Abstract
For the first time to our knowledge, we demonstrate that whole angiosperm individuals can survive gut passage through birds, and that this occurs in the field. Floating plants of the genus Wolffia are the smallest of all flowering plants. Fresh droppings of white-faced whistling duck Dendrocygna viduata (n = 49) and coscoroba swan Coscoroba coscoroba (n = 22) were collected from Brazilian wetlands. Intact Wolffia columbiana were recovered from 16% of D. viduata and 32% of Coscoroba samples (total = 164 plantlets). The viability of plants was tested, and asexual reproduction was confirmed. Wolffia columbiana is an expanding alien in Europe. Avian endozoochory of asexual angiosperm propagules may be an important, overlooked dispersal means for aquatic plants, and may contribute to the invasive character of alien species.
Keywords: Anatidae, avian vectors, duckweed, endozoochory, plant dispersal, vegetative propagule
1. Introduction
The dispersal of viable plant units is recognized as a vital ecosystem service provided by birds, but the great majority of the literature focuses on the dispersal of seeds by frugivorous birds [1]. It is widely assumed that only plants with a fleshy fruit are adapted for endozoochory, i.e. dispersal through the gut passage of animals [2]. However, studies of waterbirds as plant vectors bring into question this assumption, and show that endozoochory by non-frugivorous birds is important. Wildfowl (Anseriformes: ducks, geese, swans and screamers) disperse seeds of many angiosperms lacking a fleshy fruit [3], are excellent vectors for long-distance dispersal [4] and have been recently shown to disperse viable moss fragments and fern spores in their guts [5,6]. Here we demonstrate they can disperse entire angiosperms by endozoochory.
The floating, rootless plants of the genus Wolffia (Araceae, Lemnoideae) are the world's smallest flowering plants [7]. Like their relatives the duckweeds Lemna, they are widely assumed to disperse via waterbirds, but by epizoochory (i.e. attaching on the outside). Darwin [8] observed that when a duck emerges from a pond, whole Lemna plantlets can adhere to its feathers, and there is experimental evidence to support this [9]. Even before Darwin [8], Weddell [10] described Wolffia brasiliensis from plants he found on the feathers of Brazilian screamer Anhima cornuta. Wolffia columbiana has a similar native range to W. brasiliensis in freshwater wetlands across temperate and tropical regions from Argentina to Canada [7]. It is also alien and spreading in Europe, where it threatens native W. arrhiza [11].
Waterbirds can disperse plants to new habitats they cannot reach by other means [4]. The distribution of Wolffia in their native and introduced ranges [11,12] indicates they are effective dispersers, even though epizoochory events may be rare and constrained by desiccation of plants on plumage [13]. It has become clear that endozoochory is more frequent than epizoochory for seed dispersal by waterbirds [4,14]. Wildfowl actively feed on Lemnoideae and disperse viable Lemna seeds by endozoochory [4,6,15], although asexual Lemna somehow also disperse readily [9]. This raises the question whether endozoochory of vegetative propagules, such as Wolffia or Lemna plantlets, occurs in nature.
2. Material and methods
Fresh droppings of Dendrocygna viduata (n = 49) and Coscoroba coscoroba (n = 22) were collected between August 2017 and July 2018 (electronic supplementary material, appendix S1) in five temporary wetlands of Santa Vitória do Palmar, in southern Brazil (figure 1a; electronic supplementary material, appendices S2 and S3). These wetlands are situated among ricefields and cattle-grazed grasslands. They contain numerous species of emergent and floating aquatic plants [16]. Wolffia columbiana is common and widespread in permanent and temporary ponds, lakes and water courses. Droppings were collected from grass close to the shoreline, were not in contact with soil or water and were immediately inspected for contamination. We observed D. viduata and C. coscoroba resting close to the droppings, and given their numbers, each sample is likely to be from a different individual. Coscoroba droppings had a distinctive colour, size and texture different from those of any other waterbird in the area. Dendrocygna droppings were collected from monospecific groups.
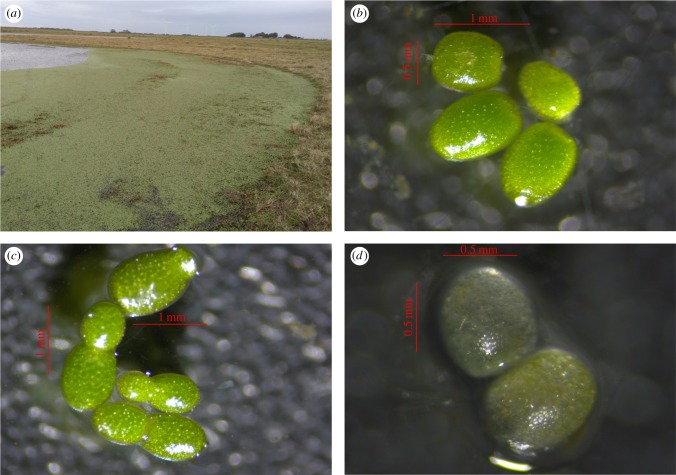
Figure 1.
(a) Wetland where faeces of D. viduata with W. columbiana were collected. (b) Intact plantlets obtained from faeces, showing a healthy appearance (bright green colour and integral structure). (c) Seven plantlets observed after 7 experimental days, confirming asexual reproduction. (d) Plants that died during the experiment lost their colour.
Samples were stored in separate tubes. In the laboratory, 34 Dendrocygna droppings and all Coscoroba droppings were frozen until inspection. Fifteen droppings of D. viduata were kept at 4°C in the fridge until a viability experiment. All samples were carefully examined under a stereomicroscope initially, to confirm the absence of any plant propagules adhered to the exterior. Frozen faeces were then defrosted in water and examined under the stereomicroscope to separate whole plants. The 15 unfrozen droppings were processed similarly later (just before the viability experiment). Only intact Wolffia plants resembling live plants (i.e. with a bright green colour and integral structure, figure 1b) were counted and removed from the samples. Fragments were also observed in some samples, but were not quantified.
Intact W. columbiana plants removed from three fresh D. viduata droppings were counted and placed in five Petri dishes. The dropping with more plants (A) was separated into three dishes (A1, A2 and A3) to facilitate the counting of new plants produced by asexual reproduction. Plants from the other two droppings (B, C) were placed in separate dishes. All dishes were filled with filtered water from the wetland where droppings were collected. The dishes were placed in a growth chamber (12 h dark at 16°C ± 2°C, 12 h light at 26°C ± 2°C). The number of living and dead individuals was counted after 7 and 14 days. An increase in the number of plants was considered to demonstrate asexual reproduction, confirming viability.
3. Results
A total of 164 intact W. columbiana were observed in faecal samples (figure 1; electronic supplementary material, appendix S1). Whole plants were observed in seven Coscoroba droppings (86 plants, frequency of occurrence = 31.8%, 4–24 plants per sample), and eight D. viduata droppings (78 plants, frequency = 16.3%, 1–31 plants per sample).
Intact W. columbiana were removed from three of 15 unfrozen D. viduata samples, and placed in Petri dishes. After 14 days, we detected vegetative reproduction in four of five dishes (with plants from two droppings). The number of living plantlets increased by 89% (figure 1 and table 1), with variation among droppings A = 93.3%, B = 150% and C = 0%.
Table 1.
Asexual reproduction of W. columbiana recovered from three D. viduata droppings, showing changes in the cumulative number of live and dead plants after 7 and 14 days.
| day 7 |
day 14 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| sample ID | initial number apparently alive | new plants | dead plants | live plants | total | new plants | dead plants | live plants | total |
| A1 | 5 | 5 | — | 10 | 10 | 10 | 1 | 14 | 15 |
| A2 | 5 | 7 | 3 | 9 | 12 | 8 | 3 | 10 | 13 |
| A3 | 5 | — | 3 | 2 | 5 | 3 | 3 | 5 | 8 |
| B | 2 | 2 | 1 | 3 | 4 | 5 | 2 | 5 | 7 |
| C | 1 | — | 1 | — | 1 | — | 1 | — | 1 |
| total | 18 | 14 | 8 | 24 | 32 | 26 | 10 | 34 | 44 |
4. Discussion
Our study provides field evidence that vegetative angiosperm propagules can be dispersed by avian endozoochory. Whole Wolffia plants were dispersed over an unknown distance between aquatic feeding sites and terrestrial loafing sites. Previously, asexual angiosperm propagules have only been reported from external parts of waterbirds [4,8,9]. Zoochory of asexual propagules allows dispersal outside the period of seed production and availability, e.g. facilitating the colonization of temporary wetlands after heavy rainfall.
Dendrocygna viduata is widespread in Central and South America, with an estimated population of one million [17]. Individuals fly an average of up to 4 km daily between different wetlands [18], making it an ideal plant vector. Coscoroba coscoroba is restricted to southern South America, with a population of 10 000–25 000 [17]. It is partially migrant, with movements of up to 1700 km [19]. Hence, endozoochory of W. columbiana by D. viduata may be a more frequent process, although C. coscoroba may be important for long-distance dispersal.
The high abundance and frequency of intact W. columbiana in faeces, and their high viability, suggest this floating plant has a high capacity to survive gut passage. Endozoochory may be more important for W. columbiana than epizoochory. The average retention time for wildfowl faeces is several hours [4], suggesting that wildfowl regularly disperse Wolffia over several kilometres during their daily movements [18]. Despite anecdotal support for epizoochory, it is unclear that floating plants both remain attached to birds and resist desiccation during extended flights. There is no risk of desiccation during endozoochory, which may provide a longer maximum retention time.
The particularly small size and simple morphology of Wolffia may promote endozoochory. Seeds with a smaller size and round shape are more likely to survive gut passage [4]. More research is needed to establish which angiosperm taxa can survive gut passage as whole plants or as viable fragments (as recently shown for bryophytes [5]). Experimental evidence suggests fragments of the invasive amphibious Crassula helmsii may disperse inside wildfowl guts [20]. Given that production of asexual vegetative propagules and an ability to grow from fragments is widespread in plants [21], dispersal of such vegetative propagules (e.g. fragments of grasses or pondweeds, or whole floating plants) by endozoochory may be an important and overlooked process. Clonality is more common in plants that establish readily outside of their native range [21], and the ability to disperse as vegetative propagules by endozoochory may increase their invasiveness. Greater resistance to desiccation has been suggested as the key to the effective dispersal of plantlets or stem fragments on the outside of animals or on boats or fishing gear [13]. Perhaps the invasive character of some species (e.g. W. columbiana or Lemna minuta) is more related to greater resistance to gut passage.
Supplementary Material
Acknowledgements
We thank F.C.S. and J.M. for helping during fieldwork.
Ethics
This work was authorized by the Brazilian agency SISBIO (no. 59225-1).
Data accessibility
Datasets supporting this article have been uploaded as electronic supplementary material.
Authors' contributions
G.G.S., A.J.G., V.W., P.H. and A.L.-K. designed this study. G.G.S. and A.J.G. led the writing and the others authors contributed equally with the text. G.G.S., V.W. and P.H. collected and analysed the data. C.S. and L.M. helped in all steps and acted as coordinators. All authors read and approved the final manuscript and agree to be accountable for all the content here presented.
Competing interests
The authors have no competing interests.
Funding
G.G.S. thanks CAPES for a doctoral scholarship. L.M. and C.S. hold Research Productivity Grants from CNPq. This research was supported by funds from UNISINOS (grant no. 02.00.023/00-0) and CNPq (grant no. 52370695-2). A.J.G. was supported by Spanish National Plan project CGL2016-76067-P (AEI/FEDER, EU).
References
- 1.Sekercioglu CH. 2006. Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471. ( 10.1016/j.tree.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 2.Vargas P, Arjona Y, Nogales M, Heleno RH. 2015. Long-distance dispersal to oceanic islands: success of plants with multiple diaspore specializations. AoB Plants 7, 1–9. ( 10.1093/aobpla/plv073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soons MB, Brochet AL, Kleyheeg E, Green AJ. 2016. Seed dispersal by dabbling ducks: an overlooked dispersal pathway for a broad spectrum of plant species. J. Ecol. 104, 443–455. ( 10.1111/1365-2745.12531) [DOI] [Google Scholar]
- 4.Green AJ, Soons M, Brochet AL, Kleyheeg E. 2016. Dispersal of plants by waterbirds. In Why birds matter: avian ecological function and ecosystem services (eds Sekercioglu ÇH, Wenny DG, Whelan CJ), pp. 147–195. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Wilkinson DM, Lovas-Kiss A, Callaghan DA, Green AJ. 2017. Endozoochory of large bryophyte fragments by waterbirds. Cryptogam. Bryol. 38, 223–228. ( 10.7872/cryb/v38.iss2.2017.223) [DOI] [Google Scholar]
- 6.Lovas-Kiss Á, Vizi B, Vincze O, Molnár VA, Green AJ. 2018. Endozoochory of aquatic ferns and angiosperms by mallards in Central Europe. J. Ecol. 106, 1714–1723. ( 10.1111/1365-2745.12913) [DOI] [Google Scholar]
- 7.Landolt E. 1986. The family of Lemnaceae – a monographic study. Vol. 1. Veröff. Geobot. Inst. ETH Stiftung Rübel Zürich 71, 1–566. [Google Scholar]
- 8.Darwin C. 1859. On the origin of species by means of natural selection. London, UK: John Murray. [Google Scholar]
- 9.Coughlan NE, Kelly TC, Jansen MA. 2017. ‘Step by step’: high frequency short-distance epizoochorous dispersal of aquatic macrophytes. Biol. Invasions 19, 625–634. ( 10.1007/s10530-016-1293-0) [DOI] [Google Scholar]
- 10.Weddell HA. 1849. Observations sur une espèce nouvelle du genre Wolffia (Lemnacees). Ann. Sci. Nat. Bot. Biol. 12, 155–173 (in French). [Google Scholar]
- 11.Ardenghi NM, Armstrong WP, Paganelli D. 2017. Wolffia columbiana (Araceae, Lemnoideae): first record of the smallest alien flowering plant in southern Europe and Italy. Bot. Lett. 164, 121–127. ( 10.1080/23818107.2017.1319293) [DOI] [Google Scholar]
- 12.Kline L, McCune B. 1987. Factors influencing the distribution of Wolffia columbiana and Wolffia punctata (Lemnaceae). Northwest Sci. 61, 41–43. [Google Scholar]
- 13.Coughlan NE, Cuthbert RN, Kelly TC, Jansen MA. 2018. Parched plants: survival and viability of invasive aquatic macrophytes following exposure to various desiccation regimes. Aquat. Bot. 150, 9–15. ( 10.1016/j.aquabot.2018.06.001) [DOI] [Google Scholar]
- 14.Reynolds C, Cumming GS. 2016. Seed dispersal by waterbirds in southern Africa: comparing the roles of ectozoochory and endozoochory. Freshw. Biol. 61, 349–361. ( 10.1111/fwb.12709) [DOI] [Google Scholar]
- 15.Green AJ, Jenkins KM, Bell D, Morris PJ, Kingsford RT. 2008. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 53, 380–392. ( 10.1111/j.1365-2427.2007.01901.x) [DOI] [Google Scholar]
- 16.Rolon AS, Maltchik L. 2006. Environmental factors as predictors of aquatic macrophyte richness and composition in wetlands of southern Brazil. Hydrobiologia 556, 221–231. ( 10.1007/s10750-005-1364-1) [DOI] [Google Scholar]
- 17.Wetlands International. 2018. Waterbird population estimates. See wpe.wetlands.org.
- 18.Don Pablo Research Team. 2012. Movements and resource utilization of four species of ducks captured in the mid-Parana River Basin, Corrientes Province, Argentina, p. 115 Cambridge, MD: USGS. [Google Scholar]
- 19.Calabuig CP, Green AJ, Menegheti JO, Muriel R, Patiño-Martínez J. 2010. Fenología del coscoroba (Coscoroba coscoroba) en el sur de Brasil y sus movimientos hacia Argentina. Ornitol. Neotrop. 21, 555–566. [Google Scholar]
- 20.Denys L, Packet J, Jambon W, Scheers K. 2014. Dispersal of the non-native invasive species Crassula helmsii (Crassulaceae) may involve seeds and endozoochorous transport by birds. New J. Bot. 4, 104–106. ( 10.1179/2042349714Y.0000000046) [DOI] [Google Scholar]
- 21.Silvertown J. 2008. The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int. J. Plant Sci. 169, 157–168. ( 10.1086/523357) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets supporting this article have been uploaded as electronic supplementary material.