Abstract
Understanding how breeding populations are spatially and temporarily associated with one another over the annual cycle has important implications for population dynamics. Migratory connectivity typically assumes that populations mix randomly; yet, in many species and populations, sex-, age- or other subgroups migrate separately, and/or spend the non-breeding period separated from each other—a phenomenon coined differential migration. These subgroups likely experience varying environmental conditions, which may carry-over to affect body condition, reproductive success and survival. We argue that environmental or habitat changes can have disproportional effects on a population's demographic rates under differential migration compared to random mixing. Depending on the relative contribution of each of these subgroups to population growth, environmental perturbations may be buffered (under-proportional) or amplified (over-proportional). Thus, differential migration may result in differential mortality and carry-over effects that can have concomitant consequences for dynamics and resilience of the populations. Recognizing the role of differential migration in migratory connectivity and its consequences on population dynamics can assist in developing conservation actions that are tailored to the most influential demographic group(s) and the times and places where they are at peril.
Keywords: migratory connectivity, differential migration, population dynamics, migration, global change, targeted conservation
1. Introduction
Many migratory populations have experienced massive declines over the past years mainly as a consequence of the single or combined effects of habitat loss and deterioration, climate change, erection of barriers and sensory pollution [1–4]. Their conservation requires understanding migratory connectivity, i.e. how breeding populations are spatially and temporarily associated with one another over the annual cycle—during breeding, migration and the non-breeding season [5–7]. The connectivity patterns have important implications for short-term population dynamics as well as long-term trends.
Migratory connectivity is quantified along a continuum from low interpopulation spread and complete segregation of different breeding populations (strong connectivity) to high interpopulation spread and thorough mixing of individuals from different breeding populations (weak connectivity) [6,8]. Under the original definition of migratory connectivity [6], the entire migratory period is overlooked including in how far individuals from one breeding populations may migrate at different times or use different routes and stopover sites, suggesting that migratory connectivity is not purely a breeding to non-breeding phenomenon. Including the migration period in the definition of migratory connectivity is vital for many species and populations especially those in which sex-, age- or other subgroups migrate separately, and/or spend the non-breeding period separately from each other—a phenomenon coined differential migration [9]. Due to this segregation, subgroups likely experience varying environmental conditions en route and at the non-breeding sites. Exposure to different environments may carry-over to influence individual physical condition potentially leading to group-specific en route mortality [10], spring arrival times [11] or reproductive success [12]. More importantly, if global and climate change alter habitats at varying rates and magnitudes, these segregated subgroups will be affected to different degrees.
We argue in the following that environmental or habitat changes can have disproportional effects on a population's demographic rates under migratory segregation compared to random mixing. Depending on the relative contribution of each subgroup to population growth, negative effects caused by environmental perturbations may be buffered (under-proportional) or amplified (over-proportional). Thus, differential migration resulting in differential mortality and carry-over effects can have concomitant consequences for the dynamics of migratory populations.
2. Differential migration
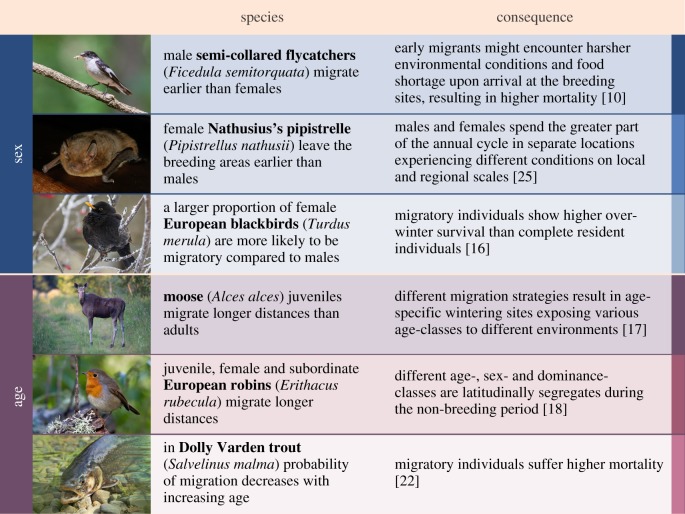
Differential migration is a widespread phenomenon in the animal kingdom and can take many forms, from differences in the timing of migration to differences in migration routes: subgroups may segregate during relatively short periods, during migration [10,13–15], during non-breeding residency [16,17] or for most of the year [18,19] (figure 1). Similarly, the spatial scales of segregation may vary from local segregation such as the use of microhabitats [20], to regional (several tens to a few hundreds of kilometres) when different stopover or wintering sites are used [16,18], to continental, when subgroups use entirely different flyways [21].
Figure 1.
Examples of differential migration from throughout the animal kingdom and its consequences for population dynamics as mediated via migratory connectivity. (Photo credits from top: Martins Briedis, Viesturs Vintulis, Edgars Smislovs, Agris Krusts, Ainārs Mankus, LoveToTakePhotos (pixabay.com).).
Probably, the most common segregation is between sex- and age-groups but other segregations, e.g. between dominants/subordinates and breeders/non-breeders may also occur (figure 1). In sexual segregation, males precede females (protandry), females precede males (protogeny), females use other habitats than males or migrate via different stopover sites to different non-breeding sites. Similarly, differential migration by age means that juveniles differ in migration timing from adults and/or use different flyways to different non-breeding sites [13,17,22,23]. Dominance status may force subordinates to undertake longer migrations [18,24] or reside in suboptimal non-breeding habitats [20]. In partially migratory populations and short-distance migrants, migratory behaviour is often female skewed with a larger proportion of females being migratory [24], leaving the breeding grounds earlier [25] or travelling longer distances compared to males [18].
These examples illustrate how the two core components of migratory connectivity—spatial and temporal site use across the annual cycle—can be essentially different for distinct groups of individuals within the same core population.
3. Cascading consequences of differential migration on migratory connectivity
Patterns of migratory connectivity can shape population dynamics on local and range-wide scales [26,27]. Strongly connected migratory populations are thought to be more vulnerable to environmental changes as all individuals cluster at certain places and times, and changes on any of those would affect the entire population. By contrast, weak migratory connectivity is thought to buffer negative consequences of environmental perturbations, adverse weather or food shortage on specific sites and times as only part of a breeding population experiences these conditions [28].
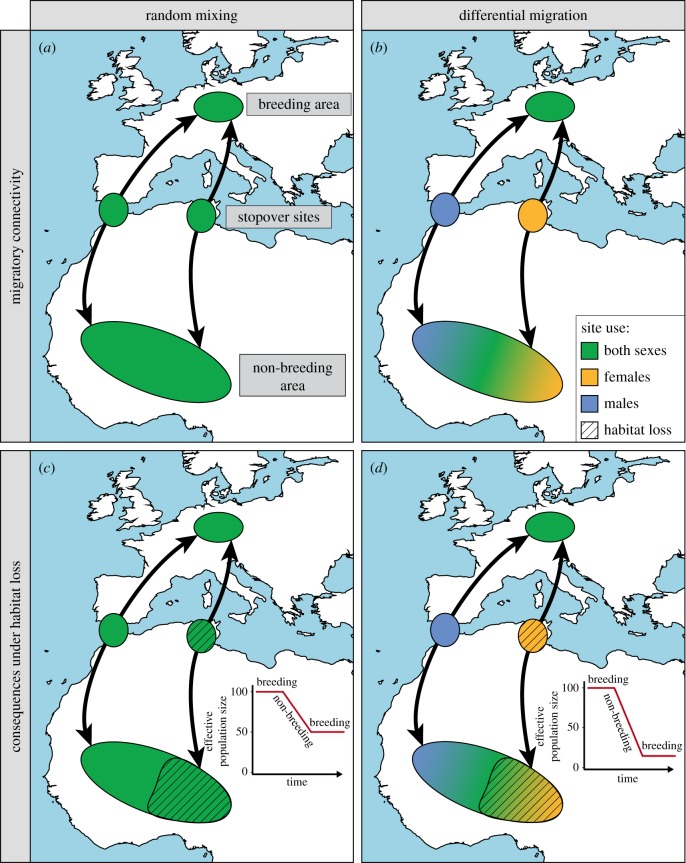
Under differential migration, connectivity patterns are stronger within groups (e.g. within sex or age classes) than between groups, leading to demography-specific vulnerability across the annual cycle (figure 2). Consequently, environmental perturbations may have under- or over-proportional effects on population dynamics if a specific demographic group is concerned, i.e. effects are much larger or smaller than what would be expected under random mixing of individuals. For instance, if males and females migrate differentially and only females suffer a high mortality, the population's sex ratio will become male-biased and its vital rates plummet even though more than half of the individuals had returned [29]. Male-biased sex ratios are particularly problematic in small populations causing local population declines [30]. By contrast, differential migration may also prevent major consequences of massive (local) habitat alterations if a demographically less significant group is affected. For instance, if young of a long-lived species experience higher mortality for a restricted period, this might show only in short-term population dynamics, but not in the long-term population trajectory [31].
Figure 2.
Schematic of (spatial) migratory connectivity in a hypothetical species under (a) random mixing of individuals and (b) sex-specific differential migration. When males and females mix randomly during migration and non-breeding residency, the consequences of habitat loss on population dynamics will be less severe (c) than under differential migration where exclusively one demographic group is affected (d).
4. Conservation implications
The widespread application of tracking devices over the past decade(s) has yielded a wealth of information on individual migration routes and schedules, and increasingly provides the possibility to determine the degree of migratory connectivity. As mounting individual tags requires the handling of individual animals, their sex and age (or other characteristics) are usually also recorded or determined retrospectively. Although we are not aware of an explicit analysis of differential migration, migratory connectivity and its link to population trends, the data to explore such links are certainly there and will continue to be accumulated [32–34].
Thus, (re-)analysing existing individual migration data with regard to differential migration is a first step in understanding demography-specific connectivity [15]. However, we also need to analyse the contribution of demographic groups to population vital rates (e.g. fecundity and seasonal survival probability) which could then be used to develop full annual cycle population models [35,36] to identify the places and times at which specific demographic groups are most at peril. Conservation and management actions could then be tailored to these places and times and targeted at the most influential demographic groups.
Thus, we call for recognizing the role of differential migration in migratory connectivity as well as its potential consequences for population trends and the implications these may have for conserving and managing migratory populations.
Acknowledgements
We are thankful to our colleagues at the Swiss Ornithological Institute for fruitful discussions of the topic and A. Krusts, A. Mankus, E. Smislovs and V. Vintulis for kindly providing images.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors contributed equally to conceptualizing and writing the manuscript.
Competing interests
We declare no competing interests.
Funding
Funding was provided by the Swiss Ornithological Institute.
References
- 1.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Antrhopocene. Science 401, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 2.Bolger DT, Newmark WD, Morrison TA, Doak DF. 2008. The need for integrative approaches to understand and conserve migratory ungulates. Ecol. Lett. 11, 63–77. ( 10.1111/j.1461-0248.2007.01109.x) [DOI] [PubMed] [Google Scholar]
- 3.Brower LP, Taylor OR, Williams EH, Slayback DA, Zubieta RR, Ramírez MI. 2012. Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv. Divers. 5, 95–100. ( 10.1111/j.1752-4598.2011.00142.x) [DOI] [Google Scholar]
- 4.Gilroy JJ, Gill JA, Butchart SHM, Jones VR, Franco AMA. 2016. Migratory diversity predicts population declines in birds. Ecol. Lett. 19, 308–317. ( 10.1111/ele.12569) [DOI] [PubMed] [Google Scholar]
- 5.Marra PP, Norris RD, Haig SM, Webster M, Royle AJ. 2006. Migratory connectivity. In Connectivity conservation (eds Crooks RK, Sanjan M), pp. 157–183. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT. 2002. Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 17, 76–83. ( 10.1016/S0169-5347(01)02380-1) [DOI] [Google Scholar]
- 7.Bauer S, Lisovski S, Hahn S. 2015. Timing is crucial for consequences of migratory connectivity. Oikos 125, 605–612. ( 10.1111/oik.02706) [DOI] [Google Scholar]
- 8.Cohen EB, Hostetler JA, Hallworth MT, Rushing CS, Sillett TS, Marra PP. 2018. Quantifying the strength of migratory connectivity. Methods Ecol. Evol. 9, 513–524. ( 10.1111/2041-210X.12916) [DOI] [Google Scholar]
- 9.Cristol DA, Baker MB, Carbone C. 1999. Differential migration revisited: latitudinal segregation by age and sex class. Curr. Ornithol. 15, 33–88. ( 10.1007/978-1-4757-4901-4_2) [DOI] [Google Scholar]
- 10.Briedis M, Hahn S, Adamík P. 2017. Cold spell en route delays spring arrival and decreases apparent survival in a long-distance migratory songbird. BMC Ecol. 17, 11 ( 10.1186/s12898-017-0121-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marra PP. 1998. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886. ( 10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 12.Rushing CS, Marra PP, Dudash MR. 2016. Winter habitat quality but not long-distance dispersal influences apparent reproductive success in a migratory bird. Ecology 97, 1218–1227. ( 10.1890/15-1259.1/suppinfo) [DOI] [PubMed] [Google Scholar]
- 13.Handel CM, Gill RE. 2010. Wayward youth: trans-Beringian movement and differential southward migration by juvenile sharp-tailed sandpipers. Arctic 63, 273–288. ( 10.14430/arctic1492) [DOI] [Google Scholar]
- 14.Morbey Y. 2000. Protandry in Pacific salmon. Can. J. Fish. Aquat. Sci. 57, 1252–1257. ( 10.1139/f00-064) [DOI] [Google Scholar]
- 15.Cohen EB, Rushing CR, Moore FR, Hallworth MT, Hostetler JA, Ramirez MG, Marra PP. 2018. The strength of migratory connectivity for birds en route to breeding through the Gulf of Mexico. Ecography, 1–12. ( 10.1111/ecog.03974) [DOI] [Google Scholar]
- 16.Zúñiga D, Gager Y, Kokko H, Fudickar AM, Schmidt A, Naef-Daenzer B, Wikelski M, Partecke J. 2017. Migration confers winter survival benefits in a partially migratory songbird. Elife 6, 1–12. ( 10.7554/eLife.28123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh NJ, Börger L, Dettki H, Bunnefeld N, Ericsson G. 2012. From migration to nomadism: movement variability in a northern ungulate across its latitudinal range. Ecol. Appl. 22, 2007–2020. ( 10.1890/12-0245.1) [DOI] [PubMed] [Google Scholar]
- 18.Catry P, Campos A, Almada V, Cresswell W. 2004. Winter segregation of migrant European robins Erithacus rubecula in relation to sex, age and size. J. Avian Biol. 35, 204–209. ( 10.1111/j.0908-8857.2004.03266.x) [DOI] [Google Scholar]
- 19.Fleming TH, Eby P. 2003. Ecology of bat migration. In Bat ecology (eds Kunz TH, Fenton MB), pp. 156–208. Chicago, IL: University of Chicago Press. [Google Scholar]
- 20.Marra PP, Holmes RT. 2001. Consequences of dominance-mediated habitat segregation in American redstarts during the nonbreeding season. Auk 118, 92 ( 10.1642/0004-8038(2001)118%5B0092:CODMHS%5D2.0.CO;2) [DOI] [Google Scholar]
- 21.Rotics S, et al. 2017. Wintering in Europe instead of Africa enhances juvenile survival in a long-distance migrant. Anim. Behav. 126, 79–88. ( 10.1016/j.anbehav.2017.01.016) [DOI] [Google Scholar]
- 22.Bond MH, Miller JA, Quinn TP, Ciannelli L. 2015. Beyond dichotomous life histories in partially migrating populations: cessation of anadromy in a long-lived fish. Ecology 96, 1899–1910. ( 10.1890/14-1551.1) [DOI] [PubMed] [Google Scholar]
- 23.Hays GC, Scott R. 2013. Global patterns for upper ceilings on migration distance in sea turtles and comparisons with fish, birds and mammals. Funct. Ecol. 27, 748–756. ( 10.1111/1365-2435.12073) [DOI] [Google Scholar]
- 24.Fudickar AM, Schmidt A, Hau M, Quetting M, Partecke J. 2013. Female-biased obligate strategies in a partially migratory population. J. Anim. Ecol. 82, 863–871. ( 10.1111/1365-2656.12052) [DOI] [PubMed] [Google Scholar]
- 25.Pētersons G. 2004. Seasonal migrations of north-eastern populations of Nathusius' bat Pipistrellus nathusii (Chiroptera). Myotis 41-42, 29–56. [Google Scholar]
- 26.Hewson CM, Thorup K, Pearce-Higgins JW, Atkinson PW. 2016. Population decline is linked to migration route in the common cuckoo. Nat. Commun. 7, 12296 ( 10.1038/ncomms12296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patchett R, Finch T, Cresswell W. 2018. Population consequences of migratory variability differ between flyways. Curr. Biol. 28, R340–R341. ( 10.1016/j.cub.2018.03.018) [DOI] [PubMed] [Google Scholar]
- 28.Taylor CM, Norris DR. 2010. Population dynamics in migratory networks. Theor. Ecol. 3, 65–73. ( 10.1007/s12080-009-0054-4) [DOI] [Google Scholar]
- 29.Lee AM, Sæther B-E, Engen S. 2011. Demographic stochasticity, Allee effects, and extinction: the influence of mating system and sex ratio. Am. Nat. 177, 301–313. ( 10.1086/658344) [DOI] [PubMed] [Google Scholar]
- 30.Morrison CA, Robinson RA, Clark JA, Gill JA. 2016. Causes and consequences of spatial variation in sex ratios in a declining bird species. J. Anim. Ecol. 85, 1298–1306. ( 10.1111/1365-2656.12556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaillard JM, Festa-Bianchet M, Yoccoz NG. 1998. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol. Evol. 13, 58–63. ( 10.1016/S0169-5347(97)01237-8) [DOI] [PubMed] [Google Scholar]
- 32.McKinnon EA, Love OP. 2018. Ten years tracking the migrations of small landbirds: lessons learned in the golden age of bio-logging. Auk 135, 834–856. ( 10.1642/AUK-17-202.1) [DOI] [Google Scholar]
- 33.López-López P. 2016. Individual-based tracking systems in ornithology: welcome to the era of big data. Ardeola 63, 103–136. ( 10.13157/arla.63.1.2016.rp5) [DOI] [Google Scholar]
- 34.Fudickar AM, Ketterson ED. 2018. Genomes to space stations: the need for the integrative study of migration for avian conservation. Biol. Lett. 14, 20170741 ( 10.1098/rsbl.2017.0741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hostetler JA, Sillett TS, Marra PP. 2015. Full-annual-cycle population models for migratory birds. Auk 132, 433–449. ( 10.1642/AUK-14-211.1) [DOI] [Google Scholar]
- 36.Rushing CS, Hostetler JA, Sillett TS, Marra PP, Rotenberg JA, Ryder TB. 2017. Spatial and temporal drivers of avian population dynamics across the annual cycle. Ecology 98, 2837–2850. ( 10.1002/ecy.1967) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.