Abstract
Aim:
This study aimed to identify genes encoding resistance to tetracycline (TE) and plasmid-mediated resistance to quinolones in Escherichia coli isolates from clinical cases of avian colibacillosis in Sukabumi, Indonesia.
Materials and Methods:
A total of 25 E. coli archive isolates were collected in 2013-2017 from clinical cases of avian colibacillosis in Sukabumi, Indonesia. All isolates were tested for TE and quinolone resistance using the disk diffusion method. TE -resistant E. coli isolates were screened for the presence of tet(A) and tet(B) genes by single polymerase chain reaction (PCR). The qnr(A), qnr(B), and qnr(S) genes were detected by multiplex PCR in quinolone-resistant E. coli isolates.
Results:
Result of this study shows that 19 of 25 (76%) E. coli isolates are resistant to oxytetracycline and 64% are resistant to TE; among them, 63.2% and 31.5% were positive tet(A) and tet(B), respectively. 13 out of 25 (52%) are resistant to ciprofloxacin and 36% are resistant to enrofloxacin either norfloxacin; among them, 61.6% were positive qnr(A), 7.7% were positive qnr(B), 23% were positive qnr(S), and 7.7% were positive both of qnr(A) and qnr(S).
Conclusion:
This study shows that a few pathogens of E. coli are resistant to TE and quinolone. The frequency of tet and qnr genes that are responsible for this resistance among avian pathogenic E. coli isolates in Sukabumi, Indonesia, was high.
Keywords: Antibiotic, colibacillosis, Escherichia coli, genes, resistance
Introduction
Avian colibacillosis is one of the most important infectious diseases in birds of all ages. This disease has an important economic impact on poultry production worldwide. Antibiotic as feed additives in an animal is required to reduce the economic consequences of bacterial disease, but it was contributed to the spread of antibiotic-resistant bacteria [1-3]. Susceptible bacteria become resistant to an antibiotic through genetic mutation or through horizontal transfer. Antibiotic exposure is a source of stress that can create stress-induced resistance among bacteria [4].
Tetracycline (TE) is one of the oldest antibiotics that is used in the livestock industry. Over the years, TE has been improved and marketed under various trade names. Importantly, they are also used as growth promoters of farm animals worldwide. It is not surprising that resistance to these antibiotics has spread in various bacterial communities [5]. The resistance mechanisms for the TE class of antibiotics fall in four categories such as energy-dependent efflux pumps, ribosomal protection proteins, enzymatic inactivation, and target modification are recognized as mediators of TE resistance in bacteria. The genes associated with an efflux mechanism, namely tet(A), tet(B), tet(C), tet(D), and tet(E), are important part of TE resistance in E. coli [6]. Other antibiotic agents that used to treat infections caused by this bacterium include fluoroquinolones, newer quinolones containing a fluorine atom. In veterinary medicine, a large proportion of these antimicrobials belongs to a group of drugs with the so-called restricted indication [7]. Quinolone usage is threatened by the rising occurrence of resistance, which has been observed in every species that is treated by this drug class. Recent work has helped to define how quinolones interact with gyrase or topoisomerase IV and how mutations in can lead these enzymes to resistance [8]. Recent studies have shown that the emergence of plasmid-determined quinolone resistance may contribute by several means to the rapid increase in bacterial resistance to quinolones. Plasmid-mediated quinolone resistance genes qnr(A), qnr(B), and qnr(S) encode pentapeptide repeat protein and has ability to protect DNA gyrase from quinolone inhibition [9].
Horizontal transfer of antibiotics resistance genes has also been confirmed, which is mediated by plasmids. Plasmid-mediated resistance widespread in poultry among Enterobacteriaceae isolates and the effect of their combination with other resistance mechanisms have been little studied in Indonesia. The aim of this study was to investigate the presence of tet and qnr genes among Escherichia coli isolates from clinical cases of avian colibacillosis in Sukabumi, Indonesia.
Materials and Methods
Ethical approval
Samples were collected from the case of colibacillosis at Sukabumi area farm from 2013 to 2017. Archival is a collection of PT Medika Satwa Laboratories, West Java, Indonesia. No live animals were used in the present study. Therefore, ethical approval was not required in this study.
Bacterial isolates and biochemical characterization
A total of 25 non-duplicated archive isolates avian pathogenic E. coli (APEC) were collected from the case of colibacillosis at Sukabumi area farm from 2013 to 2017. Archival is a collection of PT Medika Satwa Laboratories, West Java, Indonesia, and E. coli ATCC 25922 as reference isolate. Isolates were conventionally inoculated on MacConkey agar and Triple Sugar Iron Agar and then incubated at 37°C. Samples also inoculated in E. coli broth with MUG (EC-MUG broth) at 37°C for 22 h. In the presence of MUG, E. coli produces the enzyme glucuronidase that hydrolyzes MUG to yield a fluorogenic product that is detectable under UV light (366 nm). Congo red dye agar test (CR test) used to differentiate invasive and non-invasive E. coli in poultry. The colonies were streaked on CR agar and incubated for 72 h at 25°C. The identification of E. coli was based on the results of diagnostic tests, which included Gram staining, colonial morphology, gas production, and the ability to be enriched in the EC-MUG broth and compared to the standard E. coli strain, ATCC 25922 [10-12]. Identified E. coli isolate cultured on blood agar for a further step of research.
Antibiotic sensitivity identification
A total of 25 E. coli isolates were screened for TE and fluoroquinolone resistance using the disk diffusion method according to the Clinical and Laboratory Institute (CLSI) guidelines [13]. A bacterial suspension with an optical density equal to 0.5 McFarland standard (ca.1.5×108 CFU/ml) was inoculated onto Mueller-Hinton agar for disk diffusion testing. The following antibiotic disks were used for antimicrobial susceptibility testing: TE 30 µg, oxytetracycline (OT) 30 µg, ciprofloxacin (CIP) 5 µg, enrofloxacin (ENR) 5 µg, and norfloxacin (NOR) 10 µg. All the disks were placed on the plate and then incubated aerobically for 18-24 h at 35°C. The reference strain E. coli ATCC 25922 was used as a control. The zone diameters for all antibiotics were measured and interpreted as susceptible, intermediate, or resistance according to the criteria recommended by the Laboratory Standards Institute (CLSI) and manufacturer protocols (Oxoid, UK).
Preparation of DNA template
Bacterial colonies from overnight blood agar at 37°C were picked using sterile pipette tips and aseptically suspended in 500 µL of sterile distilled water in microtubes; the suspensions were boiled for 10 min. After centrifugation for 5 min at 16 060× g, 100 µL of the supernatants were taken as DNA templates for the polymerase chain reaction (PCR) reaction [11].
Resistance gene detection
Molecular detection by PCR and multiplex PCR assay was used to evaluate the presence of tet(A), tet(B), qnr(A), qnr(B), and qnr(S) resistance genes in TE - and/or quinolone-resistant strains of APEC. Single PCR reactions were performed in 10 µL reactions containing DNA template, primers (Table-1) [14], and PCR kit (KAPA2G Fast Hotstart Readymix, Wilmington, USA). The amplification condition was 3 min at 95°C and 30 cycles each consisting of 95°C for 1 min, annealing temperature (Table-1) for 30 s and 72°C for 1 min, followed by a final extension step of 5 min at 72°C. Amplified samples were analyzed by electrophoresis in 1.5% agarose gel and stained by ethidium bromide. A molecular weight marker with 100 bp (VC 100 bp Plus DNA Ladder Vivantis, Selangor, Malaysia) was used as a standard size. Multiplex PCR reactions were performed in 10 µL reactions containing DNA template, primers (Table-2) [9], and PCR kit (KAPA2G Fast Multiplex PCR Kit, Wilmington, USA). The amplification condition was 3 minutes at 95°C and 30 cycles each consisting of 95°C for 1 min, annealing temperature (Table-2) for 30 s and 72°C for 1 min, followed by a final extension step of 5 min at 72°C. Amplified samples were analyzed by electrophoresis in 1.5% agarose gel and stained by ethidium bromide. A molecular weight marker with 100 bp (VC 100 bp Plus DNA Ladder Vivantis, Selangor, Malaysia) was used as a standard size.
Table-1.
E. coli-resistant genes and primer sequences used for single PCR identification.
| Antibiotics | Resistance gene | Primer sequence | Size (bp) | Annealing temperature | Reference |
|---|---|---|---|---|---|
| Tetracycline | tet (A) | (F) 5′-GGTTCACTCGAACGACGTCA-3′ | 577 | 57°C | [14] |
| (R) 5′-CTGTCCGACAAGTTGCATGA-3′ | |||||
| tet (B) | (F) 5′-CCTCAGCTTCTCAACGCGTG-3′ | 634 | 56°C | [14] | |
| (R) 5′-GCACCTTGCTCATGACTCTT-3′ |
PCR=Polymerase chain reaction, E. coli=Escherichia coli
Table-2.
E. coli-resistant genes and primer sequences used for multiplex PCR identification.
| Antibiotics | Resistance gene | Primer sequence | Size (bp) | Annealing temperature | Reference |
|---|---|---|---|---|---|
| Quinolone | qnr(A) | (F) 5′-ATTTCTCACGCCAGGATTTG-3′ | 516 | 55°C | [9] |
| (R) 5′-GATCGGCAAAGGTTAGGTCA-3′ | |||||
| qnr(B) | (F) 5′-GATCGTGAAAGCCAGAAAGG-3′ | 469 | 55°C | [9] | |
| (R) 5′-ACGATGCCTGGTAGTTGTCC-3′ | |||||
| qnr(S) | (F) 5′-ACGACATT CGTCAACTGCAA-3′ | 417 | 55°C | [9] | |
| (R) 5′-TAAATTGGCACCCTGTAGGC-3′ |
PCR=Polymerase chain reaction, E. coli=Escherichia coli
Results
Bacterial isolates and biochemical characterization
All archive isolates APEC strains (25 isolates) showed pink colonies on MacConkey agar which is typical for E. coli and rod-shaped cell in Gram staining. In addition, biochemical characterization shows that all the strains were capable of producing a fluorogenic product, which is a typical characteristic of E. coli. Inoculation in CR test shows that all the strain positive reaction for CR test with binding dye on the colony.
Antibiotic sensitivity identification
A total 22 out of 25 (88%) APEC isolates were resistant to at least one of the tested antibiotics based on inhibition zone by disk diffusion methods (Table-3). The result showed that APEC isolates from clinical cases of avian colibacillosis in Sukabumi 76% (19 of 25 isolates) are resistant to oxytetracycline and 64% (16 out of 25 isolates) are resistant to TE. Among them, 8% (2 of 25 isolates) were intermediate to TE (Table-4). Resistance to quinolones is also known to be 52% (13 of 25 isolates) resistant to CIP and 36% (9 of 25 isolates) resistant to ENR and NOR. Among them, 8% (2 of 25 isolates), 16% (4 out of 25 isolates), and 4% (1 out of 25 isolates) were intermediate to CIP, ENR, and NOR, respectively.
Table-3.
Antibiotic resistance pattern of avian pathogenic E. coli isolates.
| No. | Isolates | TEa | OTb | CIPc | ENd | NORe |
|---|---|---|---|---|---|---|
| 1 | MSL E.C 1 | R | R | R | R | I |
| 2 | MSL E.C 2 | R | R | S | S | S |
| 3 | MSL E.C 3 | R | R | I | I | S |
| 4 | MSL E.C 4 | R | R | R | S | R |
| 5 | MSL E.C 5 | I | R | S | S | S |
| 6 | MSL E.C 6 | S | S | S | S | S |
| 7 | MSL E.C 7 | S | S | S | S | S |
| 8 | MSL E.C 9 | S | S | R | R | R |
| 9 | MSL E.C 10 | R | R | R | R | R |
| 10 | MSL E.C 11 | S | R | S | S | S |
| 11 | MSL E.C 14 | R | R | R | S | S |
| 12 | MSL E.C 15 | S | S | R | I | S |
| 13 | MSL E.C 16 | R | R | R | R | R |
| 14 | MSL E.C 17 | R | R | S | S | S |
| 15 | MSL E.C 18 | S | S | R | R | R |
| 16 | MSL E.C 19 | S | S | S | S | S |
| 17 | MSL E.C 20 | R | R | S | S | S |
| 18 | MSL E.C 28 | R | R | R | R | R |
| 19 | MSL E.C 29 | R | R | R | R | R |
| 20 | MSL E.C 30 | R | R | R | R | R |
| 21 | MSL E.C 31 | I | R | R | R | R |
| 22 | MSL E.C 32 | R | R | I | I | S |
| 23 | MSL E.C 33 | R | R | R | I | S |
| 24 | MSL E.C 34 | R | R | S | S | S |
| 25 | MSL E.C 35 | R | R | S | S | S |
TE=Tetracycline,
OT=Oxytetracycline,
CIP=Ciprofloxacin,
EN=Enrofloxacin,
NOR=Norfloxacin.
E. coli: Escherichia coli
Table-4.
Number and percentage of resistant APEC strains against antibiotics.
| Antibiotic class | Antibiotics | Isolates E. coli (n=25) | ||
|---|---|---|---|---|
| Sensitive (%) | Intermediate (%) | Resistant (%) | ||
| Tetracycline | TE | 28 | 8 | 64 |
| OT | 24 | 0 | 76 | |
| Quinolone | CIP | 40 | 8 | 52 |
| ENR | 48 | 16 | 36 | |
| NOR | 60 | 4 | 36 | |
TE=Tetracycline, OT=Oxytetracycline, CIP=Ciprofloxacin, EN=Enrofloxacin, NOR=Norfloxacin. E. coli=Escherichia coli, APEC=Avian pathogenic Escherichia coli
Resistance gene amplification
Single PCR result shows that 19 of TE-resistant isolates, 63.2% (12 out of 19 isolates) were tet(A) positive and 31.6% (6 out of 19 isolates) were tet(B) positive (Figure-1). One of 19 TE -resistant isolates (MSL E.C 31) were tet(A) and tet(B) negative. Multiplex PCR result shows that 13 of quinolone-resistant, 61.6% (8 out of 13 isolates) were qnr(A) positive, 7.7% (1 out of 13 isolates) were qnr(B) positive, and 23% (3 out of 13 isolates) were qnr(S) positive. One of 13 (7.7%) quinolone-resistant isolates (MSL E.C 15) were qnr(A) and qnr(S) positive. The result of sensitivity and gene detection indicated that all resistant isolates harbor one or more antibiotic resistance genes (Tables-4 and 5).
Figure-1.
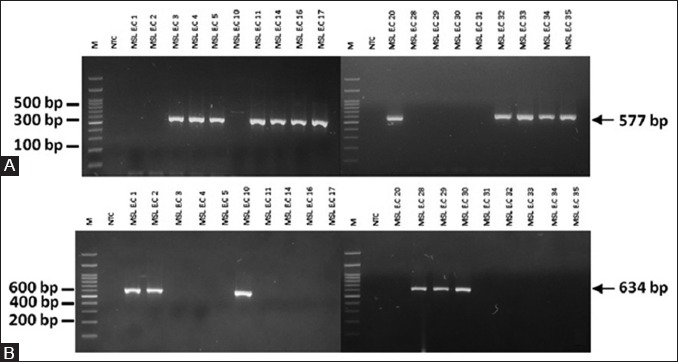
Amplification of tet(A) and tet(B) gene determinants on agarose gel. (A) An approximate 577 bp band size represents tet(A). M: 100 bp DNA ladder. NTC: Non-template control. (B) An approximate 634 bp band size represents tet(B). M: 100 bp DNA ladder. NTC: Non-template control.
Table-5.
Molecular characterization of antimicrobial resistance genotypes among APEC isolates.
| Antibiotic class | Number of resistance isolates (n=25) | Associated genes tested | Number of positive isolates (%) |
|---|---|---|---|
| Tetracycline | 19 (76%) | tet (A) | 12 (63.2) |
| tet (B) | 6 (31.6) | ||
| Quinolone | 13 (52%) | qnr (A) | 8 (61.6) |
| qnr (B) | 1 (7.7) | ||
| qnr (S) | 3 (23) | ||
| qnr (A) and qnr (S) | 1 (7.7) |
APEC=Avian pathogenic Escherichia coli
Discussion
Avian colibacillosis is an infectious disease of birds caused by E. coli, which is considered as one of the principal causes of morbidity and mortality, associated with heavy economic losses to the poultry industry by its association with various disease conditions [1]. Antibiotics treatment is often required to improve animal welfare and reduce the economic consequences of the disease. The most commonly administered antibiotic treatments include amoxicillin, OT, or ENR [2]. Antibiotic exposure is a source of stress that can create stress-induced resistance among bacteria as part of the SOS response. Under the response, mutations and genetic exchanges occur inside bacterial DNA. As a consequence of these genetic manipulations, any gene sequence, including those for antibiotic targets, can be altered, providing the possibility for the evolution of resistance [3].
In this study, the resistance rates to TE and OT were 64% and 76%, indicating a high rate of resistance. TE is frequently used as growth promoters of farm animals worldwide. Over the years, TE antibiotics have been improved and marketed under various trade names. Salehi and Bonab investigated 50 APEC strains isolated from broilers with colisepticemia and found that the rate of resistance to OT was 95% and TE was 94% [15]. The relationship between the use of TE as a growth promoter in the poultry industry and the development of resistance has been established by many authors during the past decades. Such long-term subtherapeutic use of TE antibiotics in feed is of great concern because it leads to selective pressure on the E. coli carried by the poultry and also in the farms environment, so the beneficial effects of TE will become limited as bacterial resistance increases [6]. Detection of the respective genes encoding resistance to TE raises a possibility to analyze epidemiological aspects of the resistance and to discover mobile genetic elements that may have contributed in the dissemination of the TE resistance genes (tet) in nature [5]. This gene associated with an efflux mechanism, namely tet(A), tet(B), tet(C), tet(D), and tet(E), is an important part of TE resistance in E. coli [16]. Molecular detection of tet(A) and tet(B) genes of 19 TE resistant isolates in this study shows 63.2% tet(A) positive and 31.3% tet(B) positive. This study also found one isolate (MSL E.C 31) which is negative to tet(A) or tet(B). Possibly other tet genes are involved in the resistance to TE in the isolates MSL E.C 31, among other tet(C), tet(D), and tet(E). These results supported the observation that these tet(A) and tet(B) efflux genes are the most frequent tet genes found in Enterobacteriaceae. High prevalence of these genes variant might be associated with horizontal gene transfer by conjugative or transposons that implicated in the efflux mechanism that leads to resistance phenotype of bacteria [17]. Van et al. [18] examined a current resistance profile of E. coli from poultry in Vietnam. They collected feces from two poultry farms where chickens were <1 month old. They reported that the tet(A) gene was the most prevalent of the TE resistance genes (71.1% of the isolates), followed by tet(B) (18.4%) in Vietnam. Sengelov et al. [19] found that the tet(A) and tet(B) were the most prevalent than tet(C), tet(D), or tet(E) in Denmark. The majority of isolates contained tet(A) and tet(B) which is in agreement with Al-Bahry et al. [20] findings that most of tet determinant is associated with either conjugative or mobilized elements, which explain their wide distribution among bacteria.
The resistance rates of our samples to quinolone antibiotics shows that, 52% isolates were CIP resistant and 36% isolates were ENR and NOR resistant. The World Health Organization (WHO) has classified these drugs as critically important in human medicine and said that efforts to reduce antibiotic use in farm animals should priorities the fluoroquinolones and another antibiotic class known as the modern [21]. Under current legislation, if a small number of chickens show signs of ill-health, ENR can be added to the drinking water of the whole flock for up to 10 days at a time, even when most of the birds are not ill. A recent report from The European Medicines Agency sets out statistics which show that fluoroquinolone use as oral solutions is higher than the treatment of individual animals [22]. Yeh et al. [23] reported 50.6% ENR resistance and 42.3% CIP resistance of E. coli isolates from poultry in Taiwan. The resistance of quinolone in European countries in poultry was about 52% [7]. The main mechanism of quinolone resistance is the accumulation of mutations in the bacterial enzymes targeted by fluoroquinolones to DNA gyrase and DNA topoisomerase IV. Plasmid-mediated horizontally transferable gene encoding quinolone resistance has shed light on these phenomena. Qnr proteins are capable of protecting DNA gyrase from quinolones and have been circulated for at least 20 years [24]. In this study, qnr(A), qnr(B), and qnr(S) genes were detected in APEC isolates within 61.6% qnr(A) positive, 7.7% qnr(B) positive, and 23% qnr(S) positive. Only one isolate (MSL E.C 15) was positive both of qnr(A) and qnr(S) (Figure-2). A few studies have found bacteria harboring more than one qnr gene. This occurrence has been usually but not exclusively qnr(S) with either qnr(B) or qnr(A). Whether multiple Qnr proteins have an additive effect on the MIC is still unclear [24]. Stephenson et al. [9] reported 255 fluoroquinolone-resistant Enterobacteriaceae isolates in Jamaica; the sole qnr(A), qnr(B), and qnr(S) gene locus was identified in 48%, 1%, and 10% of the isolates, respectively. Another 36% of the isolates were positive for qnr(A) and qnr(S), and two isolates (both E. coli) had all three determinants. Previous studies in Czech Republic, plasmid-mediated resistance qnr(B) and qnr(S) genes were detected in 19% and 52% of the tested samples in 86 E. coli strains isolated from poultry as compared with other European countries [8]. Rodriguez et al. [25] reported that qnr(A)- and qnr(B)-positive plasmids often harbor genes that confer resistance to β-lactams, aminoglycosides, chloramphenicol, TE, sulfonamides, trimethoprim, and rifampin. The presence of qnr genes also increased fluoroquinolone MICs. Bacteria with plasmid-mediated quinolone resistance genes are not always resistant to fluoroquinolones due to the acquisition of low-level quinolone resistance. However, it is thought that when plasmids harboring qnr genes are transferred to various bacterial species, resistance to fluoroquinolone rapidly increases [26]. In Indonesia, there are no data about plasmid-mediated quinolone resistance in E. coli strains isolated from animals, despite few studies in human.
Figure-2.
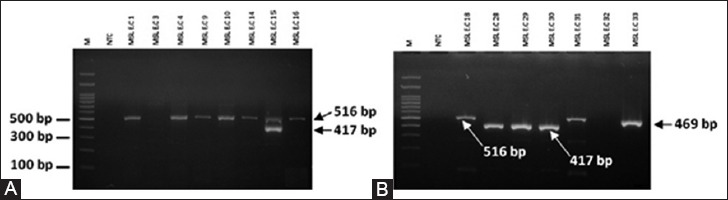
Multiplex polymerase chain reaction (PCR) amplification qnr(A). qnr(B), and qnr(S) on agarose gel. (A) M: 100 bp DNA ladder. NTC: Non-template control. PCR product MSL E.C 15 represents both qnr (A) and qnr(S) genes in this study. (B) M: 100 bp DNA ladder. NTC: Non-template control.
Conclusion
The widespread use of antimicrobial agents caused an increase in the incidence of resistance in both pathogenic and endogenous bacteria, highlighting a serious health problem to human medicine. TE and quinolone antibiotics are commonly used in poultry, but overuse and misuse of this antibiotics may lead to an increased antimicrobial resistance. The emergence of antibiotic resistance cannot be separated from the existence of resistant gene. This present study revealed a high rate of resistance TE and quinolone antibiotics in E. coli isolates from clinical cases of avian colibacillosis in Sukabumi, Indonesia. The presence of tet(A) gene is more dominant than the tet(B) gene that encodes resistance to TE. As well as, tet(A) gene and qnr(A) were higher than qnr(S) and qnr(B) that encodes resistance to quinolone. Both of these resistance genes suggestive of potential horizontal transfer of the resistance genes between strains.
Author’s Contributions
RSK designed the study and drafted the manuscript under the supervision of AI and NLPIM. AP collected samples and compiled the resource materials. RSK and AP performed the test and data analysis under the supervision of AI. RSK conducted molecular detection of resistance gene by PCR under the supervision of NLPIM. All authors have read and approved the final manuscript.
Acknowledgment
The authors would like to thank Dr. Sudarisman and Lily Natalia MS from PT Medika Satwa Laboratories, West Java, Indonesia, for help with Microbiology Laboratory facilities. This study was supported by funding of PT Medika Satwa Laboratories West Java, Indonesia.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Kabir S.M.L. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7(1):89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dheilly A, Bouder A, Devendec L, Hellard G, Kempf I. Clinical and microbial efficacy of antimicrobial treatments of experimental avian colibacillosis. Vet. Microbiol. 2011;149(3-4):422–429. doi: 10.1016/j.vetmic.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Allocati N, Masulli M, Alexeyev MF, Ilio C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health. 2013;10(12):6235–6254. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huddleston JR. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug Resist. 2014;7:167–176. doi: 10.2147/IDR.S48820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velhner M, Milanov D. Resistance to tetracycline in Escherichia coli and Staphylococcus aureus: Brief overview on mechanisms of resistance and epidemiology. Arh. Vet. Med. 2015;8(1):27–36. [Google Scholar]
- 6.Ljubojević D, Velhner M, Todorović D, Pajić M, Milanov D. Tetracycline resistance in Escherichia coli isolates from poultry. Arh. Vet. Med. 2016;9(1):61–81. [Google Scholar]
- 7.Hricová K, Röderová M, Pudová V, Hanulík V, Halová D, Julínková P, Dolejská M, Papoušek I, Bardoň J. Quinolone-resistant Escherichia coli in poultry farming. Cent. Eur. J. Public Health. 2017;25(2):163–167. doi: 10.21101/cejph.a4328. [DOI] [PubMed] [Google Scholar]
- 8.Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53(10):1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson S, Brown PD, Holness A, Wilks M. The emergence of qnr-mediated quinolone resistance among Enterobacteriaceae in Jamaica. West Indian Med. J. 2010;59(3):241–244. [PubMed] [Google Scholar]
- 10.Barrow GI, Feltham RK. In: Cowan and Steel's Manual for Identification of Medical Bacteria. 3rd ed. Cambridge: Cambridge University Press (GB); 2003. Character of gram-negative bacteria; pp. 94–149. [Google Scholar]
- 11.Qabajah M, Awwad E, Ashhab Y. Molecular characterisation of Escherichia coli from dead broiler chickens with signs of colibacillosis and ready-to-market chicken meat in the West-bank. Br. Poult. Sci. 2014;55(4):442–451. doi: 10.1080/00071668.2014.935998. [DOI] [PubMed] [Google Scholar]
- 12.Sharma KK, Soni SS, Meharchandani S. Congo red dye agar test as an indicator test for detection of invasive bovine Escherichia coli. Vet. Arh. 2006;76(4):363–366. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. Wayne (US): Clinical and Laboratory Standards Institute; 2016. pp. 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randall LP, Cooles SW, Osborn MK, Piddock L.J.V, Woodward MJ. Antibiotic resistance genes, integrons and multiple antibiotic resistances in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. Antimicrob. Chemother. 2004;53(2):208–216. doi: 10.1093/jac/dkh070. [DOI] [PubMed] [Google Scholar]
- 15.Salehi TZ, Bonab FS. Antibiotics susceptibility pattern of Escherichia coli strains isolated from chickens with colisepticemia in Tabriz Province, Iran. Int. J. Poult. Sci. 2006;5(7):677–684. [Google Scholar]
- 16.Chopra I, Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awad A, Arafat N, Elhadidy M. Genetic elements associated with antimicrobial resistance among avian pathogenic Escherichia coli. Ann. Clin. Microbiol. Antimicrob. 2016;15(59):1–8. doi: 10.1186/s12941-016-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van TTH, Chin J, Chapman T, Tran LT, Coloe PJ. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008;124(3):217–223. doi: 10.1016/j.ijfoodmicro.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Sengelov G, Halling B, Aarestrup FM. Susceptibility of Escherichia coli and Enterococcus faecium isolated from pigs and broiler chickens to tetracycline degradation products and distribution of tetracycline resistance determinants in E. coli from food animals. Vet. Microbiol. 2003;95(1-2):91–101. doi: 10.1016/s0378-1135(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 20.Al-Bahry SN, Al-Mashani BM, Al-Ansari AS, Elshafie AE, Mahmoud IY. Escherichia coli tetracycline efflux determinants in relation to tetracycline residues in chicken. Asian Pac. J. Trop. Med. 2013;6(9):718–722. doi: 10.1016/S1995-7645(13)60125-X. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Critically Important Antimicrobials for Human Medicine. 3rd ed. Geneva (CH): World Health Organization; 2011. p. 7. [Google Scholar]
- 22.EMA. Sales of Veterinary Antimicrobial Agents in 19EU/EEA Countries. 2nd ed. London (GB): ESVAC Report; 2012. [Google Scholar]
- 23.Yeh JC, Lo DY, Chang SK, Chou CC, Kuo HC. Prevalence of plasmid-mediated quinolone resistance in Escherichia coli isolated from diseased animals in Taiwan. J. Vet. Med. Sci. 2017;79(4):730–735. doi: 10.1292/jvms.16-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009;22(4):664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez MJ, Cano ME, Velasco C, Martinez ML, Pascual A. Plasmid-mediated quinolone resistance: An update. J. Infect. Chemother. 2011;17(2):149–182. doi: 10.1007/s10156-010-0120-2. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Cho JK, Kim KS. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Salmonella isolated from poultry in Korea. Avian Pathol. 2013;42(3):221–229. doi: 10.1080/03079457.2013.779636. [DOI] [PubMed] [Google Scholar]


