Abstract
Introduction
Behavioural and mental disorders have become a public health crisis and by 2020 may surpass physical illness as a major cause of disability. Early prevention is key. Two Incredible Years (IY) parent programmes that aim to enhance child well-being and development, IY Infant and IY Toddler, will be delivered and evaluated in a proportionate universal intervention model called Enhancing Social-Emotional Health and Wellbeing in the Early Years (E-SEE) Steps. The main research question is: Does E-SEE Steps enhance child social emotional well-being at 20 months when compared with services as usual?
Methods and analysis
E-SEE Steps will be delivered in community settings by Early Years Children’s Services and/or Public Health staff across local authorities. Parents of children aged 8 weeks or less, identified by health visitors, children’s centre staff or self-referral, are eligible for participation in the trial. The randomisation allocation ratio is 5:1 (intervention to control). All intervention parents will receive an Incredible Years Infant book (universal level), and may be offered the Infant and/or Toddler group-based programme/s—based on parent depression scores on the Patient Health Questionnaire or child social emotional well-being scores on the Ages and Stages Questionnaire: Social Emotional, Second Edition (ASQ:SE-2). Control group parents will receive services as usual. A process and economic evaluation are included. The primary outcome for the study is social emotional well-being, assessed at 20 months, using the ASQ:SE-2. Intention-to-treat and per protocol analyses will be conducted. Clustering and hierarchical effects will be accounted for using linear mixed models.
Ethics and dissemination
Ethical approvals have been obtained from the University of York Education Ethics Committee (ref: FC15/03, 10 August 2015) and UK NHS REC 5 (ref: 15/WA/0178, 22 May 2015. The current protocol is Version 9, 26 February 2018. The sponsor of the trial is the University of York. Dissemination of findings will be via peer-reviewed journals, conference presentations and public events.
Trial registration number
ISRCTN11079129; Pre-results.
Keywords: parent programmes, social emotional wellbeing, randomised controlled trial, proportionate universalism, infant, incredible years
Strengths and limitations of this study.
Very few studies apply a proportionate universalism approach reflecting real-world provision of services for families of very young children; within the Enhancing Social-Emotional Health and Wellbeing in the Early Years (E-SEE) intervention arm, there are three levels of intervention and four possible ‘doses’ of intervention according to need.
The study includes an economic and process evaluation, alongside the effectiveness evaluation.
The design and implementation of this trial was informed by a large randomised pilot study involving two research sites, over 200 families, and involving parent advisory committees.
The study is inclusive of co-parents (typically fathers) and will provide insights into the role of co-parents in shaping children’s social and emotional development.
The study cannot establish the effectiveness of each of the intervention’s three individual levels, that is, the study is only powered to explore the effectiveness of the overall E-SEE steps model.
Introduction
Behavioural and mental disorders have become a public health crisis and by 2020 may surpass physical illness as a major cause of disability. Early intervention and prevention of mental health and behavioural issues are more effective, and less costly, than late interventions.1 Child mental health issues are associated with significant costs to the individual and society and are associated with both short-term and long-term negative outcomes (eg, failure to thrive, school difficulties, drug/alcohol problems, juvenile delinquency, aggressive behaviour, adult mental health issues, ineffective relationship building, criminal activity), as well as becoming a young parent with the possibility of intergenerational transmission.2–4 There are clear benefits to parents, children and their families of reducing the potential for such difficulties to emerge, by improving the home environment, parenting skills, positive parent–child interactions and understanding of child development and safety issues.1 5
Recent UK policy and guidance highlight the importance of improving health and well-being in children, with an emphasis on a whole family approach including fathers and grandparents in an integrated proportionate approach.6–8 The National Institute for Health and Care Excellence (NICE) guidance further suggests that the social and emotional well-being of vulnerable young children should be tackled through home visiting, early education and childcare.9 Several Cochrane reviews have highlighted the effectiveness of group-based parent programmes to promote child and parent well-being (3 years+),5 and a review of programmes for 0–3-year-olds calls for more research with younger age groups.10 Investment in evidence-based, early years intervention has the potential for long-term effects which will benefit wider society with attendant long-term cost benefits.1
Although there is significant policy interest and increasing research in this area, the evidence gap identified by NICE still exists. For example, the recent ‘Building Blocks’ Trial in England investigated a nurse-led intensive home visitation programme—called the Family Nurse Partnership (FNP)11—to evaluate the impact on infant and maternal outcomes up to 24 months after birth. The results showed that the FNP provided no additional short-term benefits with respect to the primary outcomes assessed in the trial.
The Incredible Years (IY) parent programmes (www.incredibleyears.com) are manualised parent education and training interventions which include group-based components and parent and facilitator books and materials. IY is informed primarily by social learning theory and designed to enhance the social and emotional well-being of children aged 0–12 years. There is growing evidence of the cost-effectiveness of the IY parent programmes,12–14 as well as concomitant reductions in health, social and education service utilisation.15 16 The IY Infant (IY-I) and IY Toddler (IY-T) versions, for 0–1 and 1–3-year-olds, respectively, build on decades of development and research evidence of the IY (3 years+) programmes, but have not yet been rigorously evaluated in a UK, targeted, community-based trial. IY has the capacity to be delivered in a proportionate universalism model of varying doses according to need, and this study will be the first to evaluate such an approach in the form of our Enhancing Social-Emotional Health and Wellbeing in the Early Years (E-SEE) Steps model.
Aims and objectives
The study comprises two phases including: (1) a pilot trial; and (2) a definitive randomised controlled trial (RCT).
The pilot phase informed the main trial design and trial procedures including: (1) recruitment; (2) retention; (3) fidelity of intervention delivery; (4) model of delivery; (5) differentiation of outcome; and (6) outcome and cost-effectiveness measures. This protocol relates to the main trial only.
The main, definitive RCT is designed to: (1) establish the effectiveness of the IY programmes on clinical outcomes; (2) assess cost-effectiveness; and (3) evaluate the processes around service delivery.
Therefore, the main objectives and key questions of the trial are as follows:
Does E-SEE Steps enhance child social emotional well-being at 20 months of age when compared with services as usual?
Is IY, and the proposed delivery model, cost-effective in enhancing child social emotional well-being at 20 months when compared with services as usual?
Can IY can be delivered as a proportionate universalism model, and what are the organisational, or systems-level, barriers and facilitators to delivering in this way, with fidelity?
Methods and analysis
Design
A pragmatic two-arm RCT and economic appraisal, with an embedded process evaluation to examine the outcomes, implementation and cost-effectiveness of the intervention, as well as uptake by parents.
Setting
Participating trial sites (local authorities) will not be offering IY-I or IY-T as part of usual services and should have sufficient live birth rates to support recruitment targets.
Intervention
The E-SEE Steps model includes two IY programmes—IY-I and IY-T for parents of children aged 0–1 and 1–3 years of age, respectively. Both programmes are delivered in a universal proportionate framework, to match varying parent–infant needs at different time points. All intervention parents will receive an IY-I book (universal level). Intervention parents may then be offered the IY-I (10 weeks, 2 hours/week) and/or IY-T (12 weeks, 2 hours/week) group-based programme—based on a predefined threshold on the Patient Health Questionnaire (PHQ-9) and/or the Ages and Stages Questionnaire: Social Emotional, Second Edition (ASQ:SE-2). Figure 1 depicts the proportionate universal approach of E-SEE Steps.
Figure 1.
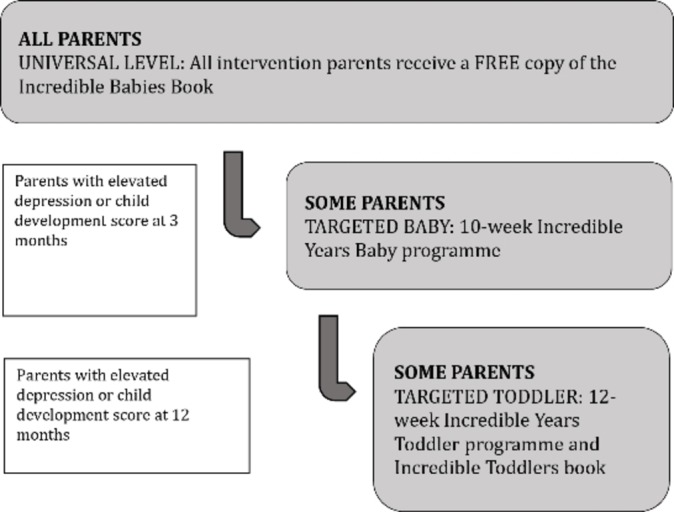
E-SEE Steps.
Delivery of IY-I and IY-T will take place in local community settings such as children’s centres, with group sizes of up to 10 parents for IY-I and 14 parents for IY-T. Sessions will be delivered by two cofacilitators—a health professional (eg, health visitor, infant mental health practitioner, speech and language therapist) and/or early years children’s services’ (or local authority commissioned) staff (eg, children centre worker or family support worker). Staff will be trained by accredited IY trainers. All intervention participants will have access to services as usual.
Controls
Control group parents will receive services as usual.
Participants
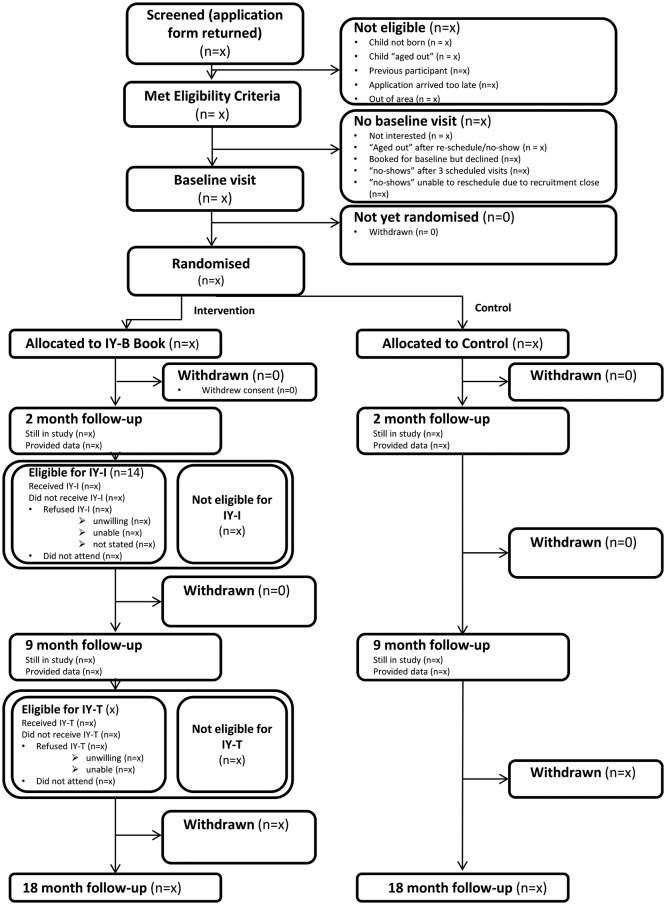
Parents (primary caregivers who have the main parenting responsibility) of children aged 8 weeks or less will be identified by health staff, such as health visitors, or children’s centre staff, or via self-referral. A range of briefing events and information resources will be made available to staff in advance of the identification period. Parent contact details will be forwarded, with consent, to the research team who will arrange a home visit to provide further information on the study and assess eligibility status, and trained researchers will obtain written, informed consent (please see online supplementary files 1 and 2 for the information sheet and consent form). Consenting parents can invite a co-parent who shares parenting responsibilities into the trial, so that we can explore the impact of co-parents on child well-being. The flow of participants through the trial is detailed in figure 2.
Figure 2.
Participant flow. IY-I, Incredible Years Infant; IY-T, Incredible Years Toddler.
Inclusion and exclusion criteria for E-SEE trial
Inclusion criteria
Parents will be included if they consent to participate, have a child aged 8 weeks or under, will be willing to be randomised and, if allocated to intervention, will be able to receive the IY services offered.
Exclusion criteria
Child has obvious, or diagnosed, organic developmental difficulties. Parent is enrolled on another group parent programme at sign-up.
Randomisation and allocation
Randomisation will be performed using a web-based randomisation system. Parents will be randomly allocated to intervention or control arms on a 5:1 ratio stratified according to level of need at baseline (BL) based on parent PHQ-9 or child ASQ:SE-2 score, gender of child and parent and recruitment site. The co-parent will automatically receive the same allocation as the randomised parent.
Methods to reduce bias
Participants, IY facilitators and some of the study team will not be blind to allocation. Data collectors will be blind to participant allocation (parents will be asked not to share their allocation status)—as will participant referrers, the chief investigator, the team statistician (until final analysis), the Trial Steering Committee (TSC) and Trial Management Group (TMG).
Families will receive shopping vouchers of a modest amount (increasing at each data collection point to retain participants) as a token of thanks for completing measures.
Primary analysis will be intention to treat (ITT); once randomised, participants will remain within their allocated group for analytical purposes even if they cross over to the other study arm, or drop out.
Sample size calculations
Sample size is calculated on the child primary outcome of social emotional well-being—the ASQ:SE-2.17 We define the clinically important difference at follow-up 3 (18 months post-BL) to be 5 units of the ASQ:SE-2 in the IY group when compared with services as usual (SAU). Assuming an SD value of 18 on the ASQ:SE-2 at follow-up 3, the correlation between BL and follow-up 3 scores is 0.26 and between pairs of measurements after BL is 0.40, the design effect of 1.25 for the IY arm, two-sided 5% significance level and 90% power, we would require the study to have retained at follow-up 3441 in IY and 92 in SAU. Allowing for 12% overall attrition, 606 should be randomised with an allocation ratio of 5:1—to ensure sufficient parents (an expected total of 48) are eligible and able to attend IY groups.
Outcome measures
A number of primary and secondary outcome measures will be completed at BL (within 10 weeks following birth) and then again at 2, 9 and 18 months post-BL (table 1). Data will be collected by trained researchers in the family home (or a venue of the participant’s choosing). Children will be 20 months at final follow-up.
Table 1.
Overview of measures
| Outcomes and timepoints | Measures | Description | BL | Fu1 | Fu2 | Fu3 |
| Social and emotional well-being | ASQ:SE-2 | Parent self-report | √ | √ | √ | √ |
| Parent or co-parent depression | PHQ-9 | Parent/co-parent self-report | √ | √ | √ | √ |
| Attachment | CARE Index | Parent observation | √ | √ | √ | √ |
| Service use | CSRI* | Data collector administered | √ | √ | √ | √ |
| Parenting skill | PSoC | Parent/co-parent self-report | √ | √ | √ | √ |
| Parent or co-parent health | EQ5D-5L | Parent/co-parent self-report | √ | √ | √ | √ |
| Demographics | Bespoke form | Data collector administered | √ | |||
| Short demographics | Bespoke form | Data collector administered | √ | √ | √ | |
| Child health (and quality of life) | PEDsQL | Parent/co-parent self-report | √ | |||
| Attachment | MPAS/PPAS† | Parent/co-parent self-report | √ | |||
| Child behaviour | SDQ | Parent/co-parent self-report | √ |
Average times to complete based on previous research carried out with similar populations by members of the research team.
*The Client Service Receipt Inventory (CSRI) description presented on p. 42 is taken from the original CSRI paper—for the E-SEE trial, we are using a revised, much shorter version, hence the variability in timings.
†Paternal Postnatal Attachment Scale (PPAS) to be used if father is the parent or co-parent.
ASQ:SE-2, Ages and Stages Questionnaire: Social Emotional, Second Edition; BL, baseline; Fu1, Follow-up 1; Fu2, Follow-up 2; Fu3, Follow-up 3; MPAS, Maternal Postnatal Attachment Scale; PEDsQL, Pediatric Quality of Life Inventory; PHQ-9, Patient Health Questionnaire; PSoC, Parenting Sense of Competence questionnaire; SDQ, Strengths and Difficulties Questionnaire 2–4 version.
Child primary outcome
Social and emotional well-being
The parent-completed ASQ:SE-217 can be used for children aged 1–72 months (with age-appropriate versions), and covers six key social and emotional development areas: self-regulation, compliance, adaptive functioning, autonomy, affect, social-communication and interaction with people. The measure is psychometrically sound with: a test-retest reliability of 89%, internal consistency of 84%, sensitivity is of 81% and specificity of 84%. The ASQ:SE-2 takes 5–10 min to complete and will be used here, along with the PHQ-9 (see below), to assess eligibility for both the IY-I and IY-T groups.
Child secondary outcomes
Attachment/interaction
Independent observation of parent–child interaction will be undertaken using the CARE Index Infant/Toddler,18 which is suitable for children aged 1–48 months. Three to five minutes of play is video recorded and later coded using an interaction classification scheme to assess global synchrony (ie, ‘At Risk’; ‘Intervention’; ‘Adequate’ and ‘Sensitive’), parent attachment and child attachment over seven subscales. Inter-rater reliability is ≥0.75 for four of the seven subscales.
Cognitive development and health (quality of life)
The parent-completed Pediatric Quality of Life Inventory (PEDsQL) Infant is a 45-item questionnaire for parents with infants aged 13–24 months.19 The measure has demonstrated internal consistency reliability for total scores (0.92) and is able to distinguish between healthy infants and those with acute and chronic illnesses.20 It takes 10 min to complete.
Child behaviour
The Strengths and Difficulties Questionnaire 2–4 version (SDQ)21 is a 25-item widely used questionnaire designed for parents of children aged 2 to 4 years old. Research has shown good internal consistency for each of the five subscales and the overall ‘Total Difficulties’ score with this age group.22 This measure takes 10 min to complete.
Parent primary outcome
Depression
PHQ-9 is a 9-item self-complete tool to assess depression using criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). The total score provides an index of overall severity of depression.23 The PHQ-9 has established good diagnostic validity evidencing 88% sensitivity and specificity for major depression.23 Cronbach alphas of 0.86 to 0.89 demonstrate good internal reliability, with a test-retest reliability at 0.84.24 The PHQ-9 takes 5 min to complete and will be used to assess eligibility to both IY-I and IY-T groups, along with the ASQ:SE-2.
Parent secondary outcomes
Maternal/paternal–child attachment/interaction
The Maternal Postnatal Attachment Scale (MPAS),24 and the Paternal Postnatal Attachment Scale (PPAS)25 contain 19 self-complete items developed to assess parent attachment to their infant. The MPAS has evidenced good internal consistency (0.78 to 0.79), high test-retest reliability (0.086) and good stability over time.24 For PPAS, internal consistency alpha levels are 0.62 to 0.81, with correlation coefficients 0.65 to 0.70, and exemplary convergent validity. MPAS/PPAS takes 10 min to complete.
Parenting skill
The Parenting Sense of Competence questionnaire (PSoC) has 17 self-complete items to assess parenting self-esteem.26 The measure has two subscales, related to parent satisfaction and parent self-efficacy. Internal consistency for the PSoC shows Cronbach’s alpha coefficients ≥0.70.26 The PSoC takes 5–10 min to complete.
Health (quality of life)
The EQ5D-5L,27 a five-item, self-complete measure that provides an index relating to quality of life over five domains: mobility, ability to self-care, ability to undertake usual activities, pain and discomfort, anxiety and depression. It also includes a visual analogue scale that records respondent’s reports of their overall health state from ‘worst imaginable health state’ to ‘best imaginable health state’. The EQ5D-5L has been validated in several countries, including the UK.28 The EQ5D-5L takes 5–10 min to complete.
Other outcomes
Demographic information will be captured via a bespoke structured interview form, including: age, ethnicity, religion, income, marital status, parent/co-parent education, housing and family composition.
Further economic evaluation outcomes
The study will examine resource use and costs based on access to health, social and educational services by parents and children as self-reported by parents using a modified Client Service Receipt Inventory.29 Costs of intervention delivery will be gathered via implementation staff and existing data sources.
Process evaluation
The embedded process evaluation will involve the completion of: weekly facilitator logs to record parental receipt of the IY-I book, and IY-I/IY-T attendance and contact rates; weekly self-rated IY checklists to assess adherence to core components; a researcher-rated Parent Programme Implementation Checklist exploring adherence, quality of delivery and participant responsiveness30; and IY Parent Satisfaction Questionnaires (modified for UK audience in collaboration with the IY developer) completed after each session, and at the end of each programme.
Statistical analysis
Statistical analyses will be conducted using validated statistical software packages. ITT and per protocol analyses will be conducted.
Treatment effectiveness
The study will examine the effectiveness of the treatment as a whole, over the three stages of the trial (2, 9 and 18 month post-BL data collection time points). We will investigate the impact of each proportionate stage of the IY intervention in a secondary analysis. The overall effectiveness of the proportionate delivery of IY will be assessed using a multilevel mixed model to examine treatment and time effects while allowing for the clustering by participant and group treatments and confounding and stratifying variables. The treatment is delivered in clusters but no cluster-based intervention occurs in the control arm. We will adhere to the most recent publication guidelines on the analysis of cluster-randomised trials.31 BL outcome measures will also be included as covariates. Missing data will be reported and multiple imputation will be used to impute missing values in the primary outcome.
Subgroup analyses will allow us to consider issues of inequalities and will include, for example, socioeconomic status, ethnicity, sex of primary caregiver, birth order of included child and co-parent outcomes to establish for whom the intervention works best, using mediator and moderator analyses.
Process evaluation/treatment processes
Service design support will facilitate the implementation of E-SEE Steps in each site, including evidenced-based strategies for engagement, retention and multiagency working.32 A service design manual for E-SEE Steps will be produced outlining programme theory, core components and intervention delivery.
A multimethod approach will assess fidelity of delivery, explore parents’, facilitators’ and service managers’ experiences of E-SEE Steps as well as the organisational, team and individual factors that facilitate or hinder its implementation. Quantitative monitoring data (see outcome section for details) will be collected for all IY-I and IY-T groups.
Additionally, facilitators will complete online questionnaires before attending training in IY and again after completing delivery of the programme/s. The pretraining questionnaire will assess facilitators’ qualifications existing experience of parenting groups and working with families, as well as perceived competence to deliver the programme and perceived organisational support. The postdelivery questionnaire will supplement the qualitative data on facilitators’ experiences of delivering IY-I/IY-T. All quantitative data will be reported descriptively.
Qualitative data will be gathered by means of 12 focus groups—half with intervention parents/co-parents and half with IY group facilitators—as well as 12 semistructured interviews with public health and children’s services managers. The focus groups and interviews will be undertaken on completion of intervention delivery in each site to avoid potentially influencing the impact of the intervention. All interviews and focus groups will be audio-recorded (with consent) and transcribed. Thematic analysis will be used to analyse qualitative data. Reporting of qualitative findings will adhere to the consolidated criteria for reporting qualitative research (COREQ).33
Economic evaluation
Cost-effectiveness analyses and cost-consequence analyses will be conducted. The latter technique is useful in the evaluation of interventions with multidimensional outcomes. Costs in both trial arms will be estimated from alternative perspectives,34 including a National Health Service and Personal Social Services (PSS) perspective (consistent with that used by NICE),35 a wider public sector perspective and a societal perspective, which includes costs to participants.36 37
Resource use estimates will be collected from a variety of sources. A microcosting of IY-I and IY-T will be conducted (building on previous IY studies) to establish programme delivery costs (including consideration of set-up and training costs). This will include collecting the details of participants’ contacts with professionals required to deliver the intervention. Wider public sector resource use data, with a particular focus on healthcare (including primary and secondary care visits), and expenditure incurred ‘out-of-pocket’ by participants and absence from employment, will be collected from trial participants using questionnaires. Costs of resources will be calculated by applying published national (UK) unit cost estimates, where available, to estimates of relevant resource use.38 39 If published unit cost estimates are not available, unit costs will be identified in consultation with the appropriate finance departments of the resource provider. Costs and effects will be discounted at 3.5% per annum in line with national guidance.35 36
The initial analysis will present incremental results for the primary outcome measures for both children (ASQ:SE-2) and adults separately (PHQ-9). These will be compared with the incremental costs measured from the alternative perspectives as above. Secondary outcomes in terms of quality-adjusted life-years (based on PEDsQL for children and EQ-5D5L for adults) will also be considered. Alternative methods for combining different primary and secondary outcomes across children and adults and across outcomes will be explored to allow for a full assessment of the benefits, which can then be compared with costs. Links between trial outcome measures and longer-term outcomes (eg, across health and education sectors) will be explored.
Probabilistic sensitivity analyses will be conducted to reflect the uncertainty around the adoption decision (depicted using cost-effectiveness acceptability curves).36 Sensitivity analyses will be performed to determine the robustness of the results to altering certain assumptions; for example, changes in the assumed discount rate could influence the results.36 40
Patient and public involvement
During the preparation of the application for funding, three discussion groups were held, two with parents who had attended, or were currently attending, a parent programme and one with parents who had not attended a programme. Their input was invaluable to the design of the study.41 Four topics were discussed: recruitment to the study/engagement; retention of participants to parenting programmes; retention to study and data collection; and public involvement in research.
Parent peers were suggested as a means to engage and retain intervention participants to the programme/study. This was seen as particularly important to overcome barriers when engaging with fathers. Regarding data collection methods, attendees suggested giving a choice to parents but should be face-to-face. The design has incorporated home-based or community-based (eg, at a children centre) data collection visits. The setting up of a parent committee was recommended.
A parent committee will be set up to:
Assist parent engagement by holding preintervention sessions in community venues to discuss parent programmes—expectations and potential benefits. Service users believed peer support important for engagement due to: mistrust of some professionals, anxiety in attending a programme/discussing feelings.
Input to the development of information/consent forms and other literature to enhance inclusivity through ease of understanding, particularly for parents with low literacy.
Assist measure selection based on user-friendliness.
Attend project steering group.
Assist in training researchers in interview/data collection methods through role play activities.
Organise a dissemination event for families to share results and encourage future programme participation.
Ancillary substudies
Four substudies are planned to explore:
The impact of co-parents on children’s social and emotional well-being.
Access to health records and frequency/severity of hospital admissions.
Statistical design and analysis of trials evaluating complex interventions.
Comparisons with complementary studies and existing data sets.
Ethics and dissemination
Ethics and governance
Participants will be informed that their personal data will be pseudonymised and related forms and questionnaires will be identified using a participant study number only. All hard copy data will be stored in a locked filing cabinet in accordance with data protection requirements for the retention of research data and study team institutional data management policies. Confidentiality would only be broken if required for safeguarding a vulnerable child or adult, with any action in accordance with the study site policies and procedures.
The ethical implications of obtaining data that may identify a participant as depressed, having suicidal thoughts and subject to domestic violence or potential child protection issues require appropriate safeguarding procedures to prevent any potential harm. Research site policies also require the reporting of potential child protection issues. Thus, we will implement the following safeguards:
Debriefing procedures
Providing information about sources of treatment
Special provisions for participants reporting severe depression, suicidal thoughts or domestic violence, and potential child protection issues
Procedures for notifying adverse events.
The trial will follow appropriate Sheffield Clinical Trials Research Unit (CTRU) Standard Operating Procedures (SOPs), and also project-specific SOPs developed collaboratively with participating sites, the research team and the Parent Advisory Committee (PAC). A data management plan details data storage and security standards and procedures.
Patient and public involvement is expected at all stages of the study. We will have PAC in each study site, comprising parents with similar demographics to the intended participants. PAC will advise and support both the study team and oversight committees about outcome tools, SOPs recruitment, retention and dissemination of results.
TSC (including a lay member), Data Monitoring and Ethics Committee (DMEC) and TMG have oversight of the trial. DMEC is independent and comprises an expert in the parenting field, statistician and health economist. Procedures are in place to notify the trial team about any adverse events identified during the course of the study, which will be reported to the oversight committees and regulatory/funding bodies as required. Sheffield CTRU conduct monitoring of trial conduct in line with an SOP.
Data statement
Requests for participant-level quantitative data and statistical codes should be made to the corresponding author and will be considered by members of the original TMG, including the chief investigator and members of Sheffield CTRU, who will release data on a case-by-case basis. Data will be shared in line with the principles for sharing patient-level data as described by Smith et al.42 The data will not contain any direct identifiers and we will minimise indirect identifiers and remove free text data to minimise the risk of identification.
Dissemination
In consultation with PAC, promotional materials were developed to assist participant recruitment and to inform participants on study progress, results and outputs. Dissemination methods include: a project website; regular newsletters; social media; a parent case-study DVD and infographics; national and international conferences, seminars and workshops; peer-reviewed publications; and other articles of professional interest. Knowledge exchange/translation events will be tailored to parents/major stakeholder groups including: policy makers, commissioners, service planners and managers, practitioners, researchers/academics.
Trial status
On Friday, 17 August 2018, 314 participants have been enrolled in the main trial phase of the study, and 249 have received the universal dose of the IY Book. This trial is ongoing (see table 2 for timeline of a selection of study milestones; Trial Registration: ISRCTN 11079129, NIHR portfolio 1 73 946).
Table 2.
Brief summary of study timeline
| Milestone | Timing |
| Main trial phase study set-up | April to September 2017 |
| Sites 1 and 2 | |
| Identification of potentially eligible participants | October to December 2017 |
| Recruitment and baseline and data collection | November 2017 to January 2018 |
| Intervention participants receive Incredible Babies book | November 2017 to January 2018 |
| Follow-up one data collection | January to February 2018 |
| Delivery of Incredible Years Baby Programme | March to May 2018 |
| Follow-up two data collection | August to September 2018 |
| Delivery of Incredible Years Toddler Programme | January to March 2019 |
| Follow-up three data collection | May 2019 to June 2019 |
| Process evaluation interviews and focus groups | July 2019 |
| Sites 3 and 4 | |
| Identification of potentially eligible participants | May to July 2018 |
| Recruitment and baseline and data collection | June to August 2018 |
| Intervention participants receive Incredible Babies book | June to August 2018 |
| Follow-up one data collection | July to September 2018 |
| Delivery of Incredible Years Baby Programme | October to December 2018 |
| Follow-up two data collection | March to May 2019 |
| Delivery of Incredible Years Toddler Programme | September to November 2019 |
| Follow-up three data collection | December 2019 to January 2020 |
| Process evaluation interviews and focus groups | February 2020 |
| Final report | July 2020 |
bmjopen-2018-026906supp001.pdf (676.8KB, pdf)
bmjopen-2018-026906supp002.pdf (514.4KB, pdf)
Supplementary Material
Acknowledgments
We would like to express our sincere thanks to all of the families who have participated in our patient and public involvement activities during the preparation of the application and to those involved in our study Parent Advisory Committee. Their input has been invaluable.
Footnotes
Contributors: All authors substantially contributed to the conception or design of the work, and/or the acquisition, analysis, or interpretation of data for the work, in addition to drafting the work or revising it critically for important intellectual content. All authors gave final approval of this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The Enhancing Social-Emotional Health and Wellbeing in the Early Years trial is funded by the National Institute for Health Research’s Public Health Research Programme (NIHR 13/93/10).
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: All authors, with the exception of TB, declare no competing interests. TB is Chair of the Board of Trustees for Children’s Early Intervention Trust (CEIT). CEIT is committed to improving the lives of children and their families by researching and delivering evidence-based programmes in community and school-based settings. Early Intervention Wales Training (EIWT). EIWT is owned by CEIT. EIWT offers training courses in a variety of programmes such as the Incredible Years. The trustees do not benefit financially from any such trainings, or other CEIT/EIWT activities. All trainings for the Enhancing Social-Emotional Health and Wellbeing in the Early Years study were arranged via the developer in Seattle and delivered by accredited IY trainers and not through CEIT/EIWT.
Patient consent: Not required.
Ethics approval: University of York Education Ethics Committee (ref: FC15/03, 10 August 2015), UK NHS REC 5 (ref: 15/WA/0178, 22 May 2015).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1. Allen G. Early intervention: smart investment, massive savings, the second independent report to Her Majesty’s government. London: The Stationery Office, 2011:1–147. [Google Scholar]
- 2. Marmot M, Allen J, Goldblatt P, et al. . Fair society, healthy lives (The Marmot Review final report0. London: University College London, 2010:1–242. [Google Scholar]
- 3. Sir Michael G Marmot, Bell RG. Improving health: social determinants and personal choice. Am J Prev Med. 2011;40:S73–7. doi: 10.1016/j.amepre.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 4. Loeber R, Farrington DP. Young children who commit crime: epidemiology, developmental origins, risk factors, early interventions, and policy implications. Dev Psychopathol 2000;12:737–62. 10.1017/S0954579400004107 [DOI] [PubMed] [Google Scholar]
- 5. Furlong M, McGilloway S, Bywater T, et al. . Behavioural/cognitive-behavioural group-based parenting interventions for children age 3-12 with early onset conduct problems: The Cochrane Library, 2010:1–14. [Google Scholar]
- 6. Department of Health. Healthy child programme: pregnancy and the first 5 years of life: Department of Health, 2009:1–77. [Google Scholar]
- 7. Allen G. Early intervention: the next steps cabinet office. London, 2011:1–179. [Google Scholar]
- 8. Leadsom A, Field F, Burstow P, et al. . The importance of the conception to age two period: the 1001 critical days. A cross-party manifesto: Wave Trust, 2013:1–12. [Google Scholar]
- 9. National Institute for Health and Care Excellence (NICE). Social and emotional wellbeing: early years public health guidance [PH40]. https://www.nice.org.uk/guidance/ph40 (Accessed 21 Aug 2018).
- 10. Barlow J, Smailagic N, Ferriter M, et al. . Group-based parent-training programmes for improving emotional and behavioural adjustment in children from birth to three years old. Cochrane Database Syst Rev 2010(3):CD003680 10.1002/14651858.CD003680.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robling M, Bekkers MJ, Bell K, et al. . Effectiveness of a nurse-led intensive home-visitation programme for first-time teenage mothers (Building Blocks): a pragmatic randomised controlled trial. Lancet 2016;387:146–55. 10.1016/S0140-6736(15)00392-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charles JM, Bywater T, Edwards RT. Parenting interventions: a systematic review of the economic evidence. Child Care Health Dev 2011;37:462–74. 10.1111/j.1365-2214.2011.01217.x [DOI] [PubMed] [Google Scholar]
- 13. Edwards RT, Céilleachair A, Bywater T, et al. . Parenting programme for parents of children at risk of developing conduct disorder: cost effectiveness analysis. BMJ 2007;334:682–6. 10.1136/bmj.39126.699421.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charles JM, Edwards RT, Bywater T, et al. . Micro-costing in public health economics: steps towards a standardized framework, using the incredible years toddler parenting program as a worked example. Prev Sci 2013;14:377–89. 10.1007/s11121-012-0302-5 [DOI] [PubMed] [Google Scholar]
- 15. Bywater T, Hutchings J, Daley D, et al. . Long-term effectiveness of a parenting intervention for children at risk of developing conduct disorder. Br J Psychiatry 2009;195:318–24. 10.1192/bjp.bp.108.056531 [DOI] [PubMed] [Google Scholar]
- 16. O’Neill D, McGilloway S, Donnelly M, et al. . A cost-effectiveness analysis of the Incredible Years parenting programme in reducing childhood health inequalities. Eur J Health Econ 2013;14:85–94. 10.1007/s10198-011-0342-y [DOI] [PubMed] [Google Scholar]
- 17. Squires J, Bricker D, Twombly E. ASQ-SE-2 User’s guide. 2nd ed Baltimore: Paul Brookes Publishing Company, 2015. [Google Scholar]
- 18. Crittenden PM. CARE-Index infancy: coding manual. unpublished manuscript. Miami, FL: The Family Relations Institute, 2010. [Google Scholar]
- 19. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–39. 10.1097/00005650-199902000-00003 [DOI] [PubMed] [Google Scholar]
- 20. Varni JW, Limbers CA, Neighbors K, et al. . The PedsQL™ infant scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 2011;20:45–55. 10.1007/s11136-010-9730-5 [DOI] [PubMed] [Google Scholar]
- 21. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 1997;38:581–6. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- 22. D’Souza S, Waldie KE, Peterson ER, et al. . Psychometric properties and normative data for the preschool strengths and difficulties questionnaire in two-year-old children. J Abnorm Child Psychol 2017;45:345–57. 10.1007/s10802-016-0176-2 [DOI] [PubMed] [Google Scholar]
- 23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Condon JT, Corkindale CJ. The assessment of parent-to-infant attachment: Development of a self-report questionnaire instrument. J Reprod Infant Psychol 1998;16:57–76. 10.1080/02646839808404558 [DOI] [Google Scholar]
- 25. Condon JT, Corkindale CJ, Boyce P. Assessment of postnatal paternal–infant attachment: development of a questionnaire instrument. J Reprod Infant Psychol 2008;26:195–210. 10.1080/02646830701691335 [DOI] [Google Scholar]
- 26. Johnston C, Mash EJ. A Measure of parenting satisfaction and efficacy. J Clin Child Psychol 1989;18:167–75. 10.1207/s15374424jccp1802_8 [DOI] [Google Scholar]
- 27. van Reenen M, Janssen B. EQ-5D-5L user guide: basic information on how to use the EQ-5D-5L instrument. Rotterdam, The Netherlands: EuroQoL Group, 2015:1–25. [Google Scholar]
- 28. Essink-Bot ML, Krabbe PF, Bonsel GJ, et al. . An empirical comparison of four generic health status measures. The Nottingham health profile, the medical outcomes study 36-item short-form health survey, the COOP/WONCA charts, and the EuroQol instrument. Med Care 1997;35:522–37. [DOI] [PubMed] [Google Scholar]
- 29. Beecham J, Knapp M. Costing psychiatric interventions : Thornicroft G, Brewin C, Wing J, Measuring mental health ne. London, England: Gaskell/Royal College of Psychiatrists, 1992:200–24. [Google Scholar]
- 30. Bywater T, Gridley N, Berry V, et al. . The parent programme implementation checklist (PPIC): the development and testing of an objective measure of skills and fidelity for the delivery of parent programmes. Child Care in Practice 2018;6:1–29. 10.1080/13575279.2017.1414031 [DOI] [Google Scholar]
- 31. Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702–8. 10.1136/bmj.328.7441.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Axford N, Berry V, Blower S, et al. . Design and Refine Developing effective interventions for children and young people: Dartington Social Research Unit, 2013:1–36. [Google Scholar]
- 33. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 34. Claxton K, Walker S, Palmer S, et al. . Appropriate perspectives for health care decisions: CHE Research Paper 54. New York: The University of York Centre for Health Economics, 2010. [Google Scholar]
- 35. NICE. Guide to the methods of technology appraisal [PMG9]. London: NICE, 2013. [Google Scholar]
- 36. Drummond MF, Sculpher MJ, Claxton K, et al. . Methods for the economic evaluation of health care programmes. Oxford: Oxford university press, 2015:1–437. [Google Scholar]
- 37. Treasury HM. The magenta book: guidance for evaluation. London: TSO, 2011. [Google Scholar]
- 38. Department of Health. NHS reference costs 2012-2013. London: Department of Health, 2013. [Google Scholar]
- 39. Curtis L, Burns A. PSSRU unit costs of health and social care. University of Kent (United Kingdom): Canterbury, 2013. [Google Scholar]
- 40. Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford University Press: Oxford, 2006. [Google Scholar]
- 41. Tracey L, Bywater TJ, Blower SL, et al. . Public involvement in research: social and emotional well-being in early years: social emotional well-being in early years. New York: Institute for Effective Education, 2014:24. [Google Scholar]
- 42. Smith CT, Hopkins C, Sydes M, et al. . Good practice principles for sharing individual participant data from publicly funded clinical trials. Trials 2015;16:O1 10.1186/1745-6215-16-S2-O1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026906supp001.pdf (676.8KB, pdf)
bmjopen-2018-026906supp002.pdf (514.4KB, pdf)