolomide.67 Ten of 30 patients were alive at 24 months. Unusually, these patients also exhibited a durable response: they were still alive at the 5-year landmark, but the overall survival curves show that none lasted past the 7-year landmark. Patients harboring MGMT promoter methylated tumors and those who had a treatment-free interval of 2 months before recurrence after initial treatment represented the subset that exhibited this positive response.
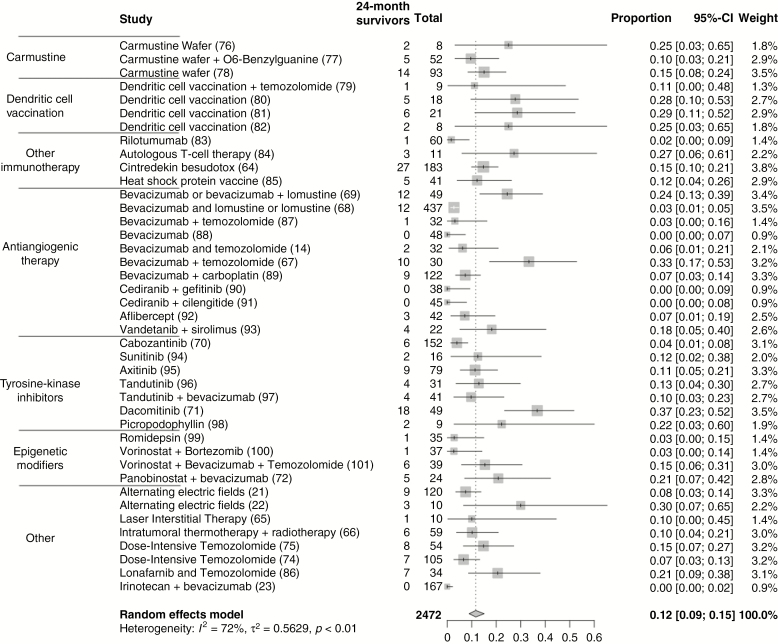
Fig. 3.
Pooled proportion of survivors in non-virotherapies trials for rGBM at (A) 24 months and (B) 36 months. Studies on non-virotherapy clinical trials reporting 24-month and 36-month overall survival for rGBM are included. Survivors represent the number of patients surviving at 24 or 36 months. Total represents the total number of rGBM patients in a trial. Given the heterogeneity of designs, pooling of proportion of patients was performed via a random effects model.
Two trials have assessed the utility of combination bevacizumab and lomustine.68,69 The larger was a phase III comparing combination bevacizumab + lomustine with lomustine alone, with 6/288 and 6/149 patients alive at 24 months in both groups, respectively. Only one patient was alive at 36 months in the bevacizumab and lomustine arm. In the phase II BELOB trial assessing the efficacy of combination bevacizumab and lomustine for rGBM, 17/148 patients remained alive at 18 months, but outcomes beyond this landmark were not reported.70
There have been a handful of studies assessing tyrosine kinase inhibitors in patients with rGBM (Supplementary Table 7). The largest was a single-arm phase II trial of cabozantinib in 152 patients.71 All patients were naive to anti-angiogenic therapy. Treatment was given orally at 2 doses. Most patients (99/152) had stable disease upon treatment. A small group of patients demonstrated partial response to treatment (23/152) and 6/152 patients were alive 24 months after treatment, with 3 patients alive at 34 months, but all deceased by 36 months. The study with the greatest proportion of patients that reached the 24-month survival landmark was reported in a phase II study using dacomitinib in patients with epidermal growth factor receptor (EGFR) amplified GBMs at first recurrence.72 Patients were divided into 2 cohorts according to EGFR variant III mutation status. Overall, 18/49 patients were alive at 24 months, with a substantial proportion remaining alive at 36 months. Other phase II studies assessing various tyrosine kinase inhibitors have also showed a similar trend of a “tail” of patients alive 24 months after treatment.
With the increasing recognition of the role of epigenetic changes in carcinogenesis, epigenetic modifiers have been trialed as antineoplastic agents in rGBM (Supplementary Table 8). The most noteworthy is a phase II trial of panobinostat, a histone deacetylase inhibitor, in combination with biweekly bevacizumab.73 Of 24 rGBM patients, 5 remained alive at the 24-month mark, with no further deaths confirmed at the 30-month landmark.
Other non-invasive, non-medical trials have been tested in the setting of rGBM (Supplementary Table 5). For example, low intensity alternating electric fields (TTFs) disrupt GBM cell division.21,22,74 One hundred and twenty patients were treated with TTF in a phase III trial. Median duration of follow-up was 39 months, 9/120 patients were alive at 24 months, and 7/120 patients were alive at 30 months, with no further confirmed deaths beyond this point (censored data). Rechallenging with dose-intensive temozolomide for rGBM has been studied in 2 trials.75,76 The larger (n = 105) demonstrated a 15% proportion of 24-month survivors and 9% proportion of 36-month survivors (DIRECTOR trial). Factors associated with favorable survival were MGMT methylation and complete resection of contrast enhancing disease.75
Summary of Landscape of Durable Responders in Virotherapy and Non-Virotherapy Clinical Trials
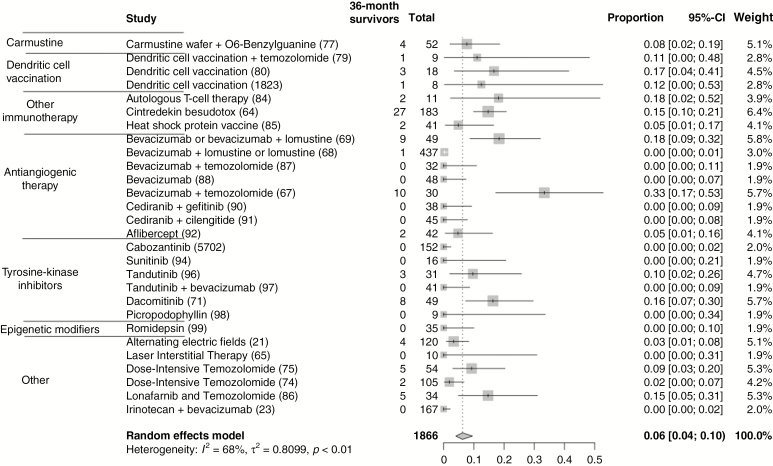
The rates of 24-month survival in non-virotherapy trials for rGBM vary from 0 to 37%. A pooled analysis of 2472 patients suggests that the overall proportion of 24-month survival is 12% (95% CI: 9–15%) and 36-month survival is 6% (95% CI: 4–10%; Figure 3). A pooled analysis of recent virotherapy trials for rGBM suggests that the overall proportion of 24-month survival is 15% (95% CI: 11–21%) and of 36-month survival, 9% (95% CI: 5–14%; Figure 4). The considerable heterogeneity in trial designs and populations precludes a pooled comparison of different treatment modalities. This analysis does highlight the existence of a subgroup of durable responders to various treatments for rGBM. Reporting of IDH mutation and MGMT status has not been consistent, emphasizing a need for a harmonized approach to facilitate pooled analyses across clinical trials. In studies that report these factors, most durable responders possess the classically favorable variables: mutation in IDH1/2, MGMT promoter methylation, young age, and good performance status. For the latest virotherapy trials, it does seem that tumor size may also be an important factor in responses. Other issues can introduce significant bias in interpreting treatment effects with early phase surgical and medical trials. These include lack of blinded randomization, perioperative steroid use, and absence of central confirmation of eligibility and treatment effects. Ultimately, discovering a scientific surrogate that correlates with an observed treatment effect could confirm therapeutic validity and provide an important advance.
Fig. 4.
Pooled proportion of survivors in recent virotherapies trials for rGBM at (A) 24 months and (B) 36 months. Studies on virotherapy clinical trials reporting 24-month and 36-month overall survival for rGBM are included. Survivors represent the number of patients surviving at 24 or 36 months. Total represents the total number of rGBM patients in a trial. Given the heterogeneity of designs, pooling of proportion of patients was performed via a random effects model.
Conclusions
The field eagerly awaits the ultimate test of virotherapy’s role as a suitable agent in rGBM in a prospective randomized trial. There were 123 patients with rGBMs that were treated with a biopsy alone followed by injection of a viral agent throughout the last two decades of clinical trials (Supplementary Table 1 and Table 1) and 19 lived for at least 24 months. Several, but not all, early phase clinical trials using a variety of modalities include a “tail” of long-term responders, the large majority of whom had tumors known to possess relatively favorable biologic and/or demographic characteristics. This implies that the design of late phase randomized trials should restrict eligibility to these subpopulations with seemingly favorable biologic and/or demographic characteristics to determine if the treatment is truly effective.
It is also evident that most rGBM patients possess one or more “unfavorable” demographic, radiologic, and/or biologic features, such as older age, poor performance, large tumors, IDH wildtype, unmethylated MGMT promoter, immunosuppression, concomitant morbidities, multifocal disease, and multiple prior treatment failures. These patients have not shown favorable efficacy responses in early phase clinical trials of any modality. These are the patients for whom the field of neuro-oncology still seeks the beginnings of a hint of what the ultimate curative treatment may look like. This will require more scientific progress in dissecting the architecture and evolution of the complexity of these tumors in the context of their genetics, epigenetics, and immunology.
Funding
E.A.C. acknowledges research support from the National Institutes of Health under awards numbers 2P01CA163205, CA069246-20, and P50CA165962. E.A.C. acknowledges research support from Amgen, Inc. P.P.P. acknowledges research support from NIH K08NS101091. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement.
E.A.C. is currently an advisor to Advantagene Inc. and DNAtrix Inc. and has equity interest in DNAtrix; he has previously advised Oncorus, Merck, Tocagen, StemCell, NanoTx., Ziopharm Oncology, and Alcyone Biosciences; he also is a named inventor on patents related to oncolytic HSV1. F.N., J.W., P.P.P., and G.Z. report no conflicts.
Supplementary Material
References
- 1. Desjardins A, Gromeier M, Herndon JE 2nd, et al. . Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017;3(6):841–849. [DOI] [PubMed] [Google Scholar]
- 3. Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ribas A, Dummer R, Puzanov I, et al. . Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 6. Yan H, Parsons DW, Jin G, et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 8. Cairncross JG, Ueki K, Zlatescu MC, et al. . Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 9. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. . Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burger PC, Green SB. Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer. 1987;59(9):1617–1625. [DOI] [PubMed] [Google Scholar]
- 11. Nitta T, Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer. 1995;75(11):2727–2731. [DOI] [PubMed] [Google Scholar]
- 12. Johnson DR, Wefel JS. Relationship between cognitive function and prognosis in glioblastoma. CNS Oncol. 2013;2(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2049. [DOI] [PubMed] [Google Scholar]
- 14. Desjardins A, Reardon DA, Coan A, et al. . Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–1312. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2048–2049. [DOI] [PubMed] [Google Scholar]
- 16. Haines IE, Gabor Miklos GL. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2048. [DOI] [PubMed] [Google Scholar]
- 17. Stupp R, Weller M. Questions regarding the optimal use of bevacizumab in glioblastoma: a moving target. Neuro Oncol. 2014;16(6):765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. . Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 19. Wen PY, Junck L. Bevacizumab for glioblastoma: what can we learn from patterns of progression?Neurology. 2014;82(19):1670–1671. [DOI] [PubMed] [Google Scholar]
- 20. Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN; AANS/CNS Joint Guidelines Committee The role of cytotoxic chemotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):501–555. [DOI] [PubMed] [Google Scholar]
- 21. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stupp R, Wong ET, Kanner AA, et al. . NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 23. Friedman HS, Prados MD, Wen PY, et al. . Bevacizumab alone and in combination with irinotecan in rGBM. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 24. Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN, Ryken TC; AANS/CNS Joint Guidelines Committee The role of targeted therapies in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):557–599. [DOI] [PubMed] [Google Scholar]
- 25. Ezzeddine ZD, Martuza RL, Platika D, et al. . Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol. 1991;3(6):608–614. [PubMed] [Google Scholar]
- 26. Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–1552. [DOI] [PubMed] [Google Scholar]
- 27. Short MP, Choi BC, Lee JK, Malick A, Breakefield XO, Martuza RL. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990;27(3):427–439. [DOI] [PubMed] [Google Scholar]
- 28. Harsh GR, Deisboeck TS, Louis DN, et al. . Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: a gene-marking and neuropathological study. J Neurosurg. 2000;92(5):804–811. [DOI] [PubMed] [Google Scholar]
- 29. Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. [DOI] [PubMed] [Google Scholar]
- 30. Puumalainen AM, Vapalahti M, Agrawal RS, et al. . Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9(12):1769–1774. [DOI] [PubMed] [Google Scholar]
- 31. Sandmair AM, Loimas S, Puranen P, et al. . Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11(16):2197–2205. [DOI] [PubMed] [Google Scholar]
- 32. Boviatsis EJ, Scharf JM, Chase M, et al. . Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1(5):323–331. [PubMed] [Google Scholar]
- 33. Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. [DOI] [PubMed] [Google Scholar]
- 34. Trask TW, Trask RP, Aguilar-Cordova E, et al. . Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. [DOI] [PubMed] [Google Scholar]
- 35. Lang FF, Bruner JM, Fuller GN, et al. . Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21(13):2508–2518. [DOI] [PubMed] [Google Scholar]
- 36. Chiocca EA, Smith KM, McKinney B, et al. . A phase I trial of Ad.hIFN-β gene therapy for glioma. Mol Ther. 2008;16(3):618–626. [DOI] [PubMed] [Google Scholar]
- 37. Markert JM, Medlock MD, Rabkin SD, et al. . Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. [DOI] [PubMed] [Google Scholar]
- 38. Rampling R, Cruickshank G, Papanastassiou V, et al. . Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. [DOI] [PubMed] [Google Scholar]
- 39. Chiocca EA, Abbed KM, Tatter S, et al. . A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. [DOI] [PubMed] [Google Scholar]
- 40. Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46(10):5276–5281. [PubMed] [Google Scholar]
- 41. Chiocca EA, Aguilar LK, Bell SD, et al. . Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29(27):3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112(8):1969–1977. [DOI] [PubMed] [Google Scholar]
- 43. Wheeler LA, Manzanera AG, Bell SD, et al. . Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18(8):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Westphal M, Ylä-Herttuala S, Martin J, et al. ; ASPECT Study Group Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. [DOI] [PubMed] [Google Scholar]
- 45. Markert JM, Liechty PG, Wang W, et al. . Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whisenhunt TR Jr, Rajneesh KF, Hackney JR, Markert JM. Extended disease-free interval of 6 years in a recurrent glioblastoma multiforme patient treated with G207 oncolytic viral therapy. Oncolytic Virother. 2015;4:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Markert JM, Razdan SN, Kuo HC, et al. . A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014;22(5):1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andtbacka RH, Kaufman HL, Collichio F, et al. . Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 49. Lawler SE, Chiocca EA. Oncolytic virus-mediated immunotherapy: a combinatorial approach for cancer treatment. J Clin Oncol. 2015;33(25):2812–2814. [DOI] [PubMed] [Google Scholar]
- 50. Ostertag D, Amundson KK, Lopez Espinoza F, et al. . Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12(5):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hiraoka K, Inagaki A, Kato Y, et al. . Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro Oncol. 2017;19(7):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mitchell LA, Lopez Espinoza F, Mendoza D, et al. . Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19(7):930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cloughesy TF, Landolfi J, Hogan DJ, et al. . Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cloughesy TF, Landolfi J, Vogelbaum MA, et al. . Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018;20(10):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kicielinski KP, Chiocca EA, Yu JS, Gill GM, Coffey M, Markert JM. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther. 2014;22(5):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Samson A, Scott KJ, Taggart D, et al. . Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med. 2018;10(422). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Geletneky K, Hajda J, Angelova AL, et al. . Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma Trial. Mol Ther. 2017;25(12):2620–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fueyo J, Alemany R, Gomez-Manzano C, et al. . Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. [DOI] [PubMed] [Google Scholar]
- 60. Fueyo J, Gomez-Manzano C, Alemany R, et al. . A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. [DOI] [PubMed] [Google Scholar]
- 61. Lang FF, Conrad C, Gomez-Manzano C, et al. . Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A. 1996;93(6):2370–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97(12):6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kunwar S, Chang S, Westphal M, et al. ; PRECISE Study Group Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. . Results of the NeuroBlate System first-in-humans phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202–1219. [DOI] [PubMed] [Google Scholar]
- 66. Maier-Hauff K, Ulrich F, Nestler D, et al. . Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Badruddoja MA, Pazzi M, Sanan A, et al. . Phase II study of bi-weekly temozolomide plus bevacizumab for adult patients with recurrent glioblastoma. Cancer Chemother Pharmacol. 2017;80(4):715–721. [DOI] [PubMed] [Google Scholar]
- 68. Wick W, Gorlia T, Bendszus M, et al. . Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 69. Weathers SP, Han X, Liu DD, et al. . A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neurooncol. 2016;129(3):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Taal W, Oosterkamp HM, Walenkamp AM, et al. . Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 71. Wen PY, Drappatz J, de Groot J, et al. . Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018;20(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sepúlveda-Sánchez JM, Vaz MÁ, Balañá C, et al. . Phase II trial of dacomitinib, a pan-human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro Oncol. 2017;19(11):1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee EQ, Reardon DA, Schiff D, et al. . Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015;17(6):862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kirson ED, Dbaly V, Tovarys F, et al. . Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104(24):10152–10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Suchorska B, Weller M, Tabatabai G, et al. . Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18(4):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Norden AD, Lesser GJ, Drappatz J, et al. . Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15(7):930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aoki T, Nishikawa R, Sugiyama K, et al. ; NPC-08 study group A multicenter phase I/II study of the BCNU implant (Gliadel(®) Wafer) for Japanese patients with malignant gliomas. Neurol Med Chir (Tokyo). 2014;54(4):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Quinn JA, Jiang SX, Carter J, et al. . Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15(3):1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Westphal M, Hilt DC, Bortey E, et al. . A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hunn MK, Bauer E, Wood CE, et al. . Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme. J Neurooncol. 2015;121(2):319–329. [DOI] [PubMed] [Google Scholar]
- 81. Yamanaka R, Homma J, Yajima N, et al. . Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11(11):4160–4167. [DOI] [PubMed] [Google Scholar]
- 82. Wheeler CJ, Black KL, Liu G, et al. . Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68(14):5955–5964. [DOI] [PubMed] [Google Scholar]
- 83. Prins RM, Soto H, Konkankit V, et al. . Gene expression profile correlates with T cell infiltration and survival in GBM patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wen PY, Schiff D, Cloughesy TF, et al. . A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13(4):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schuessler A, Smith C, Beagley L, et al. . Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74(13):3466–3476. [DOI] [PubMed] [Google Scholar]
- 86. Bloch O, Crane CA, Fuks Y, et al. . Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16(2):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yust-Katz S, Liu D, Yuan Y, et al. . Phase 1/1b study of lonafarnib and temozolomide in patients with recurrent or temozolomide refractory glioblastoma. Cancer. 2013;119(15):2747–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sepúlveda JM, Belda-Iniesta C, Gil-Gil M, et al. . A phase II study of feasibility and toxicity of bevacizumab in combination with temozolomide in patients with recurrent glioblastoma. Clin Transl Oncol. 2015;17(9):743–750. [DOI] [PubMed] [Google Scholar]
- 89. Hovey EJ, Field KM, Rosenthal MA, et al. . Continuing or ceasing bevacizumab beyond progression in rGBM: an exploratory randomized phase II trial. Neurooncol Pract. 2017;4(3):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Field KM, Simes J, Nowak AK, et al. ; CABARET/COGNO investigators Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brown N, McBain C, Nash S, et al. . Multi-center randomized phase ii study comparing cediranib plus gefitinib with cediranib plus placebo in subjects with recurrent/progressive glioblastoma. PLoS One. 2016;11(5):e0156369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gerstner ER, Ye X, Duda DG, et al. . A phase I study of cediranib in combination with cilengitide in patients with recurrent glioblastoma. Neuro Oncol. 2015;17(10):1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Groot JF, Lamborn KR, Chang SM, et al. . Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29(19):2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chheda MG, Wen PY, Hochberg FH, et al. . Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study. J Neurooncol. 2015;121(3):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pan E, Yu D, Yue B, et al. . A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J Neurooncol. 2012;110(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duerinck J, Du Four S, Bouttens F, et al. . Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol. 2018;136(1):115–125. [DOI] [PubMed] [Google Scholar]
- 97. Batchelor TT, Gerstner ER, Ye X, et al. . Feasibility, phase I, and phase II studies of tandutinib, an oral platelet-derived growth factor receptor-β tyrosine kinase inhibitor, in patients with recurrent glioblastoma. Neuro Oncol. 2017;19(4):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Odia Y, Sul J, Shih JH, et al. . A Phase II trial of tandutinib (MLN 518) in combination with bevacizumab for patients with recurrent glioblastoma. CNS Oncol. 2016;5(2):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Aiken R, Axelson M, Harmenberg J, Klockare M, Larsson O, Wassberg C. Phase I clinical trial of AXL1717 for treatment of relapsed malignant astrocytomas: analysis of dose and response. Oncotarget. 2017;8(46):81501–81510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Iwamoto FM, Lamborn KR, Kuhn JG, et al. . A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro Oncol. 2011;13(5):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Friday BB, Anderson SK, Buckner J, et al. . Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a North Central cancer treatment group study. Neuro Oncol. 2012;14(2):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peters KB, Lipp ES, Miller E, et al. . Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol. 2018;137(2):349–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.