Abstract
Metabolic syndrome (MS) is a cluster of metabolic abnormalities. Obesity and MS are always accompanied by elevated oxidative stress which might affect cellular bio-molecules such as DNA. The aim of the present study is to investigate DNA damage profile in obese premenopausal women and its relation to the risk of MS, polycystic ovary syndrome (PCOS) and history of recurrent pre-eclampsia. The study included 90 obese women included cases with MS (n = 30), PCOS (n = 30) and previous history of recurrent preeclampsia (n = 30) and, age-matched healthy non-obese control women (n = 50). The assessment of leukocyte DNA damage was done by comet assay for all cases and controls. Anthropometry and biochemical parameters have been measured. Results showed that mean percent of DNA damage was significantly higher in MS, PCOS as well as in women with the recurrent preeclampsia as compared to healthy controls. The high level of mean DNA damage frequency in obese women was significantly associated with the increased number of metabolic syndrome components. Cases with 2, 3 and 3–5 components showed significantly higher levels of DNA damage than controls. Moreover, cases with 3–5 MS components showed significant higher DNA compared to those with the two components. Regarding PCOS, significant positive association between the mean frequency of DNA damage and waist circumference was observed. The study suggests that metabolic abnormalities, PCOS and recurrent pre-eclampsia might be contributed in development of DNA damage in obese women. DNA damage can serve as an early marker for obesity complications in premenopausal women.
Keywords: DNA damage, Metabolic components, Obesity, PCOS, Recurrent preeclampsia
Introduction
MS and obesity are major health problems worldwide.1 Metabolic syndrome (MS) is a complex disorder which leads to cardiovascular disease (CVD) and type 2 diabetes (T2D).2 Central obesity and insulin resistance are the main feature of MS that ultimately cause high glucose and triglyceride levels, in addition to hypertension. Although the etiopathology of MS is still not completely determined, a combination of factors might lead to such disorder. Environmental, lifestyle and most probably genetic susceptibility factors are likely involved in the development of MS. Patients with MS are subjected to cardio-vascular diseases and cancer.3, 4 An important link seems to present between obesity and the development of PCOS, where the low-grade inflammation that concomitant to obesity most likely contributes to the pathogenesis and development of PCOS.5, 6 There is an association between oxidative stress and inflammation. The link between oxidative stress (OS) and inflammation is so strong that it is not easy to differentiate between them and both are accompanied by increased DNA damage.7 Obesity and MS patients are subjected to genetic damage.8, 9 Significant associations between BMI, WC and increased levels of oxidative DNA damage have also been reported.10 Moreover, genomic instability has been found to be associated with high BMI and insulin resistance in PCOS.11 Pre-eclampsia is a complex multi-systemic disorder that causes maternal and fetal morbidity and mortality. High oxidative stress has been noticed in Patients with pre-eclampsia.12 Consequently, oxygen free radicals that created by oxidative stress are causing several types of DNA damage.13 The imbalance status between oxidant and antioxidant could badly affect DNA and other cellular biomolecules which in turn induce diseases such as diabetes mellitus and cardiovascular disease.14
There are no previous studies, to the far of the authors' knowledge, examined the association between the DNA damage and number of metabolic components along with PCOS and recurrent preeclampsia in obese women. Therefore, the present investigation aimed to assess the DNA damage among Egyptian obese women and examine its relation with metabolic syndrome components and risk of PCOS, in addition to its relation with recurrent pre-eclampsia.
Materials and methods
This study was conducted at the National Research Centre, Egypt; Medical Research Centre of Excellence. Subjects of the study include 90 obese women divided as 30 MS, 30 PCOS and 30 with history of recurrent pre-eclampsia, in addition to 50 healthy non-obese women as a control group. All subjects aged between 25 and 35 years. The study was approved by the Ethical Committee of National Research Centre, Egypt (number = 16361), in accordance with the World Medical Association's Declaration of Helsinki. A written informed consent was obtained from each participant. Exclusion criteria include, subjects with a history of any systemic chronic disease or viral infection within the last 2 months. Subjects that received antibiotics, systemic steroids and mineral or vitamin supplements during the past 2 months were also excluded. Rotterdam consensus criteria were used to confirm the diagnosis of PCOS.15
Whereas the criteria used to diagnose the metabolic syndrome were modified from those of the National Cholesterol Education Program's (NCEP) Adult Treatment Panel III (ATP III).16 According to NCEP ATP III, the components of the metabolic syndrome are: 1) hypertension, defined as antihypertensive treatment and/or elevated blood pressure (>160 mmHg systolic or > 90 mmHg diastolic); 2) elevated plasma triglyceride ≥1.7 mmol/l, and/or low HDL cholesterol < 1.0 mmol/l concentrations; 3) WHR ratio > 0.85; 4) high fasting blood glucose ≥ 110 mg/dL. Women have the metabolic syndrome if two of these criteria are fulfilled in addition to being obese (BMI ≥ 30 (kg/m2).17
Anthropometric measurements
Anthropometric variables including height, weight, waist and hip circumference have been measured. Details on the anthropometric measurements have been reported on.18 BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Obesity is defined as a BMI of ≥30.0 kg/m2. The waist hip ratio (WHR) was calculated as WC divided by HC. All Standardized equipment have been used in anthropometric measurements and the recommendations of the International Biological Program19 have been applied.
Blood pressure (BP) was measured with the patients sitting with their left arm at heart level using a professional Riester sphygmomanometer manufactured in Japan. Average BP measurement was obtained from several measurements.
Biochemical analysis
Fasting plasma glucose and serum lipids (total cholesterol, HDL-C and triglycerides) were measured by enzymatic colorimetric methods using a Hitachi auto analyzer 704 (Roche Diagnostics Switzerland). Low density lipoprotein cholesterol (LDL-C) was calculated according to certain equation (LDL-C = Total cholesterol – Triglycerides/5+HDL-C).
Determination of DNA damage by comet assay
Cell preparation
Peripheral blood leukocytes were isolated by centrifugation (30 min at 1300 g) in Ficoll–Paque density gradient (Pharmacia LKB Biotechnology, Piscataway, NJ, USA). After centrifugation, leukocytes in the buffy coat; were aspirated and washed twice by phosphate-buffered saline at pH 7.4 (PBS).
Preparation of cell microgels on slides
The comet assay was performed based on Singh et al20 with modifications according to (Blasiak et al, 2003). Cell microgels were prepared as layers. The first layer of gel was made by applying 100 μl of normal melting point agarose (0.7%) onto a pre-cleaned microscope charged slides and cover slipped gently. The coverslip was removed after the agarose solidified at 4°C. Low melting-point agarose (0.5%) was prepared in 100 mmol/L PBS and kept at 37°C. Mononuclear cells were mixed with the low melting-point agarose and 100 μl of the mixture was applied to the first gel layer. The slides were then covered with a coverslip and placed at 4°C for solidification. After the second layer solidified, the coverslips were removed from the cell microgels. A final layer of low-melting agarose was added followed by coverslips, left to solidify for 10 min then the coverslips were removed.
Lysis of cells, DNA unwinding, gel electrophoresis, DNA staining were done.
The slides were covered with 100 ml of fresh lysis buffer at pH 10 at 4°C for 1 h. Buffer contains; 2.5 mol/L NaCl, 100 mmol/L EDTA, 1% sodium hydroxide, 10 mmol/L Tris, 1% Triton X-100 and 10% (Dimethylsulfoxide) DMSO. After draining, microgels slides were treated with DNA unwinding solution (300 mmol/L NaOH, 1 mmol/L EDTA and pH 13) for 30 min at 4°C, and placed directly into a horizontal gel electrophoresis chamber filled with DNA-unwinding solution. Gels were run with constant current (300 mA at 4°C) for 30 min. After electrophoresis, the microgels were neutralized with 0.4 M Trisma base at pH 7.5 for 10 min. The slides were stained with 20 μl ethidium bromide (10 μg/ml).
Visualization and analysis of comet slides
The slides were examined at 400 × magnification using a fluorescence microscope (Leica Microsystems, CMS GM b H, Wetzlar, Germany. Model DM 2500), equipped with an excitation filter of 549 nm and a barrier filter of 590 nm. A damaged cell is visualized as each cell had the appearance of a comet, with a brightly fluorescent head and a tail to one side formed by the DNA containing strand breaks that were drawn away.
In order to evaluate the degree of damage, comet images were scored visually. Slides were duplicated for each case. Fifty cells per slide were examined, and 100 cells were examined per case. We performed comet assays on blood samples from all obese women and healthy controls.
Statistical analysis
The Kruskal–Wallis test was used to assess the differences the differences in DNA damage among MS, PCOS, pre-eclampsia and control groups as well as among metabolic components groups (2, 3, 4–5) and controls. Mann–Whitney U test has been used to compare comet assay results between the two groups. While student t-test was applied to the anthropometric and biochemical parameters and ANOVA was used for between-group comparisons of continuous variables. Regression linear analysis was performed to evaluate the association between DNA damage frequency and waist circumference, after adjusting for age and BMI. p values less than 0.05 were considered to be statistically significant for each test. Statistical analyses have been done using the Statistical Package for Social Sciences (SPSS version 17).
Results
Table 1 shows anthropometric and biochemical parameters in MS, PCOS, recurrent preeclampsia and controls. The results of the present study have revealed the presence of significantly elevated BMI, waist circumference, waist to hip ratio and systolic/diastolic blood pressure levels in women with MS as well as with PCOS and preeclampsia as compared to controls. The mean BMI was ranged from (31.4 ± 4.4) to (29.6 ± 4.2) in cases while healthy controls had much lesser value (23.6 ± 4.4). Similar trend was observed for waist circumference, waist to hip ratio and systolic/diastolic blood pressure levels. Regarding the biochemical parameters, total cholesterol, LDL-C and triglycerides and fasting glucose were significantly higher in MS, PCOS and preeclampsia than controls, while HDL-C was significantly lower.
Table 1.
Anthropometric and biochemical measurements in MS, PCOS, recurrent preeclampsia and controls.
| MS (n = 30) | PCOS (n = 30) | Recurrent preeclampsia (n = 30) | Controls (n = 50) | P ANOVA | |
|---|---|---|---|---|---|
| Age (year) | 26.8 ± 3.4 | 28.8 ± 3.3 | 27.7 ± 4.5 | 27.7 ± 4.4 | 0.06 |
| BMI(kg/m2) | 31.4 ± 4.3* | 31.4 ± 4.4* | 29.6 ± 4.2* | 23.6 ± 4.4 | 0.02 |
| WC (cm) | 99.9 ± 8.9* | 99.9 ± 6.9** | 91.4 ± 7.6* | 80.4 ± 5.6 | 0.01 |
| WHR | 0.97 ± 0.01** | 0.92 ± 0.02* | 0.90 ± 0.04* | 0.71 ± 0.02 | 0.02 |
| SBP (mmHg) | 133.2 ± 10.5* | 133.2 ± 10.5* | 135.0 ± 11.1* | 115.0 ± 11.1 | 0.02 |
| DBP (mmHg) | 85.0 ± 9.8* | 85.0 ± 8.8* | 82.6 ± 7.8* | 72.6 ± 7.7 | 0.01 |
| Glucose (mg/dl) | 151.9 ± 18.8* | 153.9 ± 15.8* | 149.5 ± 13.3* | 87.5 ± 10.3 | 0.01 |
| Total cholesterol (mg/dl) | 249.5 ± 31.8* | 249.5 ± 30.9* | 219 ± 35.3* | 150 ± 19.3 | 0.01 |
| TG (mg/dl) | 159.2 ± 25.6* | 159.2 ± 23.6* | 135.6 ± 25.1* | 101.6 ± 15.1 | 0.03 |
| HDL-C (mg/dl) | 44.9 ± 13.3* | 47.9 ± 12.2* | 43.4 ± 12.6* | 63.4 ± 11.6 | 0.01 |
| LDL-C (mg/dl) | 159.1 ± 21.9* | 157.9 ± 20.8* | 155.0 ± 19.9* | 125.0 ± 17.8 | 0.01 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; WHR: waist to hip ratio; TG: triglycerides; TC: HDL−C: high density lipoprotein cholesterol; LDL−C: low density lipoprotein cholesterol. *p<0.05, **p<0.001 vs. controls.
Table 2 shows DNA damage as evaluated by the comet assay. The results showed significantly elevated genetic damage in obese women according to their clinical diagnosis. The mean percent of damage was significantly higher in MS, PCOS and preeclampsia cases as compared to controls. Kruskal–Wallis test revealed significant differences between the studied groups (p = 0.002), the mean of DNA damage was much higher among PCOS women followed by preeclampsia and then MS. Moreover, Mann–Whitney U-test showed significant higher values of mean of DNA damage in each of MS, PCOS and recurrent preeclampsia patients as compared to controls. No significant difference was observed between the cases groups.
Table 2.
Mean DNA damage in patients and controls.
| Group | Damage frequency Mean ± SE | p valuea (Kruskal–Wallis test) |
|---|---|---|
| MS (n = 30) | 32.55 ± 3.61* | 0.002 |
| PCOS (n = 30) | 34.89 ± 3.68** | |
| Recurrent preeclampsia (n = 30) | 32.95 ± 3.16* | |
| Controls (n = 50) | 13.81 ± 1.99 |
Mann–Whitney U-test, *p value < 0.05, **p < 0.01 vs. controls.
Among MS, PCOS, recurrent preeclampsia and controls.
Table 3 shows mean of DNA damage in relation to the presence of number of metabolic components in overall patients and controls. Mean DNA damage was found to differ significantly between metabolic components abnormalities groups and controls (p < 0.01). Cases with 4–5 components had significant higher levels of DNA damage (35.3 ± 3.7) than those with 2 components (29.3 ± 2.2). Moreover, Kruskal–Wallis test showed significant difference between the studied groups with the highest values among cases with 4–5 components and the lowest among controls.
Table 3.
Mean DNA damage according to number of components in obese women and controls.
| Group | Damage frequency Mean ± SE | p valuea (Kruskal–Wallis test) |
|---|---|---|
| Controls (N = 50) | 14.95 ± 2.0 | 0.001 |
| MS components groups | ||
| 2 (N = 40) | 29.3 ± 2.2* | |
| 3 (N = 30) | 30.3 ± 3.3* | |
| 4–5 (N = 20) | 35.3 ± 3.7**,# |
Mann–Whitney U-test, *p value < 0.05, **p < 0.01 vs. controls, #p < 0.01 vs. two metabolic components.
Among metabolic components groups (2, 3, 4–5) and controls.
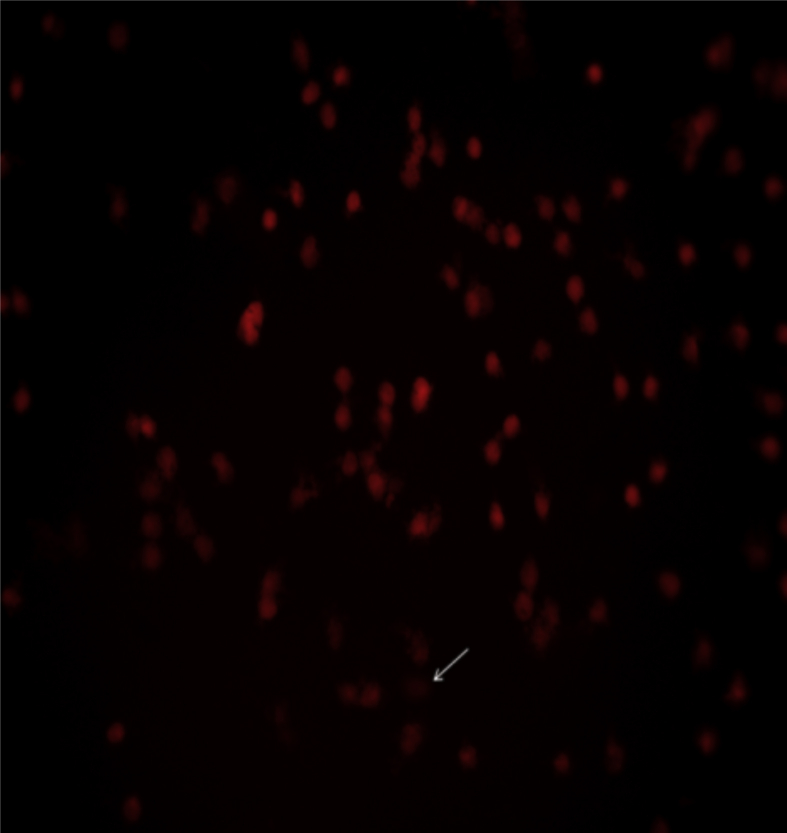
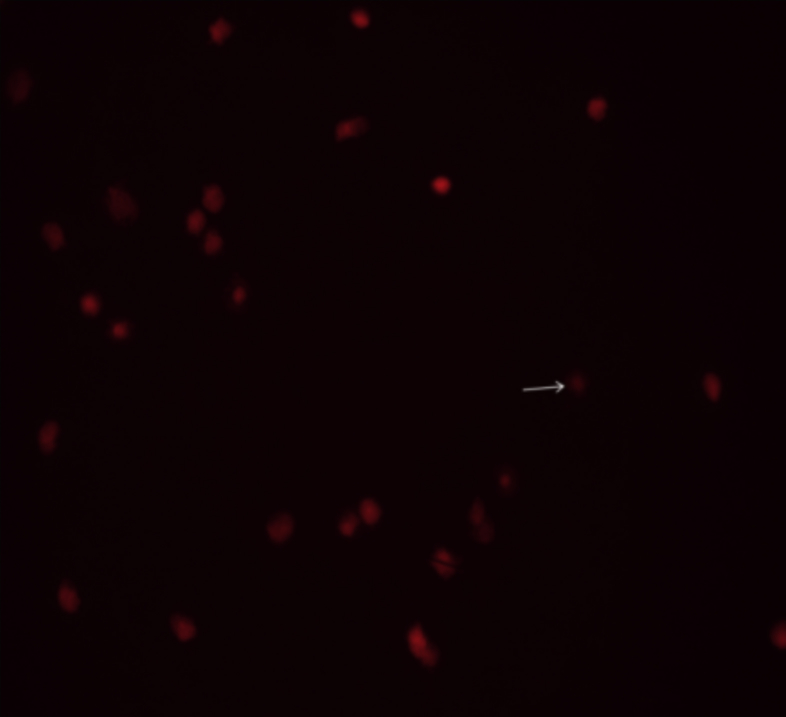
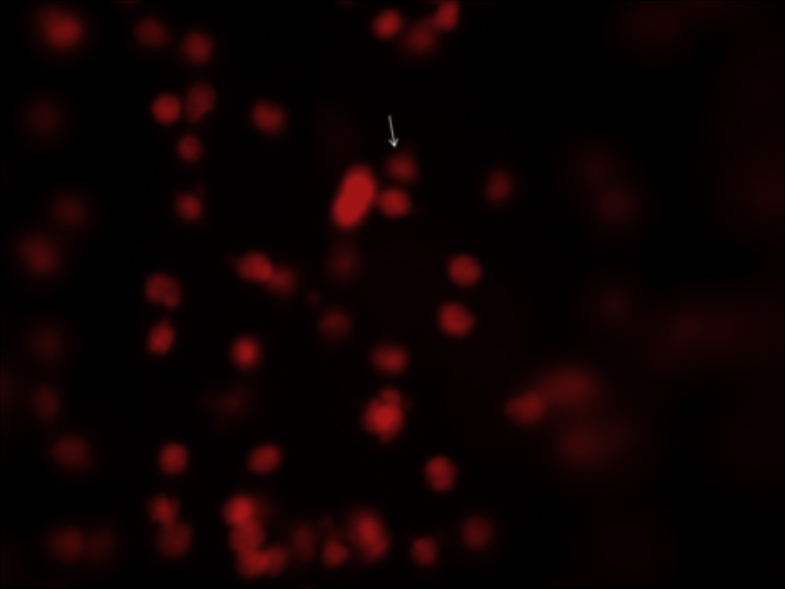
Fig. 1 show visualized damaged DNA from leukocyte cells in obese women with MS. Cells had the appearance of a comet showed a brightly fluorescent head and a tail to one side formed by the DNA containing strand breaks that were drawn away. Figure 2, Figure 3 illustrate DNA damage in PCOS and preeclampsia cases respectively.
Figure 1.
Damaged DNA (white arrow) from leukocyte cells of obese women with MS.
Figure 2.
Damaged DNA (white arrow) from leukocyte cells of obese women with PCOS.
Figure 3.
Damaged DNA (white arrow) from leukocyte cells of obese women with recurrent pre-eclampsia.
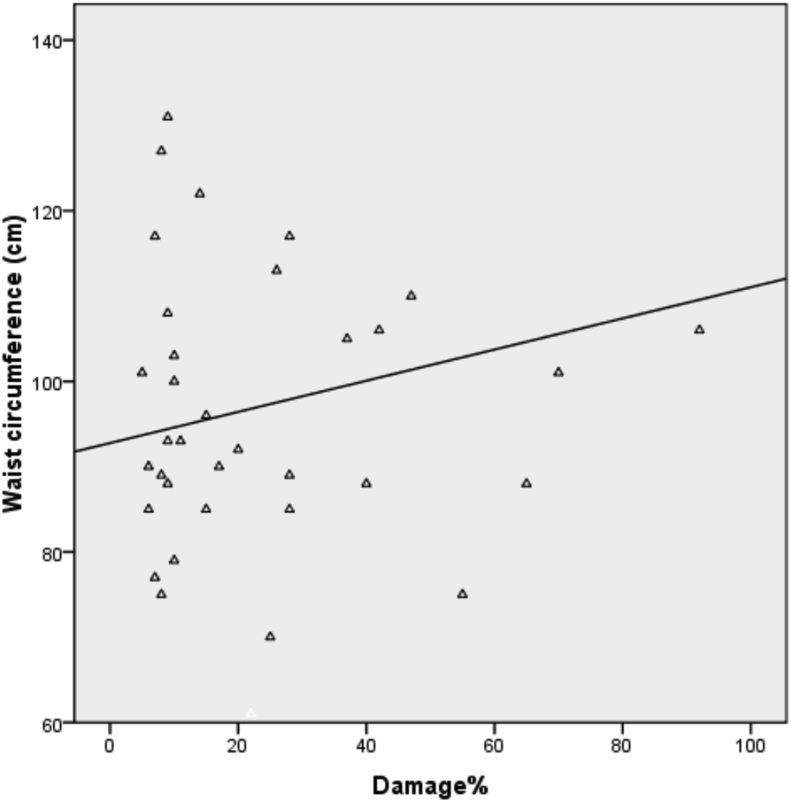
Fig. 4 shows the positive correlation between mean frequency of DNA damage and waist circumference in obese women with PCOS. The linear regression line was performed with DNA damage assays as the dependent variable and waist circumference as independent variables.
Figure 4.
Association between DNA damage frequency and waist circumference in PCOS women.
Discussion
In the present study, DNA damage has been examined in Egyptian obese women with different hormonal and metabolic abnormalities. Percent of DNA damage, anthropometric and biochemical measurements of MS, PCOS and cases with a history of recurrent pre-eclampsia have been compared to healthy non-obese women. Obesity is a growing epidemiological health problem in adults21 as well as in children.22 Obesity always leads to T2D and the development of atherosclerotic CVD as it plays a principle role in insulin resistance. There is a robust link between obesity and MS which might indicate a direct connection between their pathogenies. Associations between obesity and high levels of several oxidants have been previously reported.8 Moreover, it has been found that a significantly lower antioxidant state and higher DNA damage are concomitant to increasing the severity of MS.8 Results of the present study showed that DNA damage was significantly higher in obese subjects either with MS or PCOS or with a history of recurrent pre-eclampsia relative to the control group. Preceding investigation of the DNA damage in Egyptian obese MS women revealed significantly higher levels of DNA damage among the MS Patients compared to healthy non-obese control.18 Our findings are in accordance, as well, with previous work, where a significantly higher DNA damage was reported in obese and MS cases.9, 23 Furthermore, a statistically significant association between the frequency of DNA damage and WC in obese PCOS women has been observed from the results of the present. Elevated micronuclei frequency and DNA damage with high BMI has been previously reported,24, 25 however, controversial results were also found.26
The free radicals likely mediate the connection between higher DNA damage and obesity state. It is well known that obesity causes elevated free radicals state and these free radicals could, in turn, lead to cellular DNA damage. During obesity, adipocytes, interleukin secretions and inflammatory process are most likely involved in the development of oxidative stress.27 In addition to, high-fat diets have the potential to increase oxidative stress and, subsequently, DNA damage.28
Diabetes has been found to be associated with high oxidative DNA damage.29 The WHR is likely the major risk factor for the development of type 2 diabetes30 and it is a very important factor in determining those at high risk of prediabetes cases. Furthermore, WHR seems to be the best marker in predicting cardiovascular outcomes that result from many chronic diseases.31, 32, 33 The metabolic changes happened during hyperglycemic state might cause an increase in the reactive oxygen species (ROS) where polyol pathway is activated. Subsequently, a variety of DNA damage such as oxidized bases and DNA strand breaks occurs. Hence, oxidative stress could be a harmful process that contributes to the progression of diabetes mellitus by causing destruction to cell structures.34, 35
Results of former studies that investigated DNA damage in women with pre-eclampsia are controversial. Some studies found elevated levels of oxidative DNA damage in pre-eclamptic women either concomitant with other pregnancy complications like intrauterine growth restriction (IUGR) or not relative to controls,36 even in mildly pre-eclamptic patients without IUGR.37 Similarly, Furness and colleagues38 reported significantly increased DNA damage in women who developed PE and/or IUGR before an appearance of the symptoms.38 Other studies reported that the maximum level of DNA damage was observed in pre-eclamptic cases only when complicated by IUGR otherwise, in the case of pre-eclampsia alone the differences in the level of the DNA damage compared to the control group were not significant.39 Furthermore, other study13 found no significant difference in the extent of DNA damage between control and pre-eclampsia groups. In the present study, we have investigated cases with a history of recurrent PE and significantly higher levels of DNA damage have been found relative to control group.
Comet assay results of the present study are not only significantly different in MS compared to controls, but they are also positively correlated with several MS features, highlighting the value of these assays as biomarkers. Our results showed that obese cases having 4–5 components had significantly higher levels of DNA damage than those with 2 components with a statistically significant difference as well as compared to controls. Progressive telomere shortening in peripheral blood cells was previously reported from patients with MS, diabetes mellitus, coronary artery disease (CAD), PCOS, and premature myocardial infarction.8, 25 Nevertheless, telomere shortening and chromosomal damage roles in the pathogenesis of cardio-metabolic disease are still not clear. It is recommended in the future studies to do a clinical follow-up of MS, PCOS, pre-eclampsia patients in addition to recruiting larger populations and performing these assays on a regular basis with larger populations in order to evaluate their clinical utility.
Conclusion
The present study showed significant association of DNA damage with number of metabolic components, PCOS and recurrent pre-eclampsia in obese Egyptian women. The increase of DNA damage levels could trigger PCOS and pre-eclampsia. Our results illustrate the correlation between DNA damage and WC in PCOS women. It is important to highlight that future studies evaluating DNA damage should be carried on obese women in an early stage. DNA damage may play a role in the risk of PCOS and pregnancy complications. Comet assay for DNA damage should be considered in interventions and management strategies in obese women.
Conflict of interest
Authors declare there is no conflict of interest.
Acknowledgement
Authors acknowledge the financial assistance provided by National Research Center, Egypt.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Vassalle C., Maffei S., Ndreu R., Mercuri A. Age-related oxidative stress modulation by smoking habit and obesity. Clin Biochem. 2009;42(7):739–741. doi: 10.1016/j.clinbiochem.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E., Haffner S.M., Mitchell B.D., Stern M.P. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34(6):416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 4.Blaton V.H., Korita I., Bulo A. How is metabolic syndrome related to dyslipidemia. Biochem Med. 2008;18(2):14–24. [Google Scholar]
- 5.Alanbay I., Ercan C.M., Sakinci M., Coksuer H., Ozturk M., Tapan S. A macrophage activation marker chitotriosidase in women with PCOS: does low-grade chronic inflammation in PCOS relate to PCOS itself or obesity? Arch Gynecol Obstet. 2012;286(4):1065–1071. doi: 10.1007/s00404-012-2425-0. [DOI] [PubMed] [Google Scholar]
- 6.Kebapcilar A.G., Tatar M.G., Ipekci S.H. Cornea in PCOS patients as a possible target of IGF-1 action and insulin resistance. Arch Gynecol Obstet. 2014;290(6):1255–1263. doi: 10.1007/s00404-014-3353-y. [DOI] [PubMed] [Google Scholar]
- 7.Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Demirbag R., Yilmaz R., Gur M. DNA damage in metabolic syndrome and its association with antioxidative and oxidative measurements. Int J Clin Pract. 2006;60(10):1187–1193. doi: 10.1111/j.1742-1241.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 9.Bukhari S.A., Rajoka M.I., Nagra S.A., Rehman Z.U. Plasma homocysteine and DNA damage profiles in normal and obese subjects in the Pakistani population. Mol Biol Rep. 2010;37(1):289–295. doi: 10.1007/s11033-009-9686-0. [DOI] [PubMed] [Google Scholar]
- 10.Rytter E., Vessby B., Åsgård R. Glycaemic status in relation to oxidative stress and inflammation in well-controlled type 2 diabetes subjects. Br J Nutr. 2009;101(10):1423–1426. doi: 10.1017/s0007114508076204. [DOI] [PubMed] [Google Scholar]
- 11.Moran L.J., Noakes M., Clifton P.M., Norman R.J., Fenech M.F. Genome instability is increased in lymphocytes of women with polycystic ovary syndrome and is correlated with insulin resistance. Mutat Res Mol Mech Mutagen. 2008;639(1):55–63. doi: 10.1016/j.mrfmmm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Harma M., Harma M., Erel O. Measurement of the total antioxidant response in preeclampsia with a novel automated method. Eur J Obstet Gynecol Reprod Biol. 2005;118(1):47–51. doi: 10.1016/j.ejogrb.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Mert I., Sargın Oruc A., Yuksel S. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J Obstet Gynaecol Res. 2012;38(4):658–664. doi: 10.1111/j.1447-0756.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- 14.Andreassi M.G. DNA damage, vascular senescence and atherosclerosis. J Mol Med. 2008;86(9):1033. doi: 10.1007/s00109-008-0358-7. [DOI] [PubMed] [Google Scholar]
- 15.Jonard S., Robert Y., Dewailly D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod. 2005;20(10):2893–2898. doi: 10.1093/humrep/dei159. [DOI] [PubMed] [Google Scholar]
- 16.Williams L. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143. [PubMed] [Google Scholar]
- 17.Hunt K.J., Resendez R.G., Williams K., Haffner S.M., Stern M.P. National cholesterol education Program versus World health organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio heart study. Circulation. 2004;110(10):1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 18.Zaki M., Kamal S., Basha W.A. Assessment of DNA damage in obese premenopausal women with metabolic syndrome. Gene Rep. 2018;10:42–46. [Google Scholar]
- 19.Tanner J.M., Hiernaux J., Jarman S., Weiner J.S., Lourie J.A. Growth and physique studies. Hum Biol A Guid to F Methods IBP Handb. 1969;9:1–60. [Google Scholar]
- 20.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo-Johnson C., Mincey K.D. Obesity epidemiology Worldwide. Gastroenterol Clin North Am. 2016;45(4):571–579. doi: 10.1016/j.gtc.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ode K.L., Frohnert B.I., Nathan B.M. Identification and treatment of metabolic complications in pediatric obesity. Rev Endocr Metab Disord. 2009;10(3):167–188. doi: 10.1007/s11154-009-9115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarpato R., Verola C., Fabiani B., Bianchi V., Saggese G., Federico G. Nuclear damage in peripheral lymphocytes of obese and overweight Italian children as evaluated by the γ-H2AX focus assay and micronucleus test. FASEB J. 2011;25(2):685–693. doi: 10.1096/fj.10-168427. [DOI] [PubMed] [Google Scholar]
- 24.Violante F.S., Sanguinetti G., Barbieri A. Lack of correlation between environmental or biological indicators of benzene exposure at parts per billion levels and micronuclei induction. Environ Res. 2003;91(3):135–142. doi: 10.1016/s0013-9351(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 25.Yesilada E., Sahin I., Ozcan H., Yildirim I.H., Yologlu S., Taskapan C. Increased micronucleus frequencies in peripheral blood lymphocytes in women with polycystic ovary syndrome. Eur J Endocrinol. 2006;154(4):563–568. doi: 10.1530/eje.1.02117. [DOI] [PubMed] [Google Scholar]
- 26.Giovannelli L., Saieva C., Masala G. Nutritional and lifestyle determinants of DNA oxidative damage: a study in a Mediterranean population. Carcinogenesis. 2002;23(9):1483–1489. doi: 10.1093/carcin/23.9.1483. [DOI] [PubMed] [Google Scholar]
- 27.Park Y.-W., Zhu S., Palaniappan L., Heshka S., Carnethon M.R., Heymsfield S.B. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C.S. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Goodarzi M.T., Navidi A.A., Rezaei M., Babahmadi-Rezaei H. Oxidative damage to DNA and lipids: correlation with protein glycation in patients with type 1 diabetes. J Clin Lab Anal. 2010;24(2):72–76. doi: 10.1002/jcla.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayawardena R., Ranasinghe P., Byrne N.M., Soares M.J., Katulanda P., Hills A.P. Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health. 2012;12(1):380. doi: 10.1186/1471-2458-12-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Organization WH, WHO Multicentre Growth Reference Study Group WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva WHO. 2006;2007 [Google Scholar]
- 32.Qiao Q., Nyamdorj R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr. 2010;64(1):30–34. doi: 10.1038/ejcn.2009.93. [DOI] [PubMed] [Google Scholar]
- 33.Esteghamati A., Mousavizadeh M., Noshad S., Shoar S., Khalilzadeh O., Nakhjavani M. Accuracy of anthropometric parameters in identification of high-risk patients predicted with cardiovascular risk models. Am J Med Sci. 2013;346(1):26–31. doi: 10.1097/MAJ.0b013e31826485de. [DOI] [PubMed] [Google Scholar]
- 34.Shigenaga M.K., Ames B.N. Assays for 8-hydroxy-2′-deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med. 1991;10(3–4):211–216. doi: 10.1016/0891-5849(91)90078-h. [DOI] [PubMed] [Google Scholar]
- 35.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Fujimaki A., Watanabe K., Mori T., Kimura C., Shinohara K., Wakatsuki A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta. 2011;32(5):367–372. doi: 10.1016/j.placenta.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Hilali N., Kocyigit A., Demir M. DNA damage and oxidative stress in patients with mild preeclampsia and offspring. Eur J Obstet Gynecol Reprod Biol. 2013;170(2):377–380. doi: 10.1016/j.ejogrb.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Furness D.L.F., Dekker G.A., Hague W.M., Khong T.Y., Fenech M.F. Increased lymphocyte micronucleus frequency in early pregnancy is associated prospectively with pre-eclampsia and/or intrauterine growth restriction. Mutagenesis. 2010;25(5):489–498. doi: 10.1093/mutage/geq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiktor H., Kankofer M., Schmerold I., Dadak A., Lopucki M., Niedermüller H. Oxidative DNA damage in placentas from normal and pre-eclamptic pregnancies. Virchows Arch. 2004;445(1):74–78. doi: 10.1007/s00428-004-1024-2. [DOI] [PubMed] [Google Scholar]