Highlights
-
•
First, it is a population-based study which can provide big sample size.
-
•
Second, similar articles about chondrosarcoma using big data are lacking.
-
•
Third, the relationship between treatment type and survival outcomes was analyzed in detail.
Key words: Chondrosarcoma, Incidence, Treatment, Outcomes
Abstract
Current reports on prognostic factors for chondrosarcoma mainly involve patients in treatment centers. Few are based on multicenter or multi-eras. We analyzed existing data from the Surveillance, Epidemiology, and End Results (SEER) database to investigate the risk factors for survival outcomes. All patients with chondrosarcoma from 1973 to 2012 were identified. 3737 patients were eligible and included. In survival analysis, patient had good survival outcome if the patient was female, young, with localized stage, well grade, small tumor size, treated with surgery, while patient was male, old, with distant stage, undifferentiated grade, tumor size <50 mm, located in vertebral or pelvic bones, underwent radiation had bad survival outcome. Surgery types from having best survival outcomes to worst were local excision, radical excision, amputation, no surgery. ‘Well’ and ‘moderately’ grade seems to be suitable for local excision, but ‘poorly’ and ‘undifferentiated’ grade suitable for wide local excision. Multivariate COX regression analysis showed year of diagnosis, sex, age of diagnosis, stage, grade, tumor site, surgery, radiation were independent risk factors. Year of diagnosis, sex, age of diagnosis, stage, grade, tumor site, surgery, radiation were independent risk factors. Excision is a better treatment than amputation. Doctors can use wide local excision to treat chondrosarcoma, especially when encountering high grade chondrosarcoma or pelvic chondrosarcoma.
1. Introduction
Chondrosarcoma is the second most common malignancy of skeletal system cancers following osteosarcoma and the treatment of it is challenging for orthopedic oncologists [1]. It is less sensitive to radiation or chemotherapy and the only therapy hitherto proved to be effective is surgical excision [2]. Metastasis has been confirmed to be associated with worse survival outcome of patients with chondrosarcoma [3], [4]. However, it is difficult to correlate metastasis potential with histological features under the microscope [1]. Therefore, it is critical to study the risk factors and the effects of different treatments, on the survival outcome of chondrosarcoma.
Prognoses for chondrosarcoma have been studied in single center trials [5], meta-analysis [6] and systematic reviews [1]. Few studies analyse a sample size as large as the Surveillance, Epidemiology, and End Results (SEER) program. SEER covers 17 geographically defined registries and approximately 26% of the U.S. population, which contains a great amount of data for all kinds of tumors from 1973 to now.
In this study, we obtained data from the SEER program, and selected all patients with chondrosarcoma between 1973 and 2012. We investigated the influence of 15 variables on the survival rate of chondrosarcoma using multivariate COX regression analysis. We then performed a pairwise comparison of these factors and analyzed the impact of different therapeutic strategies on the survival rates. We aimed to identify useful factors for the prevention and treatment of chondrosarcoma.
2. Patients and methods
2.1. Data source
All data were obtained from the Surveillance, Epidemiology, and End Results (SEER) program and the SEER*Stat application (version 8.3.4) was used for analysis. We selected patients with chondrosarcoma from 1973 to 2012. Histologic Type ICD-O-3 in the application was input 9220 and Primary Site-Lableled were input C40.0–C41.9 in the software to represent chondrosarcoma, NOS. 3737 cases were identified. Survival outcomes were analyzed according to 15 variables.
2.2. Study design
These 15 factors can be split into 3 categories: patient related factors (year of diagnosis, and age of diagnosis, sex, race, CHSDA region, and whether they were from rural or urban areas), tumor related factors (stage, grade, tumor size, laterality, tumor site) and treatment related factors (surgery, surgery type, radiation, treatment).
We divided year of diagnosis into 4 groups: 1973–1982, 1983–1992, 1993–2002, and 2013–2012. Age at diagnosis was also divided into 4 groups: 00–24 years, 25–49 years, 50–69 years, 70 + years. Races determined were White, Black and Other (American Indian/AK Native, Asian/Pacific Islander). CHSDA region was divided as East, Northern Plains, Pacific Coast and Southwest. Rural or urban were defined as urban and rural according to whether the patient was in a metropolitan area. Stages of cancer were either ‘localized’, ‘regional’ or ‘distant’. In terms of grade patients were either ‘well differentiated’, ‘moderately differentiated’, ‘poorly differentiated’, or ‘undifferentiated’. We identified 4 groups regarding tumor size: <50 mm, 50–79 mm, 80–99 mm and ≥100 mm. Tumor laterality was categorized as being right or left. In respect of treatment patients either had no surgery, local excision, radical excision or amputation. For tumor site the following were identified: upper limb was the combination of C40.0 and C40.1, lower limb was the combination of C40.2 and C40.3, skull and mandible was the combination of C41.0 and C41.1, chest bones was C41.3, vertebral and pelvic bones were combinations of C41.2 and C41.4.
There is a lack of data for all 3737 patients with respect to laterality, surgery type and tumor size. Information on laterality was available for 2670 patients. Information on surgery type was only available for 2319 patients and all available records were dated from 1998. For tumor size records began from 2004 with only 1215 cases. Therefore, in the survival curve, the x-axis for surgery type and tumor size did not correspond to 40 years.
2.3. Statistical analysis
SEER*Stat application (version 8.3.4) was used and we collected data for each patient from case listing sessions. These data were then analyzed using SPSS version 17.0 (SPSS, Chicago, IL, USA). Analysis was done on five-year survival rate, log-rank testing, pairwise comparison, univariate analysis and multivariate COX regression. 15 factors were considered. Laterality, tumor size and surgery type had incomplete data. In addition, surgery type and treatment overlapped with other factors. As such these 4 factors were excluded. Only 11 factors were included and analyzed by multivariate COX regression. Model 1 includes all 11 factors whereas Model 2 only includes 8 factors as 3 factors were excluded on the basis that they had no significant difference in univariate analysis. We also compared outcomes between surgery types wholly and at the same stage and grade. Number of cases at each situation, the order from best survival outcomes to worst, and pairwise comparisons were analyzed, respectively. Relationships between surgery, radiation and metastasis were subsequently analyzed to figure out the influence of treatment type on survival.
3. Results
3.1. The five-year survival rate, univariate analysis and pairwise comparisons
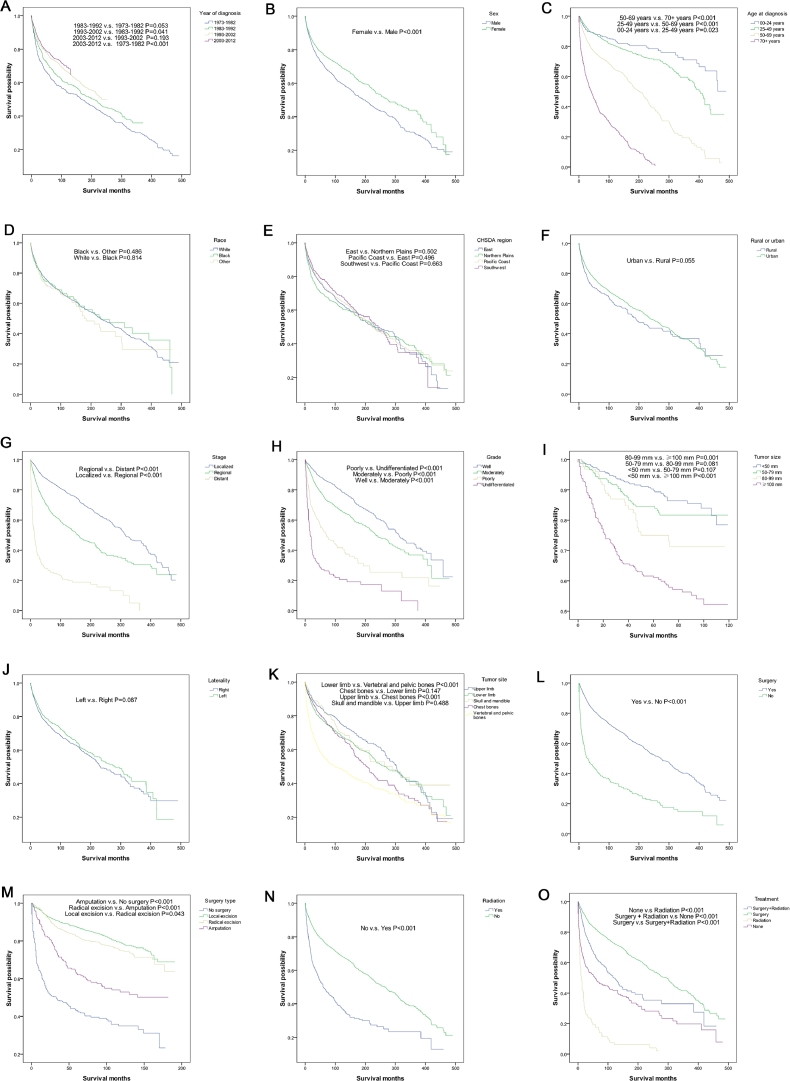
Table 1 conducted five-year survival rates and univariate analyses for 15 factors. Fig. 1 consists of drawn survival curves and presented results of pairwise comparisons on pictures. From the table and the figure, we can see prognosis of chondrosarcoma getting better when comparing outcomes by decade. 1973–1982 had the worst five-year survival rate (64.4%), whereas 2003–2012 had the best five-year survival rate (77.6%). Female patients had better survival outcome than male (P < 0.001). Survival of chondrosarcoma got worse as age increased, and each group (00–24 years, 25–49 years, 50–69 years, 70+ years) had significant difference (P < 0.05 for all). No significant differences were found between different races (P > 0.05) and different CHSDA regions (P > 0.05). There was no significant difference in survival rates for patients from rural and urban areas (P > 0.05). There were, however, significant differences for stage in pairwise comparisons and whole comparison (P < 0.001 for all). ‘Localized’ was best, followed by ‘regional’, and ‘distant’ being the worst. A similar result was shown for grade of chondrosarcoma. Patients with ‘well’ grade had better outcomes than those with ‘undifferentiated’ grade. The differences was more obvious (P < 0.001 for all). The most prominent thing shown in the 9th picture of the survival curve was that tumor size ≥100 mm had the lowest survival rate (P < 0.05). As tumor size increases prognosis becomes poorer. Whether the tumor was located on the left or right side had no effect on survival rates (P > 0.05). Tumors located in vertebral and pelvic bones had worst prognosis (P < 0.001). Those located in other sites had no differences between each other. Patients who underwent surgery had better survival rates than who not (P < 0.001). When compared with each other, the surgery type groups from having best survival outcomes to worst were local excision, radical excision, amputation, no surgery. Every pairwise comparison between surgery types shows a significant difference (P < 0.05 for all). Patient who underwent radiation had worse prognosis than those who did not (P < 0.001). When looking at every pairwise comparison, it is clear that treatment methods from having the best survival outcomes to worst were as follows: surgery, surgery + radiation, none, radiation (P < 0.001 for all). From the results of the five-year survival rate statistics it is interesting to see that ‘distant’ stage, ‘undifferentiated’ grade had a five-year survival rate lower than 30%. Moreover, more situations had five-year survival rates higher than 80%. In addition, the five-year survival rate of patients who underwent radiation but did not underwent surgery was 17.3%, which was in shark contrast to ‘radiation’ (49.9%) and ‘no surgery’ (43.0%).
Table 1.
Five-year survival rate and univariate analysis in patients with chondrosarcoma from 1973–2012.
| Variables | N (%) | Survival (95%CI) | Univariate analysis | |
|---|---|---|---|---|
| HR (95%CI) | P-value | |||
| Year of diagnosis | P < 0.001 | |||
| 1973–1982 | 444 | 64.4(59.9–68.9) | Reference | |
| 1983–1992 | 485 | 69.1(65.0–73.2) | 0.852(0.724–1.001) | 0.052 |
| 1993–2002 | 1109 | 75.3(72.8–77.8) | 0.730(0.628–0.848) | P < 0.001 |
| 2003–2012 | 1699 | 77.6(75.4–79.8) | 0.626(0.533–0.735) | P < 0.001 |
| Sex | P < 0.001 | |||
| Male | 1979 | 70.9(68.7–73.1) | Reference | |
| Female | 1758 | 77.3(75.3–79.3) | 0.746(0.672–0.828) | P < 0.001 |
| Age at diagnosis | P < 0.001 | |||
| 00–24 years | 293 | 87.6 (83.7–91.5) | Reference | |
| 25–49 years | 1420 | 87.5 (85.7–89.3) | 1.448(1.094–1.916) | 0.01 |
| 50–69 years | 1290 | 74.5 (72.0–77.0) | 3.449(2.620–4.541) | P < 0.001 |
| 70 + years | 734 | 40.7 (37.0–44.4) | 10.865(8.223–14.355) | P < 0.001 |
| Race | 0.71 | |||
| White | 3286 | 74.0 (72.4–75.6) | Reference | |
| Black | 238 | 73.8 (67.9–79.7) | 0.976(0.789–1.207) | 0.821 |
| Other | 178 | 70.9 (64.0–77.8) | 1.098(0.868–1.391) | 0.435 |
| CHSDA region | 0.702 | |||
| East | 1088 | 73.6(70.9–76.3) | Reference | |
| Northern Plains | 644 | 69.6(65.9–73.3) | 1.027(0.883–1.194) | 0.73 |
| Pacific Coast | 1641 | 74.8(72.6–77.0) | 0.961(0.846–1.091) | 0.54 |
| Southwest | 359 | 78.5(73.7–82.3) | 0.933(0.771–1.128) | 0.472 |
| Rural or urban | 0.056 | |||
| Rural | 448 | 69.3(64.8–73.8) | Reference | |
| Urban | 3240 | 74.7(73.1–76.3) | 0.863(0.741–1.004) | 0.056 |
| Stage | P < 0.001 | |||
| Localized | 1945 | 86.2(84.6–87.8) | Reference | |
| Regional | 1218 | 67.6(64.9–70.3) | 2.064(1.832–2.325) | P < 0.001 |
| Distant | 302 | 26.5(21.4–31.6) | 7.471(6.386–8.741) | P < 0.001 |
| Grade | P < 0.001 | |||
| Well | 1382 | 87.2 (85.4–89.0) | Reference | |
| Moderately | 1320 | 75.9 (73.5–78.3) | 1.562(1.363–1.791) | P < 0.001 |
| Poorly | 290 | 53.0 (46.9–59.1) | 3.524(2.924–4.247) | P < 0.001 |
| Undifferentiated | 136 | 26.7 (19.1–34.3) | 7.024(5.657–8.720) | P < 0.001 |
| Tumor size | P < 0.001 | |||
| <50 mm | 424 | 89.8 (86.5–93.1) | Reference | |
| 50–79 mm | 308 | 84.1 (79.4–88.8) | 1.400(0.922–2.127) | 0.115 |
| 80–99 mm | 129 | 75.9 (66.9–84.9) | 2.131(1.322–3.434) | 0.002 |
| ≥100 mm | 354 | 61.0 (55.3–66.7) | 4.336(3.089–6.085) | P < 0.001 |
| Laterality | 0.088 | |||
| Right | 1378 | 74.7(72.3–77.1) | Reference | |
| Left | 1292 | 77.9(75.5–80.3) | 0.894(0.786–1.017) | 0.088 |
| Primary site-lableled | P < 0.001 | |||
| Upper limb | 691 | 83.7 (80.8–86.6) | Reference | |
| Lower limb | 1201 | 77.1 (74.6–79.6) | 1.224(1.040–1.441) | 0.015 |
| Skull and mandible | 305 | 84.7 (80.4–89.0) | 1.048(0.817–1.343) | 0.714 |
| Chest bones | 612 | 79.0 (75.7–82.3) | 1.366(1.134–1.645) | 0.001 |
| Vertebral and pelvic bones | 853 | 56.0 (52.5–59.5) | 2.273(1.932–2.675) | P < 0.001 |
| Surgery | P < 0.001 | |||
| Yes | 3177 | 79.7(78.3–81.1) | Reference | |
| No | 488 | 41.5(37.0–46.0) | 3.392(2.994–3.842) | P < 0.001 |
| Surgery type | P < 0.001 | |||
| No surgery | 338 | 43.0(37.3–48.7) | Reference | |
| Local excision | 856 | 87.4(85.0–89.8) | 0.176(0.142–0.218) | P < 0.001 |
| Radical excision | 886 | 82.4(79.7–85.1) | 0.216(0.176–0.265) | P < 0.001 |
| Amputation | 239 | 62.7(56.0–69.3) | 0.470(0.368–0.600) | P < 0.001 |
| Radiation | P < 0.001 | |||
| Yes | 459 | 49.9 (45.2–54.6) | Reference | |
| No | 3203 | 77.6 (76.0–79.2) | 0.416(0.365–0.475) | P < 0.001 |
| Treatment | P < 0.001 | |||
| Surgery + radiation | 328 | 63.1(057.6–68.6) | Reference | |
| Radiation | 113 | 17.3(10.0–24.6) | 4.291(3.339–5.514) | P < 0.001 |
| Surgery | 2793 | 81.8(80.2–83.4) | 0.521(0.440–0.617) | P < 0.001 |
| None | 365 | 49.1(43.8–54.4) | 1.538(1.253–1.889) | P < 0.001 |
P values calculated by univariate analysis. Bold represents statistical significance, P < 0.05.
HR: hazard ratio; CI: confidence interval.
Fig. 1.
Survival analyses according to 15 factors and pairwise comparisons based on log-rank test in patients with chondrosarcoma less than 25 years old from 1973–2012. A. Year of diagnosis. B. Sex. C. Age. D. Race. E. CHSDA region. F. Rural or urban. G. Stage. H. Grade. I. Tumor size. J. Laterality. K. Tumor site. L. Surgery. M. Surgery type. N. Radiation. O. Treatment.
3.2. Association between surgery type and survival outcome
The relationship between surgery type and survival outcomes was further analyzed as stage and grade to avoid confusion. Results are shown in Table 2. From the table, we can see more patients at the ‘localized’ stage underwent excision and less patients underwent amputation. Patients at a ‘distant’ stage tended to not be surgically treated. Fewer patients with ‘well’ grade underwent amputation. From looking at the effect of surgery type on survival we can see the consistency of the order from best to worst (excision, amputation, and no surgery) except that we cannot determine the order of local excision and radical excision. We found that radical resection has a better outcome for higher grade chondrosarcoma (‘poorly’ and ‘undifferentiated’). But local resection appears to be better for lower grade chondrosarcoma (‘well’ and ‘moderately’).
Table 2.
Relationship between surgery type and survival outcome in patients with chondrosarcoma from 1973–2012 according to stage and grade.
| Stage |
Grade |
All (n = 2016) | ||||||
|---|---|---|---|---|---|---|---|---|
| Localized | Regional | Distant | Well | Moderately | Poorly | Undifferentiated | ||
| Number | ||||||||
| No surgery | 75 | 62 | 51 | 77 | 70 | 24 | 17 | 188 |
| Local excision | 535 | 208 | 31 | 397 | 321 | 48 | 8 | 774 |
| Radical excision | 456 | 327 | 46 | 328 | 373 | 93 | 35 | 829 |
| Amputation | 79 | 130 | 16 | 49 | 117 | 39 | 20 | 225 |
| Order of outcome | ||||||||
| Best | 2 | 3 | 2 | 2 | 2 | 3 | 3 | 2 |
| Better | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 3 |
| Worse | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Worst | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Significance | ||||||||
| 1 vs. 2 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | 0.167 | P < 0.001 |
| 1 vs. 3 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | 0.001 | P < 0.001 |
| 1 vs. 4 | 0.001 | 0.266 | 0.093 | 0.104 | P < 0.001 | 0.036 | 0.240 | P < 0.001 |
| 2 vs. 3 | 0.041 | 0.712 | 0.826 | 0.200 | 0.511 | 0.239 | 0.407 | 0.029 |
| 2 vs. 4 | 0.104 | P < 0.001 | 0.002 | P < 0.001 | 0.014 | 0.023 | 0.813 | P < 0.001 |
| 3 vs. 4 | 0.649 | P < 0.001 | 0.003 | 0.006 | 0.039 | P < 0.001 | 0.070 | P < 0.001 |
P values were calculated by log-rank tests. Bold represents statistical significance, P < 0.05.
1: No surgery; 2: Local excision; 3: Radical excision; 4: Amputation.
3.3. Association between treatment type and survival outcome
We identified patients with ‘distant’ stage in the SEER database as metastatic patients. Analysis of the relationship between treatment types and metastasis in Table 3 shows that patients who underwent single radiation had the highest metastasis rate (at 41.4% shown in the table). Patients who underwent single surgery had lowest rate of metastasis at 4.4%. Only 6.5% of all patients who did not undergo radiation were metastatic patients. 22% of all patients who did undergo radiation were metastatic patients.
Table 3.
Relationship between treatment type and metastasis in patients with chondrosarcoma from 1973–2012.
| Number (3366) | Localized | Regional | Distant | Metastasis rate (%) | |
|---|---|---|---|---|---|
| Surgery | |||||
| Yes | 2992 | 1750 | 1074 | 168 | 5.6 |
| No | 374 | 149 | 110 | 115 | 30.7 |
| Radiation | |||||
| Yes | 414 | 133 | 189 | 92 | 22.2 |
| No | 2952 | 1766 | 995 | 191 | 6.5 |
| Treatment | |||||
| Surgery + radiation | 315 | 111 | 153 | 51 | 16.2 |
| Radiation | 99 | 22 | 36 | 41 | 41.4 |
| Surgery | 2677 | 1639 | 921 | 117 | 4.4 |
| None | 275 | 127 | 74 | 74 | 26.9 |
3.4. Multivariate COX regression analysis
Through univariate analysis we can see that significantly different factors include year of diagnosis, sex, age at diagnosis, stage, grade, tumor size, laterality, tumor site, surgery, surgery type, radiation, and treatment (P < 0.05 for all). In Model 1 of multivariate COX regression analysis year of diagnosis, sex, age of diagnosis, stage, grade, tumor site, surgery, and radiation were all independent risk factors (P < 0.05 for all). Model 2 of multivariate COX regression analysis had the same independent risk factors as Model 1 (P < 0.05 for all). Results of multivariate COX regression analysis are shown in Table 4.
Table 4.
Multivariate COX regression analysis in patients with chondrosarcoma from 1973–2012.
| Variables | Model 1 |
Model 2 |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Year of diagnosis | P < 0.001 | P < 0.001 | ||
| 1973–1982 | Reference | Reference | ||
| 1983–1992 | 0.867(0.689–1.092) | 0.226 | 0.871(0.695–1.091) | 0.230 |
| 1993–2002 | 0.762(0.612–0.950) | 0.015 | 0.763(0.617–0.944) | 0.013 |
| 2003–2012 | 0.575(0.454–0.728) | P < 0.001 | 0.589(0.470–0.739) | P < 0.001 |
| Sex | P < 0.001 | P < 0.001 | ||
| Male | Reference | Reference | ||
| Female | 0.753(0.660–0.860) | P < 0.001 | 0.750(0.658–0.854) | P < 0.001 |
| Age at diagnosis | P < 0.001 | P < 0.001 | ||
| 00–24 years | Reference | Reference | ||
| 25–49 years | 1.61(1.111–2.332) | 0.012 | 1.717(1.185–2.487) | 0.004 |
| 50–69 years | 3.765(2.617–5.417) | P < 0.001 | 3.998(2.778–5.754) | P < 0.001 |
| 70 + years | 10.407(7.176–15.094) | P < 0.001 | 11.002(7.588–15.950) | P < 0.001 |
| Race | 0.503 | |||
| White | Reference | |||
| Black | 1.179(0.886–1.568) | 0.258 | ||
| Other | 1.056(0.773–1.442) | 0.732 | ||
| CHSDA region | 0.588 | |||
| East | Reference | |||
| Northern Plains | 0.884(0.717–1.091) | 0.250 | ||
| Pacific Coast | 0.967(0.815–1.148) | 0.704 | ||
| Southwest | 1.029(0.802–1.320) | 0.821 | ||
| Rural or urban | 0.754 | |||
| Rural | Reference | |||
| Urban | 0.968(0.790–1.186) | 0.754 | ||
| Stage | P < 0.001 | P < 0.001 | ||
| Localized | Reference | Reference | ||
| Regional | 1.729(1.494–2.002) | P < 0.001 | 1.720(1.489–1.987) | P < 0.001 |
| Distant | 3.952(3.139–4.976) | P < 0.001 | 3.896(3.107–4.886) | P < 0.001 |
| Grade | P < 0.001 | P < 0.001 | ||
| Well | Reference | Reference | ||
| Moderately | 1.370(1.174–1.598) | P < 0.001 | 1.365(1.174–1.587) | P < 0.001 |
| Poorly | 2.670(2.160–3.30) | P < 0.001 | 2.712(2.198–3.345) | P < 0.001 |
| Undifferentiated | 4.332(3.390–5.535) | P < 0.001 | 4.287(3.377–5.442) | P < 0.001 |
| Primary site-lableled | P < 0.001 | P < 0.001 | ||
| Upper limb | Reference | Reference | ||
| Lower limb | 1.373(1.127–1.673) | 0.002 | 1.417(1.167–1.721) | P < 0.001 |
| Skull and mandible | 0.837(0.595–1.177) | 0.306 | 0.875(0.626–1.224) | 0.437 |
| Vertebral and chest bones | 1.076(0.855–1.354) | 0.532 | 1.090(0.869–1.367) | 0.457 |
| Pelvic bones | 1.764(1.435–2.169) | P < 0.001 | 1.772(1.446–2.171) | P < 0.001 |
| Surgery | P < 0.001 | P < 0.001 | ||
| Yes | Reference | Reference | ||
| No | 2.309(1.909–2.793) | P < 0.001 | 2.403(1.994–2.898) | P < 0.001 |
| Radiation | P < 0.001 | 0.001 | ||
| Yes | Reference | Reference | ||
| No | 0.720(0.599–0.866) | P < 0.001 | 0.732(0.611–0.877) | 0.001 |
P values calculated by multivariate COX regression analysis. Bold if statistically significant, P < 0.05.
HR: hazard ratio; CI: confidence interval.
Model 1 excluded laterality, tumor size and surgery type, treatment in univariate analysis.
Model 2 further excluded 3 factors that had no significant difference in univariate analysis.
4. Discussion
Our study shows a five-year overall survival rate of 73.9% in patients with chondrosarcoma. From the survival analysis, we found that the survival of chondrosarcoma have certain characteristics. ‘Distant’ stage, ‘undifferentiated’ grade and single radiation had very low five-year survival rate, lower than 30%. Moreover, most variables have a five-year survival rate higher than 80%. Our study shows that year of diagnosis, sex, age of diagnosis, stage, grade, tumor site, surgery and radiation are all independent risk factors. Now we will further discuss them according to each variable.
In patient related factors, year of diagnosis, sex, and age of diagnosis are all independent risk factors. Azzarelli et al. [7] revealed that age was a significant prognostic factors but sex was not a prognostic factor. No other studies reported year of diagnosis, sex as risk factors for survival of chondrosarcoma, but in our population based study we found these 3 factors had obvious impact on the survival of patients with chondrosarcoma. We can see improvement of outcome with the development of the times. 1973–1982 had the worst five-year survival rate and 2003–2012 had the best five-year survival rate.
In tumor related factors, stage, grade, and tumor site are independent risk factors. Wang et al. [8] revealed high histological grade as an independent risk factor. Fiorenza et al. [9] analyzed 153 patients with non-metastatic chondrosarcoma and concluded that high histological grade was an independent risk factor but location within the body and surgery type were not. The target in our study was chondrosarcoma, NOS, which accounted for 90% all chondrosarcoma in SEER database. Dedifferentiated chondrosarcoma was a high malignant cancer and not included in our study. If it was included, it belonged to high histological grade. Liu et al. [10] studied dedifferentiated chondrosarcoma in 23 patients. They found that the median survival time was 9 months and the five-year survival rate was only 17.4%. Grimer et al. [11] conducted a retrospective study of 337 patients with dedifferentiated chondrosarcoma using data supplied by the European Musculo Skeletal Oncology Society (EMSOS). He concluded that the femur and pelvis were the most common sites, with an overall survival rate of 28% at 10 years. All above indicate that higher grade tumors have poor prognosis. With regards to sites of chondrosarcoma an axial site has poorer prognosis than an appendicular site. In our study we found that the worst sites were vertebral and pelvis. This was confirmed in other single center studies. For example, Yin et al. [12] analyzed 98 patients with spinal chondrosarcoma, with a recurrence of 90.4% in 2 years and a death rate of 67.7% in 3 years. Several studies [13], [14], [15] explained the higher recurrence rate in spinal chondrosarcoma as the anatomic constraints. Sheth et al. [16] studied 67 patients with pelvic chondrosarcoma, during a follow-up of 115 months (range, 24–288 months). His study showed a recurrence rate of 28% and a death rate of 36%.
In treatment related factors, surgery and radiation were independent factors. Reports for the effect of surgery on survival outcome mainly focus on the comparison of intralesional resection and wide resection. The largest study was from Nota et al. They conducted a systematic literature review of 13 studies and no difference was found between the two surgery types. Only 2 reports [9], [17] of the 13 showed differences. Another large study was from Chen et al. [6] who conducted a meta-analysis of 10 studies including 394 patients with central grade 1 chondrosarcoma to compared the effect of intralesional resection and wide resection. Patients who underwent intralesional resection were shown to have lower complication rates, better Musculoskeletal Tumor Society score (MSTS), but no difference in local recurrence. However, Sheth et al. [16], who studied 67 patients, suggested that the key to obtaining a favorable outcome in lower grade chondrosarcoma in the pelvis is adequate surgical excision. The study carried out by Donati et al. [18] revealed that even low-grade chondrosarcoma in the pelvis has a high local recurrence rate so it is necessary to treat it with wide local excision. The multivariate COX regression analysis in the present cohort study suggested that local excision was better (P = 0.043), and detailed analysis in Table 2 revealed that higher grade chondrosarcoma (‘poorly’ and ‘undifferentiated’) needs thorough resection whilst lower grade chondrosarcoma (‘well’ and ‘moderately’) only requires local removal. Some studies support our conclusion. One such study was carried out by Mermerkaya et al. [19] who suggested that intralesional curettage was suitable for chondrosarcoma of Grade 1. Likewise Campanacci et al. [20] studied low-grade chondrosarcoma at appendicular bones and concluded that only an aggressive biologic behavior on imaging was the indication of wide resection. The suggestion from our study that high grade tumors need wide excision is supported by Grimer et al. [11] who analyzed dedifferentiated chondrosarcoma. Their study shows inadequate margins of excision carries risks of local recurrence and therefore negatively affects the overall survival rates of patients. A recent review by Leddy et al. [1] suggests that wide local resection should be recommended even in cases of low-grade chondrosarcoma to avoid recurrence. Based on the above studies and the results in our study (the P value was 0.043 when comparing local excision with radical excision), we also recommended wide local resection as the treatment for most chondrosarcomas.
At present, the use of radiation in chondrosarcoma is very controversy. Some case reports [21], [22] showed that radiation could induce chondrosarcoma, whilst other studies showed that it could reduce recurrence of chondrosarcoma [23]. Leddy et al. [1] suggested that the effects of radiation remain unclear because chondrosarcoma is resistant to chemotherapy and radiation. Our study showed 22% patients who underwent radiation were metastatic patients, while 6.5% patients who did not undergo radiation were metastatic patients. This demonstrated that patients who select radiation with/without other treatments tend to be metastatic patients. So the reason of bad prognosis caused by radiation might be actually the metastasis lead to bad survival. Thus, this study cannot prove that radiation is harmful or beneficial to survival outcome.
We hope that this study may be of use for clinical doctors. Our study demonstrated that year of diagnosis, sex, age of diagnosis, stage, grade, tumor site, surgery and radiation are independent risk factors for survival. Although patients and tumor related factors cannot be changed treatment can be changed or adapted to obtain better outcomes. For example, excision might be recommended to treat chondrosarcoma for most patients whilst amputation might not. Amputation, a measure of thorough resection, could not improve survival outcomes, as evidenced by the worse survival outcome than local excision and radical excision in this study. Wide local excisions might be recommended especially when encountering high grade chondrosarcoma or pelvic chondrosarcoma. The effect of radiotherapy for the treatment of chondrosarcoma is uncertain so we should not use it as the main therapy. Further studies should be done to obtain a better treatment strategy.
Our study has some limitations. First, the SEER database did not provided us with detailed information of treatment. For an example, information on chemotherapy treatment and detailed surgery type was not available. Joint replacement as a detailed surgery type was not described in the database. Secondly, this study was a retrospective analysis so the accuracy of results cannot equal randomized controlled trials. Finally, this study does not include a description of tumor recurrence and therapy after recurrence. Despite the limitations, this study incorporated a large number of chondrosarcoma cases, increasing the accuracy of present study's results.
Acknowledgments
Acknowledgments
This work was supported by a National Natural Science Foundation of China (NSFC) grant (Grant No: 81672154). The Authors thank the foundation. We also thank the National Cancer Institute which provide available public data, the SEER.
Conflicts of interest
All Authors declare that they have no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.09.003.
Appendix. Supplementary materials
References
- 1.Leddy L.R., Holmes R.E. Chondrosarcoma of bone. Cancer Treat. Res. 2014;162:117–130. doi: 10.1007/978-3-319-07323-1_6. [DOI] [PubMed] [Google Scholar]
- 2.Sandberg A.A., Bridge J.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: chondrosarcoma and other cartilaginous neoplasms. Cancer Genet. Cytogenet. 2003;143(1):1–31. doi: 10.1016/s0165-4608(03)00002-5. [DOI] [PubMed] [Google Scholar]
- 3.van Maldegem A.M., Gelderblom H., Palmerini E. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer. 2014;120(20):3159–3164. doi: 10.1002/cncr.28845. [DOI] [PubMed] [Google Scholar]
- 4.Nota S.P., Braun Y., Schwab J.H. The identification of prognostic factors and survival statistics of conventional central chondrosarcoma. Sarcoma. 2015 doi: 10.1155/2015/623746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorenza F., Abudu A., Grimer R.J. Risk factors for survival and local control in chondrosarcoma of bone. J. Bone Joint Surg. Br. 2002;84(1):93–99. doi: 10.1302/0301-620x.84b1.11942. [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Yu L.J., Peng H. Is intralesional resection suitable for central grade 1 chondrosarcoma: a systematic review and updated meta-analysis. Eur. J. Surg. Oncol. 2017;43:1718–1726. doi: 10.1016/j.ejso.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Azzarelli A., Gennari L., Quagliuolo V. Chondrosarcoma–55 unreported cases: epidemiology, surgical treatment and prognostic factors. Eur. J. Surg. Oncol. 1986;12:165–168. [PubMed] [Google Scholar]
- 8.Wang J.W., Ger L.P., Shih C.H. Chondrosarcoma of bone: a statistical analysis of prognostic factors. J. Formos. Med. Assoc. 1991;90(10):998–1003. [PubMed] [Google Scholar]
- 9.Fiorenza F., Abudu A., Grimer R.J. Risk factors for survival and local control in chondrosarcoma of bone. J. Bone Joint Surg. Br. 2002;84(1):93–99. doi: 10.1302/0301-620x.84b1.11942. [DOI] [PubMed] [Google Scholar]
- 10.Liu C., Xi Y., Li M. Dedifferentiated chondrosarcoma: radiological features, prognostic factors and survival statistics in 23 patients. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0173665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimer R.J., Gosheger G., Taminiau A. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur. J. Cancer. 2007;43(14):2060–2065. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Yin H., Zhou W., Meng J. Prognostic factors of patients with spinal chondrosarcoma: a retrospective analysis of 98 consecutive patients in a single center. Ann. Surg. Oncol. 2014;21(11):3572–3578. doi: 10.1245/s10434-014-3745-z. [DOI] [PubMed] [Google Scholar]
- 13.Boriani S., De Iure F., Bandiera S. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000;25(7):804–812. doi: 10.1097/00007632-200004010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Bergh P., Gunterberg B., Meis-Kindblom J.M. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer Am. Cancer Soc. 2001;91(7):1201–1212. doi: 10.1002/1097-0142(20010401)91:7<1201::aid-cncr1120>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.York J.E., Berk R.H., Fuller G.N. Chondrosarcoma of the spine: 1954 to 1997. J Neurosurg. 1999;90(1 Suppl):73–78. doi: 10.3171/spi.1999.90.1.0073. [DOI] [PubMed] [Google Scholar]
- 16.Sheth D.S., Yasko A.W., Johnson M.E. Chondrosarcoma of the pelvis: Prognostic factors for 67 patients treated with definitive surgery. Cancer Am. Cancer Soc. 1996;78(4):745–750. doi: 10.1002/(SICI)1097-0142(19960815)78:4<745::AID-CNCR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Lee F.Y., Mankin H.J., Fondren G. Chondrosarcoma of bone: an assessment of outcome. J. Bone Joint Surg. Am. 1999;81(3):326–338. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Donati D., GA E.I., Bertoni F. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J. Bone Joint Surg. Br. 2005;87(11):1527–1530. doi: 10.1302/0301-620X.87B11.16621. [DOI] [PubMed] [Google Scholar]
- 19.Mermerkaya M.U., Bekmez S., Karaaslan F. Intralesional curettage and cementation for low-grade chondrosarcoma of long bones: retrospective study and literature review. World J. Surg. Oncol. 2014;12:336. doi: 10.1186/1477-7819-12-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campanacci D.A., Scoccianti G., Franchi A. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J. Orthop. Traumatol. 2013;14(2):101–107. doi: 10.1007/s10195-013-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obid P., Vierbuchen M., Wolf E. Radiation-induced intraspinal chondrosarcoma: a case report. Global Spine J. 2015;5(5):e74–e77. doi: 10.1055/s-0035-1546953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sule N., Xu B.O., Zein D.E. Radiation-induced Chondrosarcoma of the bladder. Case report and review of literature. Anticancer Res. 2015;35(5):2857–2860. [PubMed] [Google Scholar]
- 23.Kawaguchi S., Weiss I., Lin P.P. Radiation therapy is associated with fewer recurrences in mesenchymal chondrosarcoma. Clin. Orthop. Relat. Res. 2014;472(3):856–864. doi: 10.1007/s11999-013-3064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.