Abstract
Background
Vascular access for hemodialysis (HD) with an inappropriately high flow may underlie the onset of high output heart failure (HOHF).
The aim of this study was to determine the prevalence of high flow access (HFA) in chronic HD patients, and to determine its effects on cardiac functions.
Methods
This cross sectional study was conducted on 100 chronic hemodialysis patients through arteriovenous fistula (AVF). The study cohort was subdivided into 2 groups based on AVF flow: Group A (Non-HFA group with Qa < 2000 ml/min), and Group B (HFA group with Qa ≥ 2000 ml/min). AVF flow (Qa) was assessed using Color Doppler ultrasonography. Transthoracic echocardiography was performed for all patients to assess cardiac dimensions and functions.
Results
Prevalence of HFA among study population was 24%. Mean AVF Qa was 958.63 ± 487.35 and 3430.13 ± 1256.28 ml/min, for group A and B respectively. The HFA group demonstrated a significant dilatation in LV dimensions and volumes and significantly larger LA volume as compared to non-HFA group. A significantly lower LV ejection fraction [EF] was also observed in group B with a mean value of 57.32 ± 6.19% versus 62.90 ± 5.76%. A significant association between HFA group and high Qa/cardiac output (CO) ratio (≥20%) was also observed.
Conclusion
HFA is a prevalent hemodialysis vascular access problem. HFA was associated with dilated LV dimensions, impaired LV systolic function. High Qa/CO ratio (≥20%) was an independent predictor of high output heart failure (HOHF) in our study population.
Keywords: High flow access, High output heart failure, Vascular access, Hemodialysis
1. Introduction
On the creation of a HD vascular access, a low-resistance venous pathway is connected to the arterial circuit. AVF is the conduit of choice for dialysis. Although satisfactory vascular access flow (Qa) of an AVF is necessary for HD adequacy. The ideal access should have just enough pressure and flow to prevent thrombosis while maximizing hemodialysis efficiency.1
High access flow is associated with complications like High-output heart failure [HOHF], pulmonary hypertension, massively dilated fistula i.e., megafistula, central vein stenosis, dialysis-associated steal syndrome (or distal hypoperfusion ischemic syndrome.1
Currently, there is no precise definition for a high-flow access (HFA). National Kidney Foundation-Kidney Dialysis Outcome Quality Initiative (NKF-K/DOQI) and European best practice guidelines (EBPG) sections on vascular access did not provide reference ranges for HFA. The Vascular Access Society (VAS) on the other hand has defined a high-flow access as one with a flow (Qa) between 1000 and 1500 ml/min or a Qa that is >20% of the cardiac output (CO).
In a landmark trial in 2008 however, Basile et al., published a study-investigating patients with AV fistulas and heart failure. The study showed that upper arm AVFs are associated with an increased risk of HOHF. It was the first published study with a high predictive power for AV fistula flows greater or equal to 2000 ml/min to result in HOHF.2
Ye et al., in 2013, confirmed a 2000 ml/min threshold for treatment. They demonstrated that the risk of heart failure significantly increases when the Qa of AVF is >2000 ml/min with much higher cardiac output and lower peripheral resistance.3
1.1. Aim of the study
The aim of this study was to determine the prevalence of high flow access (HFA) in chronic HD patients, and to determine its effects on cardiac functions.
2. Methods
This cross sectional study was conducted on 100 chronic hemodialysis patients receiving regular hemodialysis for at least 3 months through a native AVF in Ain Shams University Hospital Dialysis Unit.
Patients with History of cardiovascular disease, echocardiographic evidence of structural heart disease [Valvular or congenital] were excluded from the study.
Patients’ data were collected including [A] Demographic characteristics as gender, weight, height &body mass index (BMI).
[B] Basic hemodialysis and vascular access information including date of creation of vascular access, physical findings, and dialysis procedure data. AVF flow (Qa) was assessed using Color Doppler ultrasonography [Mindray M5 system], The AVF feeding vessel diameter and mean flow velocity by Pulsed Doppler were measured for calculation of flow volume.4 The flow volume was obtained from the algorithm available on the ultrasound system. The study cohort was then subdivided into 2 groups based on AVF flow: Group A (Non-HFA group with Qa < 2000 ml/min), and Group B (HFA group with Qa ≥ 2000 ml/min).
[C] Transthoracic echocardiography Conventional echocardiographic Doppler study and tissue Doppler imaging were performed using Siemens Acuson P50 system.
Images were obtained with patients in the left lateral position at end-expiration and connected to single-lead electrocardiography (ECG) according to the recommendations of the American Society of Echocardiography. All standard measurements were obtained in the parasternal long- and short-axis views, apical four-chamber, two-chamber views, and apical long-axis view. All measurements were taken on three consecutive beats and the mean values were used.
All studies were performed within 24 h after HD session.
The following parameters were measured:
[1] LV dimensions & systolic function:
Quantification of the LV dimensions using M-mode echocardiography to measure Left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), Interventricular septal thickness & Posterior wall thickness, then using the biplane (modified Simpson method) to measure left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV)& Left ventricular ejection fraction (LVEF) was calculated. LV mass in grams was calculated according to Devereux and associates,5 then LV mass was normalized for body surface area to obtain LV mass index (LVMI in g/m2). Relative wall thickness was calculated as 2(PWT)/LVEDD.h
[2] LA volume LA volume was calculated by applying the Simpson biplane methods to the apical four and two-chamber views.
[3] Assessment of LV diastolic function:
Transmitral pulsed-wave Doppler was recorded, the peaks of both E and A waves were measured, and the E /A ratio and E wave deceleration time were calculated.
Offline color-coded tissue Doppler imaging was done in the apical four-chamber view by placing the sample volume over the septal and lateral mitral annuli, and then, early diastolic velocity (E′), and late diastolic velocity (A′) were measured. The average E′ velocities at the sepal and lateral mitral annuli were estimated, and the E/E′ ratio was calculated.
[4] Measurement of the tricuspid annular plane systolic excursion (TAPSE);
TAPSE was acquired by placing an M-mode cursor through the tricuspid annulus in the apical 4 chamber view and measuring the amount of longitudinal motion of the annulus at peak systole.
[5] Assessment of right ventricular systolic pressure;
Right ventricular systolic pressure (RVSP) was calculated by continuous-wave Doppler ultrasound examination of the maximum velocity of TR using the modified Bernoulli equation [4 × (peak velocity of TR)2] and estimation of the mean right atrial pressure from the size & collapsibility the inferior vena cava in 2-dimensional echocardiography.
[6] Cardiac output was calculated from the left ventricular outflow tract diameter (LVOTd) and velocity time integral (VTI) of LVOT flow by Doppler.6
[7] AVF Qa/CO ratio was calculated accordingly.
Statistical analysis was performed using the SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc, Chicago, Ill, USA). Categorical variables were presented as counts and percentages. Continuous variables were presented as mean ± standard deviation. Independent-samples t-test was used for between group comparisons. Chi-Square test, or Fisher Exact’s test where appropriate, was performed to determine associations. ROC curve analysis was performed to determine Qa/CO cutoff value. Pearson’s correlation test was utilized to determine correlations between AVF Qa and Qa/CO with other study parameters, and a Linear regression analysis was performed as to determine whether the observed changes in AVF Qa and/or Qa/CO may predict LV systolic function. A two-sided P value < 0.05 was considered statistically significant.
3. Results
The Study included 100 patients with chronic end stage renal disease receiving hemodialysis sessions at Ain Shams University dialysis unit. The study population comprised 66 males (66%) and 34 females (34%), with a mean age of 48.48 ± 13.75 years.
AVF flow (Qa) was assessed using Color Doppler ultrasonography and the flow volume was obtained; accordingly study population was categorized into 2 groups based on AVF Qa, where GROUP A (non-HFA group) with Qa < 2000 ml/min [76 patients], and GROUP B (HFA group) with Qa ≥ 2000 ml/min [24 patients] so the prevalence of HFA was 24%.
Mean AVF Qa for group A was 958.63 ± 487.35 ml/min, while mean AVF Qa for group B was 3430.13 ± 1256.28 ml/min.
Basic demographics for both study groups were mostly balanced with no statistically significant differences observed between them as seen in [Table 1].
Table 1.
Basic demographics, dialysis data, and lab results for both groups “Both Groups were homogenous”.
| Group A (non-HFA group) | Group B (HFA group) | P value* | |
|---|---|---|---|
| Age (years) | 47.93 ± 13.55 | 50.21 ± 14.58 | 0.482 |
| Gender | 0.566 | ||
| Male | 49 (4964.5%) | 17 (1770.8%) | |
| Female | 27 (2735.5%) | 7 (729.2%) | |
| Height (m) | 1.65 ± 0.08 | 1.61 ± 0.12 | 0.117 |
| Weight (Kg) | 66.76 ± 14.61 | 73.00 ± 20.72 | 0.104 |
| BMI (kg/m2) | 24.22 ± 4.12 | 27.29 ± 5.18 | 0.004* |
| Hypertension (no of patients) | 69 (91%) | 21 (88%) | 0.33 |
| DM (no of patients) | 20 (26.3%) | 6 (25%) | 0.64 |
| HD duration (month) | 80.82 ± 62.56 | 92.38 ± 70.58 | 0.446 |
| AVF duration (month) | 57.76 ± 50.07 | 49.63 ± 46.66 | 0.482 |
| HD time (hr) | |||
| <4 h | 67 (6777.9%) | 8 (857.1%) | |
| 4 h | 19 (1922.1%) | 6 (642.8%) | |
| >4 h | 0 | 0 | |
| HD frequency | 0.251 | ||
| 2 times/week | 4 (45.3%) | 0 (0%) | |
| 3 times/week | 72 (7294.7%) | 24 (24100%) | |
| Hb (gm/dl) | 11.6 ± 0.6 | 11.6 ± 0.6 | 0.89 |
| Pre HD urea (mg/dl) | 163.16 ± 24.78 | 157.88 ± 26.60 | 0.373 |
| Post HD urea (mg/dl) | 54.34 ± 13.91 | 53.21 ± 16.61 | 0.741 |
| HD UF rate (L) | 1.72 ± 0.86 | 1.60 ± 0.67 | 0.527 |
**Student’s t-test or Chi square test is used as indicated.
Quantitative variables are expressed as mean ± SD while qualitative variables are expressed as count (percentage).
The HFA group (Group B) demonstrated a significant dilatation in LV dimensions and volumes and significantly larger LA volume index, they also had significantly lower LV EF, significantly higher LV mass and LV mass index compared to non-HFA group (group A). The HFA group (Group B) had significantly higher E/e′ (denoting higher LV filling pressure), significantly higher COP and significantly higher SPAP as compared to non-HFA group (group A), however, no significant difference was observed in RV function as indicated by TAPSE.
Echocardiographic parameters for both groups are demonstrated in [Table 2].
Table 2.
Echocardiographic parameters for both study groups. The HFA group demonstrated a significant increase in LV dimensions, volumes, LA volume index, LV mass and LV mass index, significant decrease LV EF, The HFA group had significantly higher LV filling pressure, COP and SPAP as compared to non-HFA group, no significant difference was observed in RV function as indicated by TAPSE, compared to non-HFA group (group A).
| Group A (non-HFA group) | Group B (HFA group) | P value* | |
|---|---|---|---|
| LVEDD (cm) | 4.93 ± 0.68 | 5.67 ± 0.82 | 0.000* |
| LVESD (cm) | 3.38 ± 0.55 | 4.22 ± 0.75 | 0.000* |
| IVSd (cm) | 1.08 ± 0.18 | 1.21 ± 0.18 | 0.003* |
| PWd (cm) | 1.10 ± 0.15 | 1.20 ± 0.17 | 0.007* |
| LVEDV (ml/m2) | 63.02 ± 19.59 | 90.42 ± 20.34 | 0.000* |
| LVESV (ml/m2) | 24.37 ± 7.26 | 38.78 ± 10.59 | 0.000* |
| EF (modified Simpson’s method) (%) | 62.90 ± 5.76 | 57.32 ± 6.19 | 0.001* |
| LV mass (g) | 205.43 ± 73.23 | 291.76 ± 114.65 | 0.000* |
| BSA (m2) | 1.74 ± 0.22 | 1.79 ± 0.32 | 0.350* |
| LV mass index (g/m2) | 119.69 ± 45.10 | 169.93 ± 85.87 | 0.000* |
| RWT | 0.45 ± 0.06 | 0.43 ± 0.06 | 0.184 |
| LA volume (ml/m2) | 34.54 ± 15.03 | 48.34 ± 19.46 | 0.001* |
| SPAP (mmHg) | 29.59 ± 8.24 | 34.91 ± 7.86 | 0.006* |
| TAPSE (cm) | 2.26 ± 1.86 | 1.90 ± 0.31 | 0.351 |
| LVOTd (cm) | 2.67 ± 0.33 | 2.84 ± 0.30 | 0.025* |
| LVOT area (cm2) | 5.68 ± 1.37 | 6.41 ± 1.40 | 0.003* |
| LVOT VTI (cm) | 20.87 ± 4.60 | 23.54 ± 6.05 | 0.025* |
| SV (ml) | 119.63 ± 42.00 | 151.98 ± 55.43 | 0.003* |
| HR (bpm) | 80.78 ± 9.20 | 82.95 ± 8.51 | 0.347 |
| CO (ml/min) | 9291.24 ± 3279.33 | 12833.78 ± 4599.63 | 0.000* |
| E (m/s) | 0.63 ± 0.18 | 1.00 ± 0.67 | 0.000* |
| A (m/s) | 0.76 ± 0.64 | 0.83 ± 0.16 | 0.607 |
| E/A | 0.98 ± 0.53 | 1.20 ± 0.74 | 0.103 |
| E-dct (ms) | 78.12 ± 38.10 | 69.3932.41 | 0.325 |
| s′ (m/s) | 0.11 ± 0.13 | 0.08 ± 0.01 | 0.317 |
| e′ (m/s) | 0.08 ± 0.08 | 0.10 ± 0.12 | 0.472 |
| a′ (m/s) | 0.09 ± 0.03 | 0.15 ± 0.19 | 0.024* |
| E/e′ | 8.74 ± 3.10 | 13.98 ± 10.64 | 0.000* |
Student’s t-test.
Qa/CO was also assessed and it was significantly higher in group B compared to group A (29.89 ± 9.64% in group B as vs 11.14 ± 7.45% in group A) (P value 0.000).
A correlation between Qa and the echocardiographic parameters was performed for the whole study population. A significant positive correlation was observed between Qa and LV dimensions, volumes, wall thickness, LV mass, LV mass index and LA volume. A highly significant positive correlation between Qa and cardiac output was found. There was a significant negative correlation between Qa and LV EF. A significant positive correlation between Qa and SPAP was found, however a negative but non significant correlation was noted between Qa & TAPSE [Table 3].
Table 3.
Correlation of Qa and echocardiography parameters. A significant positive correlation between Qa and LV dimensions, volumes, wall thickness, LV mass, LV mass index and LA volume, cardiac output and SPAP. A significant negative correlation between Qa and LV EF.
| Echocardiography parameters | Pearson’s correlation (r) | P value |
|---|---|---|
| LVEDD (cm) | 0.297 | 0.003* |
| LVESD (cm) | 0.413 | 0.000* |
| IVSd (cm) | 0.263 | 0.008* |
| PWd (cm) | 0.289 | 0.004* |
| LVEDV (ml/m2) | 0.574 | 0.000* |
| LVESV (ml/m2) | 0.679 | 0.000* |
| EF (modified Simpson’s method) (%) | −0.387 | 0.001* |
| LV mass (g) | 0.346 | 0.000* |
| LV mass index (g/m2) | 0.237 | 0.017* |
| RWT | −0.025 | 0.805 |
| LA volume (ml/m2) | 0.304 | 0.004* |
| SPAP (mmHg) | 0.251 | 0.012* |
| TAPSE (cm) | −0.107 | 0.287 |
| LVOTd (cm) | 0.167 | 0.097 |
| LVOT area (cm2) | 0.172 | 0.088 |
| LVOT VTI (cm) | 0.338 | 0.001* |
| SV (ml) | 0.340 | 0.001* |
| HR (bpm) | 0.176 | 0.117 |
| CO (ml/min) | 0.454 | 0.000* |
| E (m/s) | 0.272 | 0.006* |
| A (m/s) | 0.045 | 0.660 |
| E/A | 0.104 | 0.303 |
| E-dct (ms) | −0.022 | 0.832 |
| s′ (m/s) | −0.072 | 0.479 |
| e′ (m/s) | 0.018 | 0.859 |
| a′ (m/s) | 0.250 | 0.013* |
| E/e′ | 0.250 | 0.013* |
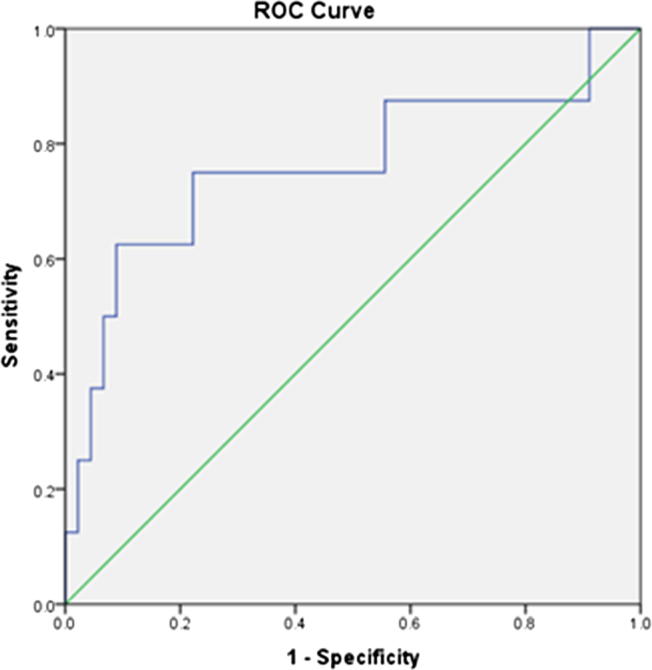
About 12% (8 patients) of our study population had impairment in LV systolic function [i.e. EF < 55%] with statistically significant association between HFA and impaired LV function. A ROC curve analysis demonstrated a cutoff value for Qa/CO of 19.27% to be associated with impaired LV systolic function (AUC 0.76, sensitivity 75%, specificity 78%) [Fig. 1].
Fig. 1.
ROC curve for determination of Qa/CO cut-off associated with impaired LV function. A significant association between HFA group and high Qa/CO ratio (≥20%).
A significant association between HFA group and high Qa/CO ratio (≥20%) was observed.
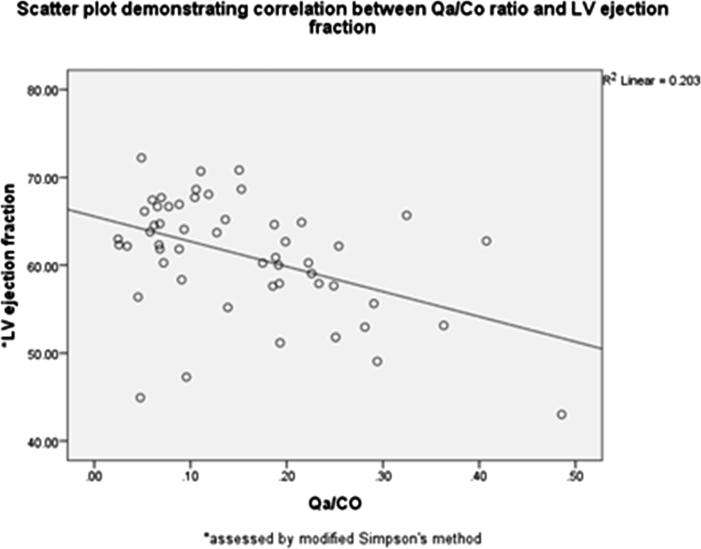
Finally to determine whether Qa and/or Qa/CO ratio can predict LV systolic dysfunction (impaired LV ejection fraction), a linear regression analysis was performed. The model for linear regression was fit (f = 6.484, p = .003). Qa/CO was an independent predictor of LV systolic dysfunction, and it accounted for 20.6% of the explained variability in LV ejection fraction Fig. 2.
Fig. 2.
Scatter plot demonstrating correlation between AVF Qa/CO and LV ejection fraction. Qa/CO was an independent predictor of LV systolic dysfunction.
4. Discussion
It is widely acknowledged that uremic syndrome is associated with high cardiovascular risk; indeed manifest congestive heart failure can be observed in 25–50% of HD patients.7 An inappropriately high flow (Qa) within the AVF may underlie the onset of HOHF.8, 9 HOHF is characterized by the occurrence of signs and symptoms of systemic congestion in the presence of an elevated cardiac output >8 l/minute or cardiac index (CI) >3.9 l/min/m2.10 Ejection fraction (EF) is usually preserved. The prevalence of this condition is not well established as many cases are not reported and remain unrecognized. Much of the current information has been derived from case reports and case series. AVFs for HD are believed to be an uncommon cause of heart failure (HF).11
Our data demonstrated that the prevalence of HFA was 24% using 2000 ml/min as cut off point with a mean flow of 3440.13 for HFA group as compared to a mean flow of 958.63 for the non-HFA group. Similarly, Schier et al.12 investigated the incidence of AVF closure due to HOHF in patients who received a kidney transplant. The authors reported in their study that 29 out of 113 patients (25.7%) needed an AV fistula closure, because of heart failure symptoms. The mean Qa in the AVF closure group was 2197.2 ml/min, whereas the mean Qa was only 850.9 ml/min in the non-intervention group.
The available case reports of HFA reported Qa/CO ranges between 23 and 57%.8, 9, 13, 14, 15, 16, 17 HF rarely occurs with normal fistula flow.8, 9
In concordance with Basile et al.,2 and based on ROC curve analysis, data from our study have demonstrated that Qa/CO ratio exceeding a lower cut off of 20% has been associated with a relative impairment in LV function as indicated by an ejection fraction that is <55%. Furthermore, we performed a linear regression model that included Qa and Qa/CO ratio as predictors for LV systolic dysfunction (impaired LV ejection fraction) which demonstrated that Qa/CO is an independent predictor of LV systolic dysfunction further emphasizing the importance of assessing Qa/CO ratio rather than absolute Qa values only.
Patients with HFA featuring increased LVEDV displayed a higher risk of developing CF.18, 19
Patients with a Qa > 2000 ml/min had a higher tendency toward an increase LVEDV with increased levels of atrial natriuretic peptide.20
In our study, patients in the HFA group manifested a significant increase in volumes of left cardiac chambers (LVEDV, LVESV, and LA volume), as well as LV dimensions. This was further supported by a strong correlation between Qa and cardiac volumes and dimensions. It was noted that Qa/CO demonstrated similar findings.
The causes underlying the evolution of a volume overload into overt congestive heart failure remain to be clarified. Specific AVF or patient characteristics may predispose towards the development of heart failure. Undeniably, an anastomosis of >4–6 mm in a proximal AVF may represent a major trigger. However, even low Hb levels or inadequate extracellular volume control and arterial hypertension may all enhance the onset of congestive heart failure, particularly in the presence of preexisting cardiac injury.21
In our study, HFA group of patients exhibited a significantly lower ejection fraction with a mean value of 57.32% as compared to 62.90% for the non-HFA group. In presence of HF, the decision for an intervention to reduce the AVF Qa should be supported by good control of other potential risk factors known to precipitate the disease such as: anemia, hypertension, excess inter-dialytic weight gain comprising a fluid volume overload. If clinical signs of HF (e.g. stress induced or resting dysnea, orthopnea, asthenia, and oedma) persist, then an intervention procedure to correct an AVF with persistently high Qa (≥2000) should be undertaken.22
Data from our study supports using this cutoff value for AVF Qa; in addition to Qa/CO ratio (determined by means of transthoracic echocardiography). In fact, our data suggests that Qa/CO; rather than absolute AVF Qa; was an independent predictor for a relative impairment in LV systolic function.
Several techniques have been adopted for AVF Qa reduction, all of which are based on an attempt to increase resistance to the anastomosis or in venous outflow (e.g. reduction of anastomsis caliber, interposition of a graft, banding). An intraoperative assessment of Qa by means of Doppler ultrasonography should be undertaken.22
Attempts should be made to avoid excessive reduction in the blood vessel caliber, which may lead to a drastic decrease in AVF Qa eventually precipitating thrombosis and VA failure. In some cases where possibility of creating a contralateral distal access is present, AVF ligature may be considered. In more severe or resistant cases of HF, closure of the AVF may be required and insertion of a hemodynamically inert tunneled venous catheter may constitute the only possible mean of VA.
With regards to the effect of HFA on pulmonary arterial pressure, The prevalence of pH varies from 40% to 48% in the ESRD population.23, 24
In a cohort of chronic hemodialysis (HD) patients (all with AVFs), Yigla et al. sated that the incidence rate of pH was significantly higher after initiation of HD with four out of six patients eventually developing PH. As opposed to this, pulmonary artery pressure (PAP) dropped in four out of five HD patients with PH after kidney transplantation following AVF closure. It was noted that mean PAP also dropped with AVF compression (from 52 to 41 mmHg) along with a drop in CO.23
Our data support these findings through demonstrating a strong correlation between Qa and Qa/CO with SPAP. Yet as for RV function, indicated by TAPSE, there was no statistical difference in TAPSE values between both study groups although a less mean value was observed in the HFA group.
In conclusion, HFA is a prevalent HD vascular access problem affecting about 24% of chronic hemodialysis patients. HFA was associated with a significant dilatation of left cardiac chambers (LA and LV) along with a relatively lower ejection fraction and a higher SPAP, compared to subjects with a non-HFA. Our data suggests that HFA is an under-recognized problem in dialysis units in Egypt that may lead to HOHF, a disease that has been associated with increased morbidity and mortality in this group of patients. Screening for AVF Qa with Doppler ultrasonography combined with a transthoracic echocardiography estimation of CO and calculation of Qa/CO is recommended.
Conflict of interest
We have no conflict of interest to declare.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
Contributor Information
Wael Mahmoud El Kilany, Email: wael.kilany@gmail.com.
Tamer Wahid El Said, Email: drtamer_elsaid@med.asu.edu.eg.
References
- 1.Sequeira A., Tan T.W. Complications of a high-flow access and its management. Semin Dial. 2015;28:533–543. doi: 10.1111/sdi.12366. [DOI] [PubMed] [Google Scholar]
- 2.Basile C., Lomonte C., Vernaglione L. The relationship between the flow of arteriovenous fistula and cardiac output in hemodialysis patients. Nephrol Dial Transplant. 2008;23:282–287. doi: 10.1093/ndt/gfm549. [DOI] [PubMed] [Google Scholar]
- 3.Ye W.L., Fang L.G., Ma J., Li X.M. Long-term effects of arteriovenous fistula on cardiac structure and function in non-diabetic hemodialysis patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013;35:95–101. doi: 10.3881/j.issn.1000-503X.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Zamboli P., Fiorini F., D’Amelio A., Fatuzzo P., Granata A. Color Doppler ultrasound and arteriovenous fistulas for hemodialysis. J Ultrasound. 2014;17:253–263. doi: 10.1007/s40477-014-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux R.B., Alonso D.R., Lutas E.M. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 6.McLean A.S., Needham A., Stewart D., Parkin R. Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care. 1997;25:250–254. doi: 10.1177/0310057X9702500307. [DOI] [PubMed] [Google Scholar]
- 7.Harnett J.D., Foley R.N., Kent G.M. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 8.Ahearn DJ, Maher JF. Heart failure as a complication of hemodialysis arteriovenous fistula. Ann Intern Med. 1972. Supported in part by a grant from Missouri Regional Medical Programs and grant RR00287-06, Clinical Research Center, National Institutes of Health, Bethesda, Md.
- 9.Engelberts I., Tordoir J.H., Boon E.S., Schreij G. High-output cardiac failure due to excessive shunting in a hemodialysis access fistula: an easily overlooked diagnosis. Am J Nephrol. 1995;15:323–326. doi: 10.1159/000168857. [DOI] [PubMed] [Google Scholar]
- 10.Anand I.S., Florea V.G. High output cardiac failure. Curr Treat Options Cardiovasc Med. 2001;3:151–159. doi: 10.1007/s11936-001-0070-1. [DOI] [PubMed] [Google Scholar]
- 11.Dillard M.G., Alexander P.C. High output cardiac failure secondary to a Brescia-Cimino fistula. J Natl Med Assoc. 1979;71:285–287. [PMC free article] [PubMed] [Google Scholar]
- 12.Schier T., Göbel G., Bösmüller C., Gruber I., Tiefenthaler M. Incidence of arteriovenous fistula closure due to high-output cardiac failure in kidney-transplanted patients. Clin Transplant. 2013;27:858–865. doi: 10.1111/ctr.12248. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C.B., Codd J.R., Graff R.A. Cardiac failure and upper extremity arteriovenous dialysis fistulas. Case reports and a review of the literature. Arch Int Med. 1976;136:292–297. [PubMed] [Google Scholar]
- 14.MacRae J.M., Pandeya S., Humen D.P., Krivitski N., Lindsay R.M. Arterovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis. 2004;43:e17–e22. doi: 10.1053/j.ajkd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Khreiss M., Haddad F.F., Musallam K.M. High-output cardiac failure secondary to a large arteriove- nous fistula: a persistent threat to the dialysis and kidney transplant patient. NDT Plus. 2009;2:147–148. doi: 10.1093/ndtplus/sfn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oe K., Araki T., Katano K. Impact of inflow reduction of arteriovenous fistula on systemic hemodynamics in a patient with high-output heart failure during hemodialysis: a case report. J Cardiol Cases. 2010;1:e98–e101. doi: 10.1016/j.jccase.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis G.J., Hirsch A.T., Come P.C. Detection and treatment of high- output cardiac failure resulting from a large hemodialysis fistula. Cathet Cardiovasc Diagn. 1988;14:263–265. doi: 10.1002/ccd.1810140409. [DOI] [PubMed] [Google Scholar]
- 18.Seals D.R., hagberg G.M., Spina R.J. Enhanced left ventricular performance in endurance trained older man. Circulation. 1994;89:198–205. doi: 10.1161/01.cir.89.1.198. [DOI] [PubMed] [Google Scholar]
- 19.MacRae J.M., Do T.H., Rosenbaum D., Levin A., Kiaii M. High flow fistulas and cardiac hemodysnamics. J Am SocNephrol. 2004;15:369A. [Google Scholar]
- 20.Iwashima Y., Horio T., Takami Y. Effects of the creation of arterovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 2002;40:974–982. doi: 10.1053/ajkd.2002.36329. [DOI] [PubMed] [Google Scholar]
- 21.London G.M. Left ventricular hypertrophy: why does it happen? Nephrol Dial Transplant. 2003;18(Suppl. 8) doi: 10.1093/ndt/gfg1083. viii 2-6. [DOI] [PubMed] [Google Scholar]
- 22.Tellioglu G., Barber I., Kilicoglu G. Doppler ultrasonography-guided surgery for high-flow hemodialysis vascular access: preliminary results. Transplant Proc. 2008;40:87–89. doi: 10.1016/j.transproceed.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Yigla M., Nakhoul F., Sabag A. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–1582. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 24.Bos W.J., Zietse R., van den Meiracker A.H., Schalekamp M.A., Wei- mar W. Hemodynamic consequences of Cimino fistulas studied with finger pressure measurements during fistula compression. Kidney Int. 1995;48:1641–1645. doi: 10.1038/ki.1995.459. [DOI] [PubMed] [Google Scholar]