Abstract
Background
Epicardial fat, in addition to its secretory function, may have an important role in predicting and stratifying cardiovascular risk. There is a paucity of data regarding correlation between epicardial fat thickness and coronary artery disease in Egypt.
Aim of the study
To study the relationship between epicardial fat thickness (EFT) measured by trans-thoracic echocardiography (TTE) and severity of coronary artery disease (CAD) and its distribution in Egyptian population.
Methods
Our study was a prospective observational case control study that was conducted upon 150 patients with stable CAD presented to the cardiology departments in Ain Shams University hospitals and Al-Zaitoun Specialized hospital from March to October, 2015. EFT was measured by TTE for all patients at the same day of performing invasive coronary angiography (CA). We studied the statistical correlation between EFT and presence of CAD, also we tried to find if EFT is related to severity of CAD (according to Gensini score) or its distribution.
Results
The study population was divided according to CA results to 2 groups; patients’ group having atherosclerotic CAD consisting of 100 patients and control group consisting of 50 patients with normal coronaries. All the well- known risk factors of CAD (male sex, smoking, hypertension, diabetes, dyslipidemia, increased body mass index) were significantly more prevalent in the patients’ group. Patients had significantly lower systolic and diastolic functions. EFT was significantly correlated to presence of CAD (P < 0.001) with a cut-off value of 5.5 mm. EFT was significantly correlated to severity of CAD assessed by Gensini score (P < 0.001). Also we found a significant positive correlation between EFT and number of vessels affected (P < 0.001).
Conclusion
EFT is a good predictor of CAD severity and multivessel affection in Egyptian patients. It is also a potentially promising predictor for the presence of CAD.
Abbreviations: CAD, Coronary artery diseases; WHO, world health organization; MI, myocardial infarction; VAT, visceral adipose tissue; EAT, epicardial adipose tissue; EFT, epicardial fat thickness; BMI, body mass index; WC, waist circumference; CA, coronary angiography
1. Introduction
Atherosclerotic Coronary artery diseases (CAD) are the leading cause of death worldwide according to the latest data published by the world health organization (WHO).1 It is also the leading cause of death in Egypt.2 CAD worldwide is largely driven by modifiable risk factors, as The INTERHEART study showed that smoking, dyslipidemia, Diabetes, hypertension, obesity, physical inactivity, alcohol consumption, psychological factors and a high risk diet were responsible for over 90% of myocardial infarction (MI) risk.3 Primary prevention is paramount for the large number of people who are at high risk for acquiring CAD. In view of limited resources in developing countries, finding low-cost prevention strategies is a top priority.
The association between visceral obesity and cardiovascular risk has been well described.4 Visceral adipose tissue (VAT) which is distributed around the viscus or hollow muscular organs of the body is now well established as being associated with the development of metabolic syndrome and CAD.5
The mechanism of these effects of VAT are not entirely understood, but could be mediated by release of free fatty acids causing direct ‘lipotoxicity’.4 Adipose tissue, especially the VAT, also acts as an endocrine organ, releasing numerous proinflammatory and proatherogenic cytokines and hormones affecting endothelial function.4, 6, 7
Epicardial adipose tissue (EAT) fills the area between the pericardium and the myocardium, and has the same embryologic origin as intra-abdominal adipose tissue.8 The adipose tissue of the heart is divided into two layers: epicardial fat (the visceral layer) which lies between the myocardium and visceral layer of the pericardium (Fig. 1) and pericardial fat situated external to the parietal layer of the pericardium9 Each layer has its own embryological origin and different blood supply.10 Epicardial fat is the visceral fat depot of the heart.11 Under physiological conditions, EAT acts as a buffer that protects the heart from lipotoxicity and provides the myocardium with the lipids needed to obtain energy through β-oxidation of fatty acids in addition to its mechanical protective effect. Under pathological conditions, as in metabolic syndrome, EAT dysfunction occurs, leading to the loss of its cardioprotective effect.12 Measuring the amount of EAT might represent a novel parameter that is inexpensive and easy to obtain and may be helpful in cardiovascular risk stratification. However, the relationship between epicardial fat and determinants of coronary artery disease is not well established.
Fig. 1.
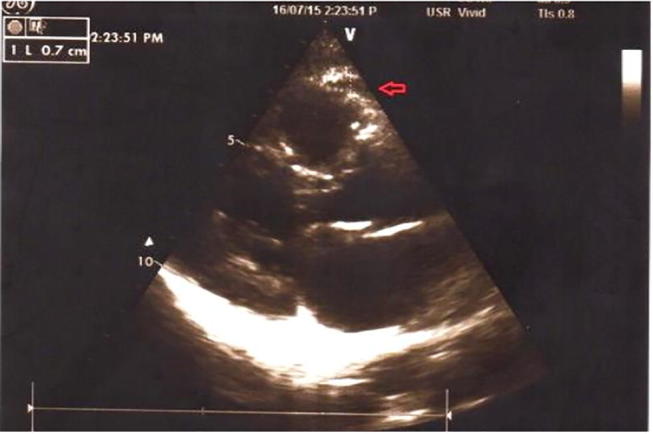
Echocardiographic epicardial fat anatomy at parasternal long axis view as echo free space on the free wall of Rt. Ventricle. (Case no. 19; patients group).
In our study we tried to detect if there is a relationship between epicardial fat thickness (EFT) and severity of coronary artery disease in Egyptian population or not.
2. Methods
This study was a prospective observational case control study that was conducted upon 150 patients presented to the cardiology departments in Ain Shams University hospitals and Al-Zaitoun Specialized hospital in the time interval from March to October, 2015.
The study was approved by the local ethics committee and all patients signed an informed consent for participation in the study. All patients underwent coronary angiography at the same day of performing a full echocardiographic study. Patients were divided into two groups according to their coronary angiographic findings; group I (patients group), that included 100 patients who had documented CAD by coronary angiography (CA) and group II (control group), that included 50 patients who had normal coronaries confirmed by CA.
The study included all stable patients that were indicated for invasive CA according to the appropriateness criteria.13
2.1. Exclusion criteria
Extremes of age (less than 18 or more than 70 years), patients with debilitating diseases, patients with poor acoustic windows, patients with normal coronaries despite having previously documented ischemic insult, patients with acute coronary syndromes, patients refusing to participate and patients with coronary ectasia were excluded from the study.
Clinical data, including the medical history, cardiovascular risk factors, and associate comorbidities, were collected by history taking from the patients followed by thorough clinical examination including calculation of body mass index (BMI) by the standard formula [weight (kg)/height (m)2]14 and waist circumference (WC) measurement that is made at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest.15 12 lead surface ECG was obtained from all study subjects in order to assess presence of new or old ischemic changes.
Trans-thoracic Echocardiography was performed using a high-performance cardiovascular ultrasound system Vivid S5 machine (GE healthcare Company, USA). Two independent investigators analyzed the recordings blinded to clinical data and the results of coronary angiography. Systolic functions were assessed by the Biplane disk summation volume method. Diastolic functions were assessed according to the 2009 American Society of Echocardiography guidelines 2009.16 Epicardial fat (which is defined echocardiographically as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium) was measured perpendicularly on the free wall of the right ventricle in the parasternal long-axis view at mid ventricle at end-diastole in 3 cardiac cycles at the point at which the ultrasound beam is oriented in a perpendicular manner, using the aortic annulus as an anatomic landmark.12 There is no consensus till now about whether EFT is to be measured at end-systole or end-diastole and data are conflicting regarding this issue.
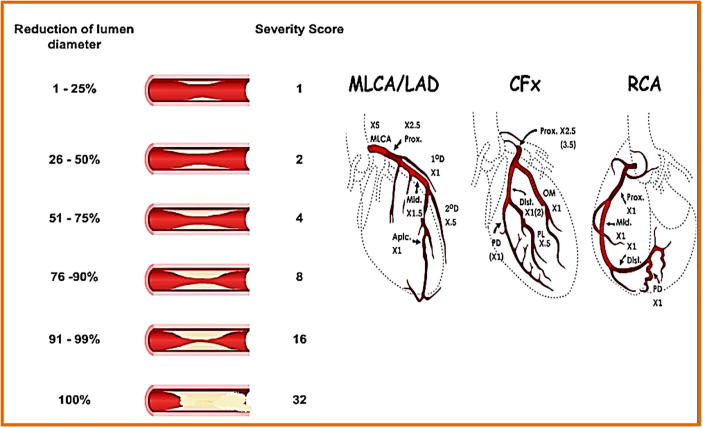
All patients underwent CA according to our approved institutional protocol. Two independent operators analyzed the CA data blinded to clinical and echocardiographic data of all patients. The “Gensini” severity score which calculates severity of CAD by using both site and degree of stenoses17, 18 was used to assess severity of CAD in the patients group (Fig. 2).
Fig. 2.
Gensini score of coronary artery disease severity 18.
2.2. Statistical analysis
Data were analyzed using package for special science (SPSS) software version 20.0 (IBM, Armonk, NY, USA). T-test was used for comparison of quantitative variables. Chi-square test (or Fisher’s exact test when appropriate) was used for comparison of distribution of qualitative variables. The receiver operating characteristic (ROC curve) was used to find cutoff value.
P value was used to determine the statistical significance. A value of 0.05 is arbitrary a cut off value; below it, the relation between variables is considered statistically significant, and above it the relation is considered statistically not significant.
3. Results
3.1. Demographic, anthropometric and clinical characteristics
Demographic, Anthropometric and clinical data of our study population are shown in (Table 1), there was a significant difference between both groups in most of these variables.
Table 1.
Demographic, anthropometric and clinical characteristics.
| Demographic/clinical variable | Patients (Group I) (n = 100) |
Controls (Group II) (n = 50) |
P value | ||
|---|---|---|---|---|---|
| Conventional risk factors |
Male 86 (57%) |
65 (65%) | 21 (42%) | 0.007 | |
| Age | 57.81 ± 8.18 | 44.82 ± 6.58 | <0.001 | ||
| Smoking | Non-smoker | 37 (37%) | 38 (76%) | <0.001 | |
| Smoker | 53 (53%) | 9 (18%) | |||
| Ex-smoker | 10 (10%) | 3 (6%) | |||
| HTN | 88 (88%) | 1 (2%) | <0.001 | ||
| DM | 67 (67%) | 0 (0%) | <0.001 | ||
| Dyslipidemia | 56 (56%) | 0 (0%) | <0.001 | ||
| Body mass index | 35.98 ± 4.31 | 27.38 ± 2.03 | <0.001 | ||
| Waist circumference | 99.26 ± 10.08 | 81.8 ± 6.79 | <0.001 | ||
| Ejection fraction | 44.9 ± 8.59 | 64.4 ± 6.9 | <0.001 | ||
| Left ventricular mass index | 84.4 ± 29.0 | 80.9 ± 28.8 | 0.494 | ||
| Diastolic functions | Normal diastolic function | 3 (3%) | 50 (100%) | <0.001 | |
| Grade I DD | 37 (37%) | 0 (0%) | <0.001 | ||
| Grade II DD | 36 (36%) | 0 (0%) | <0.001 | ||
| Grade III DD | 20 (20%) | 0 (0%) | <0.001 | ||
| Grade IV DD | 4 (4%) | 0 (0%) | <0.001 | ||
| Epicardial fat thickness | 7.4 ± 1.3 | 4.12 ± 0.82 | <0.001 | ||
Bold values means either a variable or a significant P value.
3.2. CA data of the patients group
39% of patients in the patients group were found to have single vessel affection, 33% had two vessels affection, while 28% had three vessels affection, we found that LAD affection contributes to 79% of reported coronary artery stenosis, while RCA affection to contribute to 63%. Severity score “Gensini” showed a mean value of 54.25 ± 49.29. CA data are shown in (Table 2).
Table 2.
CA data in patients group.
| CA data | Patients (n = 100) | |
|---|---|---|
| Gensini “Severity Score” | 54.25 ± 49.29 | |
| Number of affected coronary vessels | Single vessel | 39 (39%) |
| Two vessel | 33 (33%) | |
| Three vessel | 28 (28%) | |
| Coronary Artery Disease distribution |
LM Disease | 10 (10%) |
| LAD affection | 79 (79%) | |
| LCX affection | 42 (42%) | |
| RCA affection | 63 (63%) | |
| LAD + LCX | 30 (30%) | |
| LAD + RCA | 46 (46%) | |
| LCX + RCA | 33 (33%) | |
| LM + RCA | 8 (8%) |
3.3. Epicardial fat thickness measurements
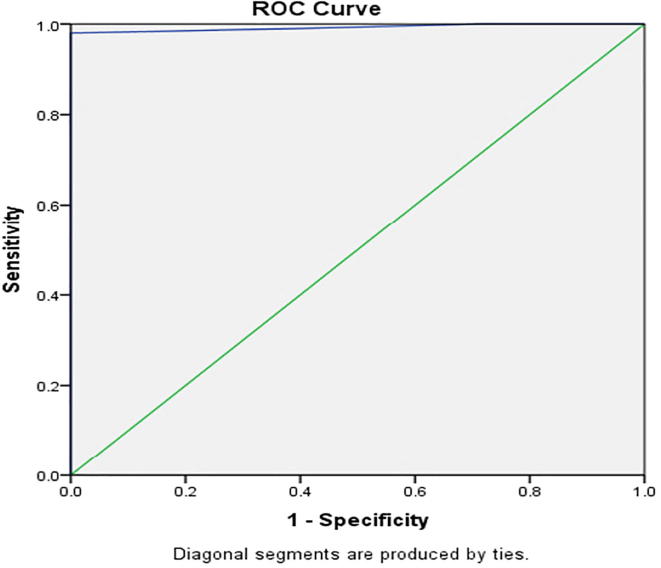
A statistically significant difference between both groups was found regarding Epicardial fat thickness with mean thickness of 7.4 ± 1.3 mm for the patients group ranging from 4 mm to 9 mm and mean thickness of 4.12 ± 0.82 mm for the control group ranging from 3 mm to 5 mm (P < 0.001) (Table 1). Using Receiver Operating characteristic Curve (ROC) we found a statistically significant cut-off point of 5.5 mm epicardial fat thickness that refers to presence of CAD with a significant area under the curve of 0.992%, 98% sensitivity, 100% specificity, 95% CI of 0.98–1.00 and P value <0.001 (Fig. 3).
Fig. 3.
Roc curve for sensitivity and specificity of epicardial fat thickness cut-off point for a presence of CAD.
3.4. Epicardial fat thickness in relation to different study variables
Epicardial fat thickness was found to have a statistically significant correlation to age (P < 0.001, r = 0.53), Smoking (P < 0.001), hypertension (P < 0.001), diabetes (P < 0.001), dyslipidemia (P < 0.001), BMI (P < 0.001, r = 0.68), systolic functions (P < 0.001, r = −0.62) and diastolic functions (P < 0.001) in the whole study population. Correlation between epicardial fat thickness and coronary anatomy detected by CA in the patients group revealed a significant correlation between epicardial fat thickness and the severity “Gensini” score (p < 0.001). Also post hoc analysis showed a significant positive correlation between epicardial fat thickness and number of vessels affected (p < 0.001).
4. Discussion
Identification and treatment of atherosclerotic plaques before narrowing of coronary artery lumen or causing acute coronary syndromes can halt and possibly even reverse the progression of CAD, returning the plaques to a stable form.19 EAT secretes pro-inflammatory cytokines that have been shown to play an important role in the development of CAD.20 Correlation of EFT to incidence and severity of CAD can yield an interesting possible low cost screening method for prediction and prevention of CAD.
Our study was an observational prospective case-control study that included 150 subjects; divided according to CA findings into (100) patients that have a documented CAD, and (50) controls with documented normal coronaries.
Our study population comprised 86 males (57.3%) with 65 (65%) males in the patients group and 21 (42%) males in control group. Mean age was 57.81 ± 8.18 years for the patients group and 44.82 ± 6.58 years for the control group. The risk of developing CAD increases with age and this goes with another similar study done by Aydin et al.21 Also risk is more in males despite the increased risk in females after menopause. This gender difference is attributed to the protective effect of the estrogen in females.22, 23
The incidence of smoking, Hypertension, diabetes and dyslipidemia in the patients group was significantly higher than in the control group, a finding that goes well with the already known fact that these factors are among the most important risk factors of CAD. Mustelier et al. in a study similar to ours, showed the same significant correlation between all these risk factors (except diabetes) and CAD.24 Also, in our study, the mean BMI was 35.98 ± 4.31 kg/m2 for the patients group versus 27.38 ± 2.03 kg/m2 for the control group and mean WC was 99.26 ± 10.08 cm for the patients group versus 81.8 ± 6.79 cm for the control group, yielding a significant difference between the 2 groups. Faghihi et al. showed a similar difference between the 2 groups in a study similar to ours,25 also Iacobellis et al. showed similar results.12
Regarding echocardiographic assessment of the 2 groups; there was a significant difference between both groups as regards both systolic and diastolic functions. On the other hand there was no significant difference as regards LVMI. Systolic and diastolic functions can be highly affected by ischemic conditions while LV mass may be more affected by factors rather than ischemia as genetics, race and loading conditions that faces the ventricles in the course of chronic cardiovascular conditions.
4.1. Analysis of epicardial fat thickness
EFT showed a significant correlation with age, smoking, HTN, DM, Dyslipidemia, BMI and WC; all of which are settled predictors of CAD. These results agree with the results obtained from many of other similar studies.21, 26, 27 The causality relationship between HTN, DM, Dyslipidemia, BMI, WC and increased epicardial fat seems convincing given the strong evidence that epicardial adipose tissue has the same origin as visceral adipose tissue and that Epicardial fat thickness in subjects with metabolic syndrome is significantly higher than that observed in subjects without metabolic syndrome.28 Also, Epicardial fat thickness is inversely associated with insulin sensitivity.29
Our study showed a significant difference between EFT in the patients group versus controls (7.4 ± 1.3 mm versus 4.12 ± 0.82 mm). A similar study done by Eroglu et al. found the same significant correlation 31. Despite epicardial fat may release factors that blunt the toxic effects of high fatty acid levels on the myocardium,31 it may also release factors that promote harmful coronary artery and myocardial changes. A body of evidence shows that epicardial fat is an extremely active organ that produces several bioactive adipokines.32, 33 Some studies utilizing MSCT have shown similar significant correlation between epicardial fat and coronary artery disease.34 We found that EFT of 5.5 mm is the cut-off value at which we can expect presence of CAD with a high sensitivity and specificity. Finding such a value has been a concern for many similar studies done before. Faghihi et al., found an EFT cut-off value of 2.95 mm in their study with a sensitivity of 83% and specificity of 75%,25 Eroglu et al. found a cut-off point of 5.2 mm with 85% sensitivity and 81% specificity.30 Mustelier et al. postulated an EFT cut-off point of 5.2 mm with 65.4% sensitivity and 61.5% specificity.24 A point to be put in mind is that some of these investigators measured EFT at end-systole while others used end-diastolic frames and this may explain differences between them. Detection of such cut-off point is very appealing as it may be used after validation in other studies performed on larger number of patients and exclusion of the effect of other confounding factors as a simple, rapid and low cost method in screening patients for CAD; an idea similar to that of coronary calcium scoring done by multislice computed tomography but without exposing patients to hazards of radiation.35, 36, 37, 38, 39
We found a significant correlation between EFT and severity of CAD in the patients’ group calculated by the “Gensini” score. Eroglu et al.30 and Meenakshi et al.40 showed similar significant correlations in their studies. Moreover, we found that there is a significant correlation between EFT and number of vessels affected and a significant correlation between LM disease and EFT. We thought that finding and confirming a relationship between EFT and severity of CAD in the Egyptian population would be of utmost importance given that the prevalence of abdominal obesity in men is 37.1% and in women 50.8%41 and based on available data from the Egyptian National Hypertension Project42 and since abdominal obesity is a cornerstone of the metabolic syndrome; the link between EFT (which seems to have a strong correlation with visceral obesity) and CAD can open the door for a new, simple and cheap method for detection of atherosclerosis even in its early phases to halt its progression and reduce any morbidity or mortality caused by it. Such approaches are extremely needed in developing countries to reduce healthcare expenses. Also the role of EFT repeated assessments to evaluate results of life style modifications used for patients at risk of CAD is a possible field for future research.
From the above data we conclude that EFT measurement using 2D echocardiography is a good test for prediction of severity of CAD. EFT is also positively correlated to increased number of coronary vessels affected and it is a potentially good predictor for the presence of CAD. Our study limitations were that more accurate assessment of EFT using MSCT or cardiac magnetic resonance was not available, both allow more accurate 3D quantification; also our results needed larger number of patients with homogenous distribution of the other known risk factors for CAD between both study groups to detect the exact effect of EFT in prediction of CAD. Also future studies will be needed to correlate EFT with different scores that are commonly used for assessment of CAD severity (as the SYNTAX score for example) with possible use of intravascular ultrasound (IVUS) for detection of mild atherosclerosis that is not detectable by CA. Our study results were sometimes compared (in the discussion section) with other studies that were based on EFT measurement in end-systole, this difference in methodologies may have an effect on accuracy of comparison. Our study population was all from Caucasian race, future studies with multiracial population will be needed. Also, the effect of gender will need to be assessed in future studies. Again, future studies including patients presenting with acute coronary syndromes will be needed.
Conflicts of interest
No conflicts to declare.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.The top 10 causes of death worldwide: WHO January 2017 update. (Available at http://www.who.int/mediacentre/factsheets/fs310/en/) Accessed December 2017.
- 2.World Health Rankings 2014. (Available at <http://www.worldlifeexpectancy.com/egypt-coronary-heart-disease>) Accessed December 2017.
- 3.Yusuf S., Hawken S., Ounpuu S. On behalf of the INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Saleh R. Abdominal obesity and cardiovascular disease. Adv Obes Weight Manag Control 2015; 3(2): 00046.
- 5.Freedland E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutr Metab. 2004;1:12. doi: 10.1186/1743-7075-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arcaro G., Zamboni M., Rossi L. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 7.Bastard J.P., Maachi M., Lagathu C. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 8.Wang T.D., Lee W.J., Shih F.Y. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis. 2010;213:279–287. doi: 10.1016/j.atherosclerosis.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Corradi D., Maestri R., Callegari S. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardio vasc Pathol. 2004;13(6):313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Moore KL, Persaud TVN. The developing human. Clinically oriented embryology. 7th ed. Philadelphia (PA): WB Saunders; 2003.
- 11.Iacobellis G. Epicardial fat: a new cardiovascular therapeutic target. Curr Opin Pharmacol. 2016;27:13–18. doi: 10.1016/j.coph.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G., Lonn E., Lamy A. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146(3):452–454. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]
- 13.Patel M.R., Bailey S.R., Bonow R.O. ACCF/SCAI/AATS/ AHA/ ASE/ASNC/HFSA/HRS/SCCM/SCCT/ SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;59:1995–2027. doi: 10.1016/j.jacc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Stensland S.H., Margolis S. Simplifying the calculation of body mass index for quick reference. J Am Diet Assoc. 1990;90(6):856. [PubMed] [Google Scholar]
- 15.WHO. STEPwise approach to surveillance (STEPS) World Health Organization 2008. Available at <http://www.who.int/chp/steps/en/> Accessed March 21, 2015.
- 16.Nagueh S.F., Appleton C.P., Gillebert T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Gensini G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983:606–651. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan R., Marwick H., Freedman S. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J. 1990:1262–1267. doi: 10.1016/s0002-8703(05)80173-5. [DOI] [PubMed] [Google Scholar]
- 19.Baigent C., Keech A., Kearney P.M. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 20.Verhagen S.N., Visseren F.L. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Aydın A.M., Kayalı A., Poyraz A.K. The relationship between coronary artery disease and pericoronary epicardial adipose tissue thickness. J Int Med Res. 2015;43(1):17–25. doi: 10.1177/0300060514558323. [DOI] [PubMed] [Google Scholar]
- 22.Rossouw J. Estrogens for prevention of coronary heart disease. Putting the brakes on the bandwagon. Circulation. 1996;94:2982–2985. doi: 10.1161/01.cir.94.11.2982. [DOI] [PubMed] [Google Scholar]
- 23.Lennep H.W., Westerveld H.T., Zwinderman A.H. Differential effect of female gender on coronary artery disease and peripheral artery disease. Neth Heart J. 2002;10:500–505. [PMC free article] [PubMed] [Google Scholar]
- 24.Mustelier J.V., Rego J.O., González A.G. Echocardiographic parameters of epicardial fat deposition and its relation to coronary artery disease. Arq Bras Cardiol. 2011;97(2):122–129. doi: 10.1590/s0066-782x2011005000068. [DOI] [PubMed] [Google Scholar]
- 25.Faghihi S., Vasheghani-Farahani A., Parsaee M. Association between epicardial fat thickness and premature coronary artery disease: a case control study. Res Cardio Vasc Med. 2015;4(2):e25679. doi: 10.5812/cardiovascmed.4(2)2015.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yañez-Rivera T.G., Baños-Gonzalez M.A., Ble-Castillo J.L. Relationship between epicardial adipose tissue, coronary artery disease and adiponectin in a Mexican population. Cardiovasc Ultrasound. 2014;12:35. doi: 10.1186/1476-7120-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong J.W., Jeong M.H., Yun K.H. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71(4):536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 28.Iacobellis G., Willens H.J., Barbaro G. Threshold values of high risk echocardiographic epicardial fat thickness. Obesity. 2008;16:887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 29.Iacobellis G., Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 30.Eroglu S., Sade L.E., Yildirir A. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardio vasc Dis. 2009;19(3):211–217. doi: 10.1016/j.numecd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Sacks H.S., Fain J.N., Holman B. Uncoupling protein-1 and related mRNAs in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94(9):3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 32.Mazurek T., Zhang L., Zalewski A. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 33.Fain J.N., Sacks H.S., Buehrer B. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32(5):810–815. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi N., Yamamoto H., Horiguchi J. Association between visceral adipose tissue area and coronary plaque morphology assessed by CT angiography. JACC Cardiovasc Imag. 2010;3:908–917. doi: 10.1016/j.jcmg.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Brindle P., Beswick A., Fahey T. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752–1759. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakoski S.G., Greenland P., Wong N.D. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Int Med. 2007;167:2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 37.Greenland P., LaBree L., Azen S.P. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 38.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. NEJM. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 39.Shaw L.J., Raggi P., Schisterman E. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 40.Meenakshi K., Rajendran M., Srikumar S. Epicardial fat thickness: a surrogate marker of coronary artery disease – assessment by echocardiography. Indian Heart J. 2016;68:336–341. doi: 10.1016/j.ihj.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasr G.M., Sliem H., Gamal A. Screening for diabetes and cardiovascular risk factors among Egyptian population. Clin Diabetes (middle east edition) 2010;9:127–135. [Google Scholar]
- 42.Ibrahim MM. Hypertension surveys in the developing world. Lessons from the Egyptian National Hypertension Project (NHP). J Human Hypertens 1997;11:709–26. [DOI] [PubMed]