Abstract
Background
Diabetes mellitus (DM) is one of the most common diseases found across the world. Aster koraiensis extract (AKE) has a protective effect on diabetic complications such as diabetic retinopathy. However, the effects of AKE on hyperglycemia-linked impairment of wound healing during DM have not been elucidated. In this study, we investigated the effects of AKE on delayed wound healing induced by DM.
Methods
DM was induced by intraperitoneal administration of streptozotocin (STZ; 75 mg/kg) to Sprague Dawley (SD) rats. Next, a wound was induced on the back of rats after administration of STZ. Further, AKE was prepared using an alcoholic extraction of A. koraiensis and orally administered daily for 18 days. Wound healing was evaluated using an in vitro migration assay and measuring the wound area in vivo. Skin tissue thickness was evaluated using hematoxylin and eosin staining. Matrix metalloprotease (MMP) activity and expression were detected using zymography and immunohistochemistry.
Results
AKE administration improved the delayed migration of keratinocytes in hyperglycemic animals. It also attenuated an increase in keratinocyte MMP-2/9 activity induced by hyperglycemia. AKE protected against DM-induced impaired wound healing in rats and prevented the degradation of skin tissue induced by DM. In addition, AKE attenuated DM-induced increase in MMP-2/9 expression in skin tissue.
Conclusions
In conclusion, AKE may promote wound healing by re-epithelization via promotion of keratinocyte migration and by attenuating the disruption of the skin tissue layer via MMP-2/9 inhibition during hyperglycemia.
Keywords: Aster koraiensis extract, Diabetes mellitus, Hyperglycemia, Matrix metalloprotease, Wound healing
1. Introduction
Diabetes mellitus (DM) is a metabolic disease that occurs due to a malfunction in insulin sensitivity or secretion. DM is a critical disease that affects quality of life in modern society, and its prevalence has recently increased globally.1 DM leads to many diseases, also called diabetic complications, including diabetic retinopathy, diabetic neuropathy, and diabetic foot ulcers.2 Additionally, the development of microvascular and macrovascular complications is induced by chronic hyperglycemia in patients with DM.3 In particular, delayed wound healing is one of the most serious diabetic complications.4 Several research studies have demonstrated that wound healing is delayed by hyperglycemia during DM.5, 6, 7
A skin wound is a common occurrence in human life and results in the activation of protective wound healing mechanisms.8 The loss of this critical protective function exposes our body to microbial infections. Poor wound healing may result in serious infections requiring amputations in patients with chronic diabetic foot ulcers.9 Therefore, it is important to improve the process of wound healing by treating the wound appropriately.
Aster koraiensis, also known as Korean starwort, was first described by Nakai Takenoshin in 1909. It is a widely cultivated perennial plant native to Korea and belongs to the Asteraceae (Compositae) family.10 Traditionally, A. koraiensis extract (AKE) has been used as an herbal medication for chronic bronchitis, pneumonia, and pertussis.11 In a previous study, we found that the AKE improved diabetic complications such as diabetic retinopathy.10 Several studies have reported that skin wound healing is impaired and delayed in hyperglycemia.4, 5, 12 However, the effect of AKE on hyperglycemia-induced impaired wound healing has not been investigated and the mechanisms underlying these effects remain unknown. Therefore, in this study, we investigated whether AKE improves hyperglycemia-induced delayed wound healing via increased keratinocyte migration and inhibition of skin tissue degradation.
2. Methods
2.1. Preparation of A. koraiensis extract and standard solution
In 2011, aerial parts of A. koraiensis were purchased from Gongju, Republic of Korea. The preparation of AKE was performed according to a previously reported method.11 Briefly, 2.5 kg of dried and ground A. koraiensis was extracted with EtOH (3.2 L) by maceration for 3 days. AKE was stored in a deep freezer at −70°C. The resultant AKE was characterized using chlorogenic acid (Sigma, MO, USA) and 3,5-di-O-caffeoylquinic acid (Sigma, MO, USA) as reference compounds using high-performance liquid chromatography (HPLC) as described in our previous study.10 A voucher specimen (KIOM-83A) has been deposited in the herbarium of the Korea Institute of Oriental Medicine, Republic of Korea.
2.2. Animals
Seven-week-old male Sprague Dawley (SD) rats were purchased from Orient Bio (Seoul, Republic of Korea). All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Korea Institute of Oriental Medicine (Daejeon, South Korea; IACUC approval no. 13-060). Rats were divided into three groups: (1) normal group, (2) DM group, and (3) AKE-treated group (100 mg/kg of body weight, p.o.). To induce DM (as defined by the presence of a blood glucose concentration greater than 300 mg/dL), 75 mg/kg of streptozotocin (STZ, Sigma Aldrich, St. Louis, MO) was administered intraperitoneally. Age-matched normal SD rats were administered saline. Eighteen days after STZ injection, a wound was produced on the back of each rat and then AKE extract was administered orally once daily for 18 days.
2.3. Wound area measurement
The wound area was photographed daily for 18 days, and these images were used to measure wound size. The size of the wound area was captured and analyzed using a microscope (Olympus, Tokyo, Japan).
2.4. Hematoxylin and eosin staining
Skin tissue was dissected and used for histopathologic evaluation. Samples were fixed in neutral 10% formalin solution for 24 hours at room temperature, dehydrated in ethanol, cleared in xylene, and embedded in paraffin. Hematoxylin and eosin-stained paraffin sections were obtained for histological examination. Paraffin section images were observed under an optical microscope.
2.5. Immunohistochemistry
Dissected skin tissue samples were fixed, dehydrated, cleared, and paraffin-embedded as mentioned previously. Resultant samples were incubated with anti-MMP-9 antibody (1:500; Abcam, Cambridge, UK) overnight and then stained with DAB staining solution. Counterstaining was performed using hematoxylin. All samples were then observed under a microscope.
2.6. Cell culture
Human keratinocytes (HaCaT) were obtained from Huons Co. Ltd, (Gyeonggi-do, Korea) and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), at 37°C in a 5% CO2 incubator. Cells were treated with AKE (3 μg/mL, 10 μg/mL, 30 μg/mL, or 100 μg/mL) under hyperglycemic conditions (200mM glucose in media).
2.7. Migration assay
Keratinocyte migration assays were performed as an in vitro assessment of wound healing. Keratinocytes were grown in Culture-Insert 2 Well apparatuses (Ibidi, Martinsried, Germany). After removing the Culture-Insert, cells were treated with AKE (3 μg/mL, 10 μg/mL, 30 μg/mL, or 100 μg/mL) under hyperglycemic conditions (200mM glucose in media). After 10 hours of treatment, cell migration into wound areas was captured and analyzed using a microscope (Olympus, Tokyo, Japan).
2.8. Gelatin zymography
Gelatin zymography was performed by modifying procedures described previously.13 Media were collected eight hours after AKE treatment and added to 4X non-reducing sample buffer. The samples were then loaded on a zymogram gel (Invitrogen, Carlsbad, CA, USA) and analyzed for degradation of gelatin. Next, the zymogram gels were rinsed for 30 minutes in a renaturing buffer (Invitrogen) at room temperature with gentle agitation, followed by rinsing for 30 minutes in a development buffer (Invitrogen) at room temperature with gentle agitation. Thereafter, development buffer was replaced with fresh development buffer and zymogram gels were incubated for 16–18 hours at 37°C. After incubation, MMP-9 activity was visualized using a GelDoc™ imager (Bio-Rad, Hercules, CA) after staining with a Colloidal Blue Staining Kit (Invitrogen).
2.9. Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by multiple comparison tests for comparisons between three or more groups. Additionally, we utilized Student t-test to compare between two groups. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). p-values less than 0.01 or 0.05 were considered statistically significant.
3. Results
3.1. The effect of AKE on in vivo wound healing in DM
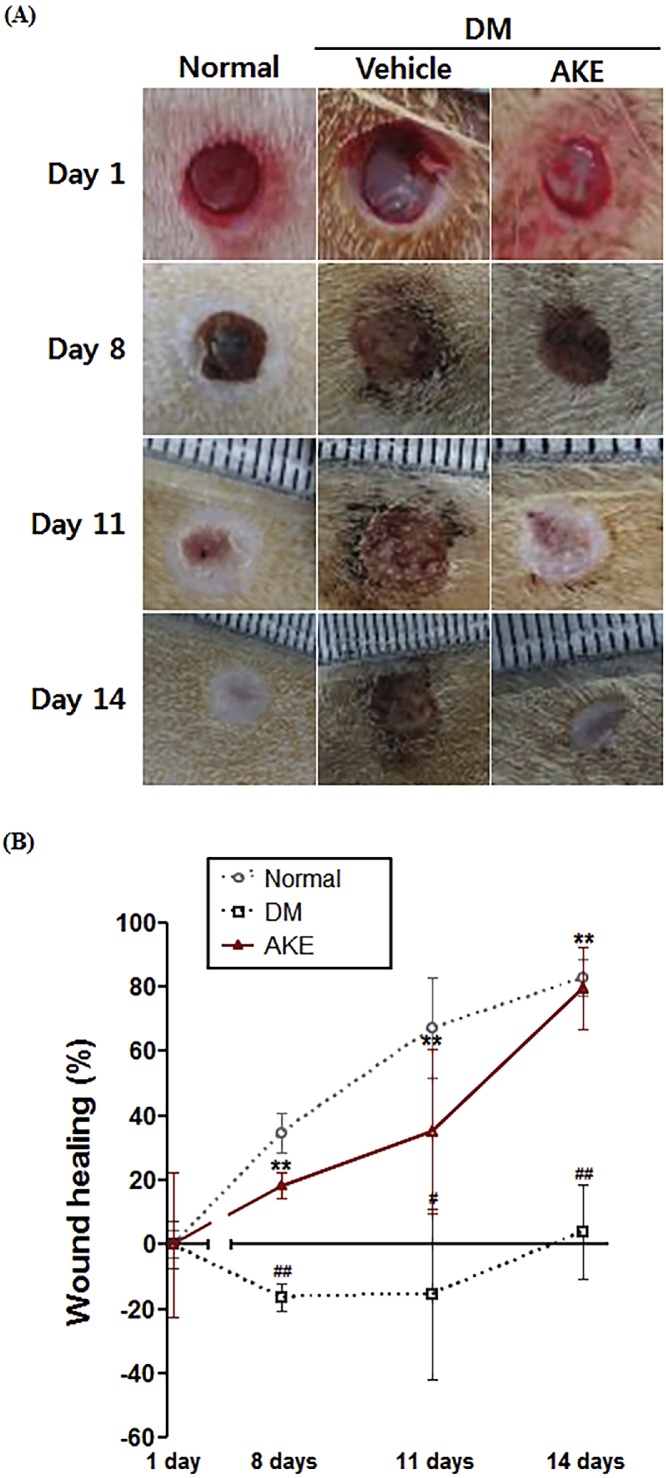
To investigate the effect of AKE on wound healing in vivo, we performed a wound healing assay using wounded AKE-treated rats (100 mg/kg, p.o.). Normal rats naturally healed by 14 days after wound induction while diabetic rats healed slowly. The wound area in the AKE-treated rats after 14 days was similar in size to those of the normal rats (Fig. 1A). As shown in Fig. 1B, wound healing in normal rats increased in a time-dependent manner. On the other hand, wounds in diabetic rats healed slower than in the normal rats. However, wounds in AKE-treated rats healed faster than in untreated diabetic rats. At 14 days after wound induction, wound healing extent in AKE-treated rats (80 ± 6.4%) was similar to normal rats (83 ± 2.8%), whereas diabetic rats did not exhibit any healing (4 ± 7.3%).
Fig. 1.
The effect of Aster koraiensis extract on wound healing in diabetic rats. The wound area was observed at 0, 8, 11, and 14 days after wound induction in diabetic rats treated with AKE (100 mg/kg, p.o.) or vehicle. (A) Representative photographs of wound areas. (B) Graph of wound healing. Wound healing is expressed as the percentage of area occupied by skin in the total wound area. Data are expressed as mean ± SEM. **=p < 0.01 vs. Normal, #=p < 0.05 or ##=p < 0.01 vs. Diabetes Mellitus (DM) (n = 4).
3.2. The effect of AKE on in vitro wound healing under hyperglycemic conditions
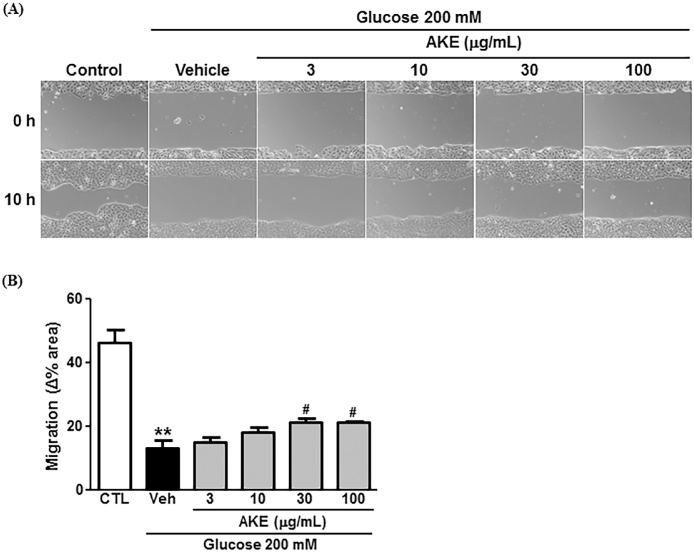
To investigate the effect of AKE on wound healing in vitro, we performed a migration assay in HaCaT cells (keratinocytes). As shown in Fig. 2A, keratinocytes subjected to hyperglycemic conditions (200mM glucose) migrated slowly relative to cells under normal conditions (control). We also observed that the AKE treatment increased keratinocyte migration relative to vehicle-treated cells under hyperglycemic conditions. Migration area change was significantly reduced under hyperglycemic conditions (13 ± 2.6%) compared with control cells (46 ± 3.8%). AKE administration at 30 μg/mL (21 ± 1.5%) and 100 μg/mL (21 ± 0.6%) doses significantly reversed the hyperglycemia-induced decrease in cell migration (Fig. 2B).
Fig. 2.
The effect of Aster koraiensis extract on in vitro wound healing under hyperglycemic conditions. The migration of HaCaT cells was observed after 10 h incubation under hyperglycemic conditions after treatment with AKE (100 μg/mL) or Vehicle. (A) Representative photographs of cell migration. (B) Changes in cell migration. Migration of HaCaT cells was measured by the percentage of area occupied by cells in the total area. Data are expressed as mean ± SEM. ** = p < 0.01 vs. Control (CTL), #=p < 0.05 vs. Vehicle (Veh) (n = 4).
3.3. The effect of AKE on in vivo thickness of skin tissue in diabetes mellitus rats
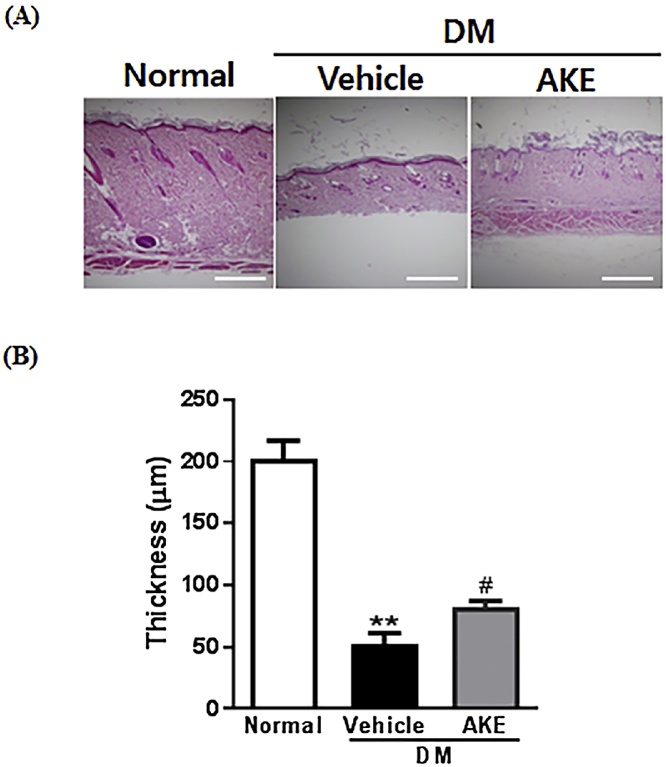
To investigate whether AKE protected against DM-induced skin tissue disruption in DM, we performed immunohistochemistry using paraffin skin sections. Skin thickness was measured using hematoxylin and eosin staining. Skin layers were disrupted in diabetic rats (52 ± 9.0 μm) compared to normal rats (202 ± 15.1 μm), and this disruption was significantly reversed in AKE-treated rats (82 ± 4.9 μm) (Fig. 3).
Fig. 3.
The effect of Aster koraiensis extract on disruption of skin tissue in diabetic rats. Skin tissue around wounds was observed at 18 days after wound induction in diabetic rats which received either AKE (100 mg/kg, p.o.) or Vehicle. (A) Representative photographs of skin tissue. Skin tissue was stained by H&E. (B) The quantitative analysis of skin tissue thickness. Scale bar = 50 μm. Data are expressed as mean ± SEM. **=p < 0.01 vs. Normal, # = p < 0.05 vs. Diabetes Mellitus (DM) (n = 4).
3.4. The effect of AKE on in vivo MMP-2/9 expression in diabetes mellitus rats
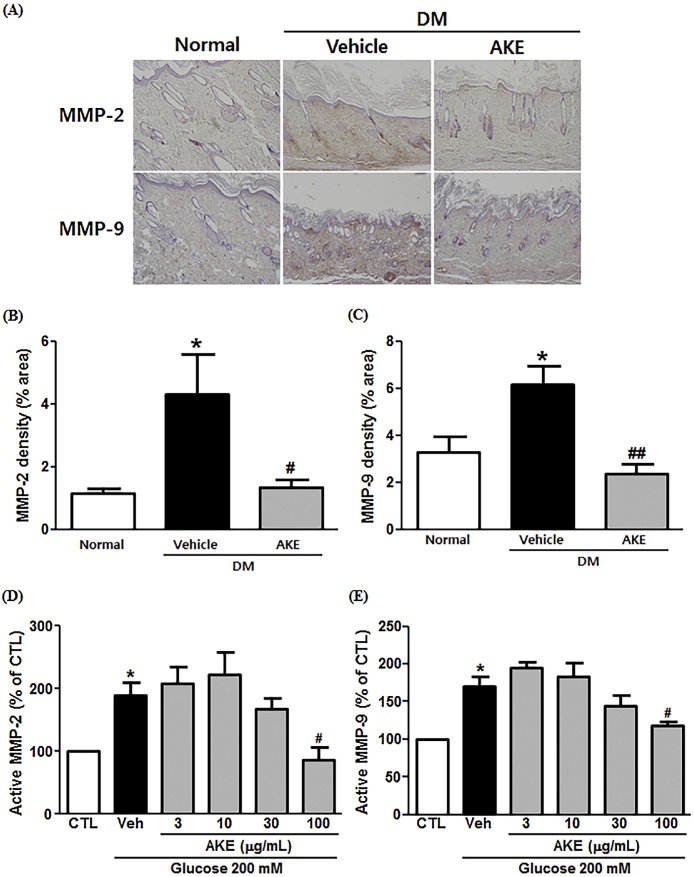
To examine the involvement of MMP-2/9 in wound healing during DM, we determined the MMP-2/9 density in skin sections. As shown in Fig. 4A, the expression of MMP-2/9 was increased in diabetic rats compared to normal rats, and the MMP-2/9 expression in AKE-treated rats was decreased relative to diabetic rats. MMP-2 expression density in diabetic rats (4.3 ± 1.3) was significantly increased compared to that in normal (1.1 ± 0.2) rats, with AKE administration lowering skin MMP-2 expression (1.3 ± 0.3) (Fig. 4B). Similar to MMP-2, MMP-9 density in diabetic rats (6.1 ± 0.8) was significantly increased compared with normal (3.3 ± 0.7) rats, and AKE administration lowered skin MMP-9 expression (2.3 ± 0.4) (Fig. 4C).
Fig. 4.
The effect of Aster koraiensis extract on MMP-2/9 expression in diabetic rats. The expression of MMP-2/9 in the wound area was observed at 18 days after wound induction in diabetic rats administered either AKE (100 mg/kg, p.o.) or Vehicle. (A) Representative photographs of MMP-2/9 expression (Red) in skin tissue. MMP-2 and MMP-9 were stained with DAB. Quantitative analysis of MMP-2 (B) and MMP-9 (C) expression in skin tissue. Data are expressed as mean ± SEM. *=p < 0.05 vs. Normal, #=p < 0.05, or ##= p < 0.01 vs. Diabetes Mellitus (DM). MMP-2/9 activity was observed after 8 h incubation under hyperglycemic conditions, in the presence of AKE (100 μg/mL) or Vehicle. Quantitative analysis of MMP-2 (D) and MMP-9 (E) activity is expressed as mean ± SEM, *=p < 0.05 vs. Control (CTL). #=p < 0.05 vs. Vehicle (Veh) (n = 4).
3.5. The effect of AKE on MMP-2/9 activity in hyperglycemia
To determine the effect of AKE on MMP-2/9 activity, we performed gelatin zymography in keratinocytes. The quantitative values of active MMP-2 activity under hyperglycemic conditions (190 ± 19.7%) was significantly elevated relative to cells under normal conditions (control; 100 ± 0.0%). AKE (100 μg/mL) significantly reduced this hyperglycemia-induced elevation in MMP-2 activity (87 ± 19.8%) (Fig. 4D). Similarly, active MMP-9 amounts were significantly elevated in hyperglycemic cells (170 ± 13.2%) compared to normal cells (control; 100 ± 0.0%) and this elevation was significantly reduced by AKE (100 μg/mL; 119 ± 5.1%) (Fig. 4E).
4. Discussion
Our findings demonstrated that AKE protects against impaired wound healing induced by hyperglycemia.
The skin is the first barrier of defense in our body and protects the body from various harmful agents including chemicals and microorganisms. When the skin barrier is destroyed by a wound, the skin repair process, including re-epithelization, spontaneously occurs to protect from microbial infection.8 Generally, there are two types of DM, type 1 diabetes mellitus (T1D) and type 2 diabetes mellitus (T2D). In T1D, insulin is not secreted because of numerous reasons such as autoimmune diseases and viruses. Alternatively, in T2D, insulin is secreted but presents reduced activity. Blood glucose levels are elevated in patients with either T1D or T2D, resulting in delayed skin wound healing.4 Wound healing is a complex and dynamic process which can be divided into three phases: the inflammatory phase, proliferative phase, and remodeling phase.8 Of these phases, it is well known that re-epithelization is involved in the proliferative phase of wound healing.14, 15, 16 In the present study, our in vivo results exhibited that hyperglycemia impaired the wound healing process on the backs of rats, while AKE accelerated the wound healing process. Consistent with the in vivo results, we observed that hyperglycemia delayed the migration of keratinocytes and AKE reversed this phenomenon. These results reveal that hyperglycemia negatively affects the wound healing process and AKE may improve the wound healing process during hyperglycemia. Additionally, re-epithelization is affected by the proliferation and migration of keratinocytes.8 We observed the effects of AKE on HaCaT cell migration and proliferation during hyperglycemia. However, AKE was not able to increase cell proliferation (data not shown). These findings indicate that AKE has a protective effect on re-epithelization by improving migration but not proliferation.
MMPs have a different role at each phase of wound healing process.17 In the inflammatory phase, MMPs remove temporary ECM and damaged proteins. During the proliferation phase, MMPs break down the capillary basement membrane to promote cell migration and angiogenesis, and in the remodeling phase, they have a role in remodeling and contracting. In addition, it is well known that MMPs degrade or cleavage ECM proteins. Among the MMPs, MMP-2/9 (gelatinases) are particularly involved in the wound healing process as ECM degraders.17 Hence, in the present study, we examined whether AKE inhibited the expression and activation of MMP-2/9 under hyperglycemic conditions. Our results showed that the expression and activity of MMP-2/9 were elevated and skin tissue thickness was reduced under hyperglycemic conditions, and that AKE attenuated skin tissue degradation. In addition, we demonstrated that AKE decreased hyperglycemia-induced increase in MMP-2/9 expression in the skin and MMP-2/9 activity in keratinocytes. These findings show that AKE exhibits significant anti-MMP activity and thus is a potent therapeutic agent for MMP-mediated diseases.
AKE consists of two major compounds, chlorogenic acid (12.4 ± 0.22 mg/g) and 3,5-di-O-caffeoylquinic acid (22.5 ± 0.51 mg/g), as described in our previous study.10 These compounds have therapeutic effects against diabetic complications such as diabetic retinopathy.10 Specifically, several studies have reported that chlorogenic acid has a protective effect on skin wound healing in DM.12, 18, 19 To our knowledge, no study has reported the effects of 3,5-di-O-caffeoylquinic acid on diabetic wound healing. From these reports, it can be hypothesized that the effects of AKE on wound healing can be attributed to chlorogenic acid that is major active compound in AKE. Further study is needed to investigate this hypothesis, as this study was limited in its ability to elucidate whether chlorogenic acid in AKE could improve the wound healing process.
Chlorogenic acid, protective agent for skin wound healing in DM, is the major phenolic compound found not only in coffee and various fruits20 but also in AKE (extract from plant). As in the case of AKE in this study, in addition, it has been reported that plant extracts in several studies, including Morinda tinctoria Roxb leaves, Ipomoea carnea Jacq., and Acanthus polystachyus Delile, exert protective effects on wound healing.21, 22, 23 These may reveal that AKE may be a potent therapeutic extract for wound healing as other extracts from plants.
In conclusion, we demonstrated that the AKE improved wound healing through the promotion of accelerated keratinocyte migration. Our findings also show that AKE protected against skin tissue disruption in the wound area by inhibiting the expression and activity of MMP-2/9. Therefore, we suggest that AKE has potential as an antidiabetic compound and promotes diabetic wound healing. The consumption of AKE may also offer clinical benefits to improve wound healing process.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bioindustry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (Grant no. 316023-05-3-HD020). This research was also supported by the Korea Institute of Oriental Medicine (K18270).
Contributor Information
Jin Sook Kim, Email: jskim@kiom.re.kr.
Chan-Sik Kim, Email: chskim@kiom.re.kr.
References
- 1.McGuire S. Centers for Disease Control and Prevention. State indicator report on Physical Activity, 2014. Atlanta, GA: U.S. Department of Health and Human Services. Adv Nutr. 2014;5:762–763. doi: 10.3945/an.114.007211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlanga-Acosta J., Mendoza-Mari Y., Martinez M.D., Valdes-Perez C., Ojalvo A.G., Armstrong D.G. Expression of cell proliferation cycle negative regulators in fibroblasts of an ischemic diabetic foot ulcer. A clinical case report. Int Wound J. 2013;10:232–236. doi: 10.1111/j.1742-481X.2012.12000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamagishi S., Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–2299. doi: 10.2174/1381612054367300. [DOI] [PubMed] [Google Scholar]
- 4.Okonkwo U.A., DiPietro L.A. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18:e1419. doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X.N., Ma Z.J., Wang Y., Sun B., Guo X., Pan C.Q. Angelica Dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS One. 2017;12:e0177862. doi: 10.1371/journal.pone.0177862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kido D., Mizutani K., Takeda K., Mikami R., Matsuura T., Iwasaki K. Impact of diabetes on gingival wound healing via oxidative stress. PLoS One. 2017;12:e0189601. doi: 10.1371/journal.pone.0189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu S.C., Lan C.E. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci. 2016;84:121–127. doi: 10.1016/j.jdermsci.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Yeh C.J., Chen C.C., Leu Y.L., Lin M.W., Chiu M.M., Wang S.H. The effects of artocarpin on wound healing: in vitro and in vivo studies. Sci Rep. 2017;7:15599. doi: 10.1038/s41598-017-15876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim J., Jo K., Lee I.S., Kim C.S., Kim J.S. The extract of Aster koraiensis prevents retinal pericyte apoptosis in diabetic rats and its active compound, chlorogenic acid inhibits age formation and AGE/RAGE interaction. Nutrients. 2016;8:e585. doi: 10.3390/nu8090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn E., Kim J., Kim C.S., Kim Y.S., Jang D.S., Kim J.S. Extract of the aerial parts of Aster koraiensis reduced development of diabetic nephropathy via anti-apoptosis of podocytes in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 2010;391:733–738. doi: 10.1016/j.bbrc.2009.11.129. [DOI] [PubMed] [Google Scholar]
- 12.Sarandy M.M., Novaes R.D., Xavier A.A., Vital C.E., Leite J.P.V., Melo F.C.S.A. Hydroethanolic extract of Strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. Biomed Res Int. 2017;2017:9538351. doi: 10.1155/2017/9538351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X., Beeton C. Detection of functional matrix metalloproteinases by zymography. J Vis Exp. 2010:e2445. doi: 10.3791/2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farghali H.A., AbdElKader N.A., Khattab M.S., AbuBakr H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci Rep. 2017;37 doi: 10.1042/BSR20160503. eBSR20160503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousselle P., Montmasson M., Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2018 doi: 10.1016/j.matbio.2018.01.002. [In press] [DOI] [PubMed] [Google Scholar]
- 16.Yang L., Zheng Z., Zhou Q., Bai X., Fan L., Yang C. miR-155 promotes cutaneous wound healing through enhanced keratinocytes migration by MMP-2. J Mol Histol. 2017;48:147–155. doi: 10.1007/s10735-017-9713-8. [DOI] [PubMed] [Google Scholar]
- 17.Ayuk S.M., Abrahamse H., Houreld N.N. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res. 2016;2016:2897656. doi: 10.1155/2016/2897656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagdas D., Etoz B.C., Gul Z., Ozyigit M.O., Cinkilic N., Inan S. Chlorogenic acid enhances abdominal skin flap survival based on epigastric artery in nondiabetic and diabetic rats. Ann Plast Surg. 2016;77:e21–e25. doi: 10.1097/SAP.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 19.Bagdas D., Etoz B.C., Gul Z., Ziyanok S., Inan S., Turacozen O. In vivo systemic chlorogenic acid therapy under diabetic conditions: wound healing effects and cytotoxicity/genotoxicity profile. Food Chem Toxicol. 2015;81:54–61. doi: 10.1016/j.fct.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Clifford M.N., Johnston K.L., Knight S., Kuhnert N. Hierarchical scheme for LC–MSn identification of chlorogenic acids. J Agric Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 21.Demilew W., Adinew G.M., Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae) Evid Based Complement Alternat Med. 2018;2018:2047896. doi: 10.1155/2018/2047896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex Jeya Rajkumar S., Gnanavel G., Muthukumar Nadar M.S.A., Sankaranarayanan R. wound healing activity of Morinda tinctoria Roxb aqueous leaf extract. 3 Biotech. 2018;8:343. doi: 10.1007/s13205-018-1361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla R., Gupta G., Kashaw S.K., Jain A.P., Lodhi S. Wound healing effect of ethanolic extract from Morning Glory (Ipomoea carnea Jacq.) leaves by using different models in rats. Pak J Pharm Sci. 2018;31:1355–1361. [PubMed] [Google Scholar]