Abstract
Herbivory is strongly influenced by different sources of plant variation, from traits such as secondary metabolites to features associated with population- and community-level variation. However, most studies have assessed the influence of these drivers in isolation. We conducted a large-scale study to evaluate the associations between multiple types of plant-based variation and insect leaf herbivory in alder (Alnus glutinosa) trees sampled in riparian forests throughout northwestern Spain. We assessed the associations between insect leaf herbivory and alder mean production of leaf secondary metabolites (phenolic compounds), variation among neighbouring alder trees in leaf phenolics and community-related features including alder relative size and frequency and tree species phylogenetic diversity. Structural equation modelling indicated that increasing concentrations of alder leaf flavonoids (but not other types of phenolic compounds) and increasing variation in phenolics among neighbouring alders were both significantly negatively associated with herbivory. In addition, increasing relative frequency of alder was positively associated with leaf damage, whereas the size of alders relative to other trees and phylogenetic diversity were not significantly associated with herbivory. These results demonstrate the concurrent and independent influences of different sources of plant-based variation on insect herbivory and argue for further future work simultaneously addressing multiple plant-based bottom-up controls.
Keywords: Alnus glutinosa, herbivory, phenolic compounds, resource concentration, riparian forests
1. Introduction
It has long been recognized that quantitative and qualitative variation in secondary metabolites plays a key role in shaping insect herbivore loads and damage levels [1]. At the same time, it also well known that features of plant populations (e.g. conspecific density) and communities (e.g. species diversity) strongly influence herbivory [2]. The Resource Concentration Hypothesis [3] posits that as the frequency of a focal species decreases with increasing community diversity, the ability of specialist insects to locate host plant patches will diminish (i.e. associational effects [2]). Likewise, plants that are easier to find either because they are larger in size, more abundant or long-lived (i.e. more apparent) are expected to experience higher levels of damage [4,5].
Plant secondary metabolites and population- and community-level features likely exert concurrent influences on herbivory. For instance, stronger competition by neighbours on focal plants in denser patches may influence defences if plants allocate preferentially towards growth to avoid being outcompeted (e.g. [6]), and plant species diversity may influence secondary metabolite concentrations through changes in the abiotic environment (e.g. light availability) and in doing so indirectly affect herbivory on focal plants [2]. However, efforts to simultaneously address these multiple features under a single study remain scarce, leading to a potentially biased or incomplete understanding of bottom-up controls on herbivory. Recent work has, on the one hand, looked at the effects of plant secondary metabolites on herbivory at the community level using community-weighted trait means (e.g. [7]), and on the other correlated plant intra- or interspecific chemical diversity and composition with herbivory (e.g. [8]). Still, the former approach does not allow separation of the independent effects of plant traits related to herbivore resistance at the community level versus other community-related attributes such as species diversity and species relative frequencies, whereas the latter approach in plant diversity studies has usually not separated effects of plant chemistry from other community-level variables (but see [7]). It is therefore not possible to ascertain whether variation in plant secondary metabolites mediates plant population and community effects on herbivory or if these higher-order effects take place independently of plant secondary metabolites via some other mechanisms.
Here we assessed plant secondary metabolites (phenolic compounds), population- and community-related factors potentially associated with insect leaf herbivory on alder, Alnus glutinosa (Betulaceae), a dominant tree in riparian habitats throughout northern Spain. Specifically, we tested for associations between insect herbivory and mean alder tree-level phenolics, variation among neighbouring alder trees in phenolics, alder relative size and frequency and tree species phylogenetic diversity. This study therefore provides a unifying evaluation of the relative importance of multiple bottom-up factors associated with herbivory in a tree species structuring an endangered and biodiverse habitat.
2. Material and methods
(a). Natural history
The European black alder A. glutinosa is a fast-growing deciduous tree native to most of Europe and is a dominant species in riparian forests. At our field sites (Galicia, northern Spain), it is attacked mainly by the specialist alder leaf beetle Agelastica alni (Coleoptera: Chrysomelidae) and the alder moth Acronicta alni (Lepidoptera: Noctuidae) (see electronic supplementary material, appendix A for details).
(b). Field sites
From late September to mid-October 2017, we surveyed six riparian forest sites in Galicia with similar climatic conditions. Distance between sites ranged from 4 to 46 km. Alder is dominant in riparian habitats of this region, representing ca. 60% of the individuals sampled at our field sites. At each site, we established a 1.1 km transect parallel to the river along which we sampled 10 plots, each 20 × 8 m (long side parallel to the river), separated by 100 m (60 plots total).
(c). Insect herbivory
Within each plot, we quantified insect leaf herbivory for all alder trees with diameter at breast height greater than 4 cm. The number of sampled alder trees per plot ranged from 4 to 15 and was on average (±s.d.) 7.68 ± 0.32. We randomly selected two low-hanging branches (2–3 m from the ground) of each alder tree and collected 10 leaves per branch. For each leaf, we visually estimated per cent leaf area removed by chewers (‘leaf herbivory’ hereafter [4]) (see electronic supplementary material, appendix B) and then averaged values across all leaves per branch and across branches to obtain a single value per tree. We used the mean value across trees per plot for analyses (electronic supplementary material, table SM1).
(d). Bottom-up factors associated with insect herbivory
Phenolic compounds have been reported to confer resistance against leaf herbivores in A. glutinosa [9]. Within each plot, we collected four fully expanded leaves per alder tree from the same branches sampled for herbivory. To be consistent, we collected all leaves growing under shaded conditions between 09.00 and 13.00 h. We collected leaves with little (less than 5%) or no herbivory to minimize variation in phenolics due to site-specific induction. Leaf samples were oven-dried for 48 h at 40°C, ground, and pooled into a single sample per tree for chromatographic analyses using an ultra-high-performance liquid chromatograph (see electronic supplementary material, appendix C for details). We quantified flavonoids as rutin equivalents, condensed tannins as catechin equivalents, hydrolysable tannins as gallic acid equivalents and lignins as ferulic acid equivalents. Total phenolics were calculated as the sum of concentrations for all four groups and expressed in milligrams per gram of tissue on a dry weight basis.
We assessed associations between insect herbivory and phenolics in two ways: (a) using the mean value across alder trees per plot for each group of phenolic compounds, which measures the association between mean tree-level production of phenolic compounds and insect herbivory at the plot level (electronic supplementary material, table SM1), and (b) using the amount of total phenolics per alder tree relative to the total amount of phenolics summed across all alder trees per plot, and transforming these values to proportions for each tree to calculate the Shannon–Wiener Index for each plot (electronic supplementary material, table SM1). Higher values of this latter measure reflect a greater number of individuals contributing more similarly to the total (summed) amount of phenolics per plot (hereafter ‘conspecific diversity in total phenolics’). Thus, while this variable is necessarily correlated with alder abundance or relative frequency, it also accounts for variation in relative defence levels among alder trees (i.e. evenness in phenolic levels) which may contribute independently to variation in herbivory.
By documenting the identity and size of all trees with diameter greater than 4 cm, for each plot we also calculated alder relative frequency (number of alder individuals/total number of individuals of all species), alder relative size (size of alder trees relative to mean size of heterospecifics) and the phylogenetic diversity (see electronic supplementary material, appendix B for details on calculations). Finally, we estimated forest canopy cover by taking five digital photographs per plot and measuring the percentage of canopy cover for each photograph (electronic supplementary material, appendix B).
(e). Statistical analyses
We used structural equation modelling (SEM) to investigate direct associations between alder insect herbivory and individual-level phenolics (mean tree-level concentration of flavonoids, lignins, condensed and hydrolysable tannins), alder conspecific diversity in total phenolics, alder relative size and frequency and phylogenetic diversity using data at the plot level. We also included in the model direct associations between canopy cover and each group of phenolics and on conspecific diversity in total phenolics, as well as direct and indirect (via phenolics) associations between plant cover and herbivory (see electronic supplementary material, appendix D for details). Although the plot sample size was moderately large, it is important to note that it was still rather limited for conducting SEMs for which 10–20 times as many replicates as variables in the model is recomended. We therefore excluded some direct associations (following criteria informed by preliminary analyses; see electronic supplementary material, appendix D) and focused on the previously described ‘core’ associations of interest justified based on the study goals as well as relevant patterns reported by previous work in other systems. We ran this SEM model retaining significant coefficients as well as non-significant coefficients that improved model fit. This reduced model had a better fit (lower AICc) than the full model including all non-significant effects. All four groups of phenolic compounds and conspecific diversity in total phenolics were not normally distributed and therefore log-transformed to satisfy this assumption. The analysis was run using PROC CALIS and the RAM statement in SAS 9.4 (SAS System, Cary, NC).
3. Results
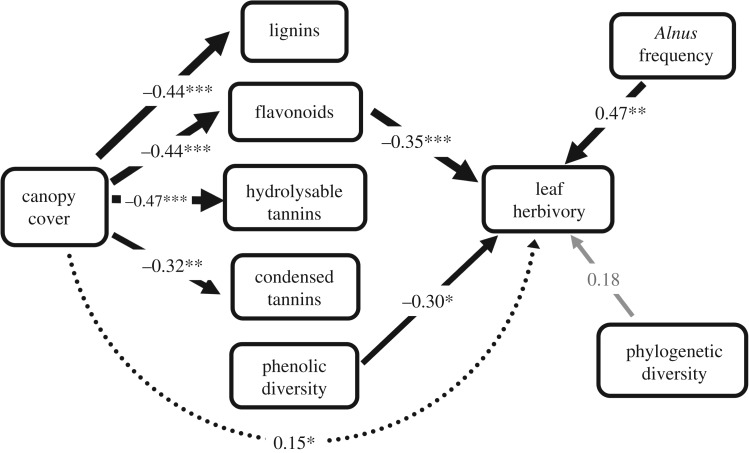
Results from the structural equation model indicated a significant negative association between flavonoids and leaf herbivory; all other groups of leaf phenolics were not significantly associated with leaf herbivory (figure 1). We also found a significant negative association between alder conspecific diversity in total phenolics and leaf herbivory, as well as a significant positive association between alder relative frequency and leaf herbivory (figure 1). However, neither alder relative size (not retained in the model) or phylogenetic diversity were significantly associated with leaf herbivory (figure 1). There were also significant negative associations between canopy cover and mean tree-level values of all the groups of phenolic compounds, and there was a significant indirect association (mediated by mean tree-level phenolic compounds) between canopy cover and leaf herbivory, but no direct association between cover and insect damage (figure 1).
Figure 1.
Diagram showing results from the structural equation model testing for associations between plant phenolics (flavonoids, lignins and condensed and hydrolysable tannins), alder (Alnus glutinosa) diversity in total phenolics, alder relative frequency and community-level phylogenetic diversity and insect leaf herbivory on alder trees at the plot level. Alder trees were sampled from 60 plots across six riparian sites located in northwestern Spain. Values next to each arrow represent path coefficients (i.e. standardized regression coefficients). Continuous arrows indicate direct associations whereas broken arrows indicate indirect associations, in this case of canopy cover on leaf herbivory via phenolic compounds. Arrow thickness indicates the magnitude of the path coefficient. The model also accounted for covariation between groups of phenolic compounds, but these estimates are not shown for ease of visualization. Significant (*p < 0.05, **p < 0.01, ***p < 0.001) and non-significant path coefficients (text and arrows) are in black and grey, respectively. Explained variance: herbivory = 0.30; flavonoids = 0.20; lignins = 0.19; condensed tannins = 0.10; hydrolysable tannins = 0.22. Model χ2 (absolute) = 22.15, p = 0.391, GFI = 0.436, RMSEA estimator = 0.030, AICc = 90.14.
4. Discussion
Mean tree-level concentration of leaf flavonoids was strongly negatively associated with leaf herbivory on alder trees, indicating a role of these compounds in herbivore resistance. Previous studies have demonstrated that these phenolic compounds act as feeding deterrents, digestibility reducers and toxins across many plant taxa [1], but to our knowledge, there are no previous reports on the influence of flavonoids on insect herbivory for A. glutinosa. Interestingly, we ran additional linear mixed models at the plant level (controlling for plot and site) testing separately for associations between each group of phenolics and herbivory (see electronic supplementary material, table SM2) and found no significant effect of flavonoids. This suggests that associations between these compounds and insect herbivory are scale-dependent and potentially underlain by different mechanisms in each case.
Our measure of diversity of phenolic levels among neighbouring alder trees was also negatively associated with leaf herbivory in alder trees, suggesting that an increasing number of similarly defended conspecific trees reduces plot-level leaf damage. This pattern could contribute to explain effects of plant intra-specific diversity paralleling those reported previously for interspecific diversity (e.g. via associational resistance) in secondary metabolites [8] as well as interspecific variation in nutritional traits [10]. Many of these studies, particularly those focusing on the diversity of chemical defences, have found negative effects of locally occurring interspecific variation in composition or diversity of compound types on herbivory. Our measure of intra-specific diversity, however, relates to variation in overall defence levels (i.e. production or concentration) among conspecific trees. Interestingly, the fact that diversity in defence levels was significantly associated with herbivory after accounting for alder relative frequency suggests that the former dictated herbivory mainly through differences in defence levels among alder trees (rather than through differences in alder abundance). This interpretation should, however, be taken with caution considering that alder relative frequency and diversity in phenolic levels were strongly positively correlated because Shannon index values account for alder abundance, and this could have limited our ability to tease apart the effects of these predictors.
The positive association between leaf herbivory and alder relative frequency agrees with theoretical expectations that reductions in the frequency of focal species lead to a concomitant decrease in encounter rates between specialist herbivores and their host plants [3]. Further, by considering plant secondary metabolites, we were able to separate the effects of alder relative frequency from those of individual groups of phenolic compounds. By contrast, previous work testing for resource concentration effects has usually not measured plant defences. Accordingly, we show that these factors exhibit concurrent and independent associations with herbivory. Alder relative size, on the other hand, was not associated with herbivory, suggesting that processes such as physical or chemical interference by heterospecifics were of lesser importance in influencing alder herbivory. In addition, contrary to previous reports of negative effects of phylogenetic diversity on herbivory in trees (e.g. [11]), we found no association between this factor and alder herbivory. It is likely that the effects of plant diversity on specialist insects are strongly mediated by changes in the relative frequency of focal host species, as any associated species would be seen as non-host [12].
There are several key aspects to consider in future alder work which are relevant to other systems. First, multiple types of chemical (e.g. terpenes) and physical (e.g. trichomes, toughness) traits, as well as nutrients (e.g. nitrogen and phosphorus), should be considered to better characterize the plant's defensive phenotype. Second, separating damage by generalist and specialist insects would allow the competing explanations for the observed patterns to be tested. Third, a characterization of herbivory under a broader range of conditions and habitat types would be valuable. To the extent, that generalist insects are important under some conditions, measuring secondary metabolites for the other dominant tree species would be desirable to test for effects of interspecific chemical variation. Fourth, a better characterization of the abiotic environment and its direct and indirect effects (e.g. via effects on plant traits) on herbivory is necessary to fully integrate the observed findings. Overall, we consider that future work that simultaneously accounts for multiple sources of plant intra- and interspecific drivers will improve our understanding of bottom-up controls on herbivory.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
The research did not involve manipulations of humans or animals. No specific permissions were required for our fieldwork. The study did not involve endangered or protected species.
Data accessibility
Electronic supplementary material, table SM4 reports all data used for statistical analyses.
Authors' contributions
X.M. and L.A.-R. formulated the idea of the manuscript and designed the experiment. X.M. and A.G. performed the experiment. M.F. and X.M. Performed the chemical analyses. X.M. Contributed reagents/materials/analysis tools. X.M., L.A.-R. and B.C. Analysed the data. X.M. and L.A.-R. wrote the manuscript. B.C. contributed critically to the writing. M.F. and A.G. edited the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This research was financially supported by a Regional Government of Galicia grant (IN607D 2016/001), a Spanish National Research grant (AGL2015-70748-R) and the Ramón y Cajal Research Programme (RYC-2013-13230) to X.M.
References
- 1.Agrawal AA. 2011. Current trends in the evolutionary ecology of plant defense. Funct. Ecol. 25, 420–432. ( 10.1111/j.1365-2435.2010.01796.x) [DOI] [Google Scholar]
- 2.Moreira X, Abdala-Roberts L, Rasmann S, Castagneyrol B, Mooney KA. 2016. Plant diversity effects on insect herbivores and their natural enemies: current thinking, recent findings, and future directions. Curr. Opin. Insect Sci. 14, 1–7. ( 10.1016/j.cois.2015.10.003) [DOI] [PubMed] [Google Scholar]
- 3.Root RB. 1973. Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol. Monog. 43, 95–124. ( 10.2307/1942161) [DOI] [Google Scholar]
- 4.Castagneyrol B, Giffard B, Pére C, Jactel H. 2013. Plant apparency, and overlooked driver of associational resistance to insect herbivory. J. Ecol. 101, 418–429. ( 10.1111/1365-2745.12055) [DOI] [Google Scholar]
- 5.Feeny P. 1976. Plant apparency and chemical defense. Recent Adv. Phytochem. 10, 1–40. ( 10.1007/978-1-4684-2646-5_1) [DOI] [Google Scholar]
- 6.Kim TN, Underwood N. 2015. Plant neighborhood effects on herbivory: damage is both density and frequency dependent. Ecology 96, 1431–1437. ( 10.1890/14-1097.1) [DOI] [PubMed] [Google Scholar]
- 7.Schuldt A, Assmann T, Bruelheide H, Durka W, Eichenberg D, Härdtle W, Kröber W, Michalski SG, Purschke O. 2014. Functional and phylogenetic diversity of woody plants drive herbivory in a highly diverse forest. New Phytol. 202, 864–873. ( 10.1111/nph.12695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumann MC, Van-Dam NM, Beran F, Harpole WS. 2016. How does plant chemical diversity contribute to biodiversity at higher trophic levels? Curr. Opin. Insect Sci. 14, 46–55. ( 10.1016/j.cois.2016.01.003) [DOI] [PubMed] [Google Scholar]
- 9.Ren X, He T, Chang Y, Zhao Y, Chen X, Bai S, Wang L, Shen M, She G. 2017. The genus Alnus, a comprehensive outline of its chemical constituents and biological activities. Molecules 22, E1383 ( 10.3390/molecules22081383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetzel WC, Kharouba HM, Robinson M, Holyoak M, Karban R. 2016. Variability in plant nutrients reduces insect herbivore performance. Nature 539, 425–427. ( 10.1038/nature20140) [DOI] [PubMed] [Google Scholar]
- 11.Webb CO, Gilbert GS, Donoghue MJ. 2006. Phylodiversity-dependent seedling mortality, size structure, and disease in a Bornean rain forest. Ecology 87, S123–S131. ( 10.1890/0012-9658(2006)87%5B123:PSMSSA%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 12.Castagneyrol B, Jactel H, Vacher C, Brockerhoff EG, Koricheva J. 2014. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. Appl. Ecol. 51, 134–141. ( 10.1111/1365-2664.12175) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Electronic supplementary material, table SM4 reports all data used for statistical analyses.