Abstract
Objective
To analyse the independent and combined associations of postlunch napping duration and night-time sleep duration with risk of cognitive impairment among Chinese elderly.
Design
A cross-sectional study.
Setting
We analysed the data from Zhejiang Ageing and Health Cohort, a population-based survey of seven counties located in Zhejiang province in eastern China.
Participants
10 740 participants aged 60 years or older were included in final analysis.
Primary and secondary outcome measures
Cognitive impairment was assessed through Mini-Mental State Examination. Data on sleep-related characteristics was collected in the behavioural habits section within the questionnaire.
Results
Relative to participants with 1–30 min of postlunch napping, those who did not nap and who napped longer had significantly higher risks for cognitive impairment. OR of cognitive impairment were 1.41 (95% CI 1.14 to 1.75) for participants with longer night-time sleep duration (≥9 hours), compared with those sleeping 7–8.9 hours. In addition, combined effects were further identified. Participants with both longer night-time sleep duration (≥9 hours) and longer postlunch napping duration (>60 min) (OR=2.01, 95% CI 1.30 to 3.13), as well as those with both longer night-time sleep duration (≥9 hours) and appropriate postlunch napping duration (1–30 min) (OR=2.01, 95% CI 1.20 to 3.38), showed significantly higher risk of cognitive impairment than those with sleeping 7–8 hours and napping 1–30 min. Meanwhile, a 34% increase in odds of cognitive impairment was observed in participants with both shorter night-time sleep duration (5–6.9 hours) and no napping.
Conclusion
Both postlunch napping duration and night-time sleep duration were independently and jointly associated with cognitive impairment, which needs verification in prospective studies.
Keywords: cognitive impairment, sleep duration, post-lunch napping, combined effects, Chinese elderly
Strengths and limitations of this study.
As far as we know, this is the first study to examine the independent effects of postlunch napping duration, night-time sleep duration and their combined effects on risk of cognitive impairment simultaneously, a valuable extension on previous studies.
Our study was based on a large community-based sample of the elderly, with adjustment of a wide range of important confounders, which makes the results relatively robust.
A limitation of the study was cross-sectional data, and the results need verification in prospective studies.
Information on duration of postlunch napping and night-time sleep was obtained from subjective report, so objective measurement is needed in future studies on this topic.
Introduction
Diseases related to cognitive impairment, especially dementia and its most common cause Alzheimer’s disease (AD), are becoming a prominent health threat and bring enormous social burden in elders worldwide. It is estimated that more than 35.6 million people lived with dementia worldwide in 2010; this number is likely to be doubled by 2030, and tripled by 20501. Based on estimation, the prevalence of dementia and AD among individuals aged 65 years and older in China were around 5.14% and 3.21%, respectively,2 and are increasing nationwide.
Accumulating evidence showed that behavioural factors may modify the risk of cognitive impairment and AD.3 Sleep is essential in many physical, cognitive and psychological processes.4 In recent years, the impact of sleep duration on cognition gradually raise the researchers’ interests, including optimal duration of night-time sleep and daytime napping especially. Unfortunately, epidemiological data on the association between night-time sleep duration and cognitive impairment is still inconsistent and limited. The majority of studies supported that longer self-reported night-time sleep duration was associated with cognitive impairment.5 On the contrary, some others observed no association.6 7 Moreover, an increased risk of cognitive impairment was also found in shorter night-time sleepers.8 Additionally, few studies has been conducted in Asian populations in this field, although previous study revealed that sleep duration in Asians was generally different from that in Caucasians.9
In the meantime, prior research likewise focused on the relation of total daytime napping duration and cognitive performance, with mixed findings in different studies.6 8 More importantly, postlunch period is recognised as the most common time for naps,10 and postlunch naps are highly prevalent among the Chinese elderly.11 However, only one cross-sectional study12 assessed the association of postlunch napping with cognitive function, which reported the beneficial effect of moderate postlunch napping. As a consequence, more epidemiological studies are needed to confirm this association.
It is important to note that combined effects of napping and night-time sleep actually existed in other diseases,13 14 which worth further study in relation with cognitive impairment. Thus in this study, we used the cross-sectional data from a large population-based cohort study to explore whether postlunch napping duration and night-time sleep duration were independently and jointly associated with risk of cognitive impairment among the Chinese elderly.
Methods
Participant involvement
The data was from Zhejiang Ageing and Health Cohort Study, a community-based dynamic cohort study focusing on ageing and health among elderly in Zhejiang province, China. It was conducted by Zhejiang Provincial Center for Disease Control and Prevention. Seven counties were randomly selected from a total of 90 counties in Zhejiang province. One town in each county and several communities in each town were then randomly selected. All permanent residents aged 60 years old and older in these selected communities were eligible and expected to be included in the study. The baseline survey was finished at 2015, and 10 911 elderly were recruited and completed the survey. One year later, the second round survey was finished in which 10 801 elderly completed the survey (among 10 911 baseline participants, 187 died and 9458 were successfully re-interviewed. In addition, 1343 was newly recruited).
A face-to-face interview based on a self-designed questionnaire was performed by trained research assistants for each participant both at baseline survey and second round survey. The questionnaire included the information of demographic characteristics, family status, reproductive history, medical disease, behavioural habits, diet habits, injury, depressive symptoms, self-care ability and cognitive function. In the questionnaire of second round survey, sleep characteristics were added into the behavioural habits section, which were not involved in the questionnaire of baseline survey. Therefore, this study used the dataset of second round survey (n=10 801).
Data was primarily checked by staff at Zhejiang Provincial Center for Disease Control and Prevention. Missing data and logical errors were fed back to the initial interviewer who would try to complete the dataset by reinvestigating the participants. In our analysis, we excluded participants with incomplete information on cognitive function (n=57) and sleep characteristics (n=4), resulting in a final study sample of 10 740 participants.
Cognitive assessment
Cognitive function was assessed using the Chinese version of the Mini-Mental State Examination (MMSE) in the cognitive function section within the questionnaire, which includes 30 items. The maximum score is 30 points, with higher MMSE scores indicating better cognitive function. The widely accepted cut-off point to define cognitive impairment in China (MMSE Chinese Standard)15 is education-specific: 17/18 for illiteracy, 20/21 for people with primary education level, 24/25 for people with higher than primary education level. We defined cognitive impairment according to this standard.
Sleep characteristics
Data on sleep-related characteristics was collected in the behavioural habits section within the questionnaire, including postlunch napping duration and night-time sleep duration. Postlunch napping duration was assessed by the four-category question, ‘During the past year, how long did you take a nap after lunch in general (0, 1–30, 31–60, >60 min)’. Night-time sleep duration was assessed by the question, ‘During the past year, how many hours of sleep did you get at night in average’. Participants were divided into four groups: very short sleeper (<5 hours), short sleeper (5–6.9 hours), normal sleeper (7–8.9 hours) and long sleeper (≥9 hours).
Covariates
Based on findings reported in the literature, variables described below were considered as potential confounders and were included in our analysis: age (years, continuous variable), gender (‘man’ and ‘women’), race (‘Han ethnicity’ and ‘minority’), education level (‘lower than primary’, ‘primary’, ‘junior middle’, ‘senior middle’ and ‘college and above’), marital status (‘single’, ‘married’ and ‘divorced/widowed’), family income (‘≤10 000’, ‘10 001–20 000’, ‘20 001–50 000’, ‘50 001–100 000’ and ‘>100 000’ Chinese Yuan), smoking (‘never’, ‘past’ and ‘current’), alcohol drinking (‘never’, ‘past’ and ‘current’), body mass index (kg/m2, continuous variable), physical activity (‘yes’ and ‘no’), hypertension (‘presence’ and ‘absence’), diabetes (‘presence’ and ‘absence’), coronary heart disease (‘presence’ and ‘absence’), Patient Health Questionnaire-9 Scale scores (continuous variable) and Activities of Daily Living Scale scores (continuous variable). Detailed information on some of these variables were described as follows.
(1) Physical activity was assessed on the basis of the response (yes or no) to a single question about doing exercise regularly. (2) Medical disease section of the questionnaire contained the items on the presence or absence of 16 common diseases, which are supposed to be formally diagnosed. Hypertension, diabetes and coronary heart disease (CHD) were considered in this study. (3) Depressive symptoms was evaluated by Patient Health Questionnaire-9 Scale (PHQ-9),16 nine-question version of the Primary Care Evaluation of Mental Disorders measured by self-reporting. Total score for the nine items ranges from 0 to 27, with greater values indicating increased severity. (4) The self-care ability was evaluated by the Barthel index of Activities of Daily Living Scale (ADL).15 It consists of self-feeding, bathing, dressing, toilet hygiene and functional mobility, with the total score of 100.
Statistical analysis
Descriptive statistics were applied to illustrate the general characteristics of included participants. The associations between general characteristics and cognitive impairment were examined by t-test, Χ2 test or Kruskal-Wallis test as appropriate to variables. The general characteristics that were associated with cognitive impairment were considered as potential confounders. Preliminary logistic regression model (model 1) was generated by including all identified potential confounders, to separately assess the association of two sleep characteristics (postlunch napping duration, night-time sleep duration) and cognitive impairment. In advanced logistic regression model (model 2), we further adjusted for night-time sleep duration for the nap–cognitive impairment relationship, and adjusted for postlunch napping duration for the sleep–cognitive impairment relationship. Results were presented as ORs, 95% CIs and corresponding p values. Postlunch napping duration of 1–30 min and night-time sleep duration of 7–8.9 hours were set as the reference groups, based on literatures suggesting that 7 ~<9 hours sleep and 1–30 min nap were beneficial or optimal.9 17 Interactions between sleep characteristics and all potential confounders were checked simultaneously through the addition of cross-product terms in models. Additionally, non-linear trend of cognitive impairment risk through the range of night-time sleep duration (continuous variable) was tested by restricted cubic spline logistic regression18 using four knots placed at the 5th, 35th, 65th and 95th percentiles, with 7 hours as the reference group. Finally, we evaluated combined effects of postlunch napping duration and night-time sleep duration on risk of cognitive impairment. A new categorical variable was constructed, which was all 16 combinations of night-time sleep and postlunch napping duration categories (4×4). This variable was evaluated in logistic regression with night-time sleep of 7–8.9 hours and postlunch napping of 1–30 min as the reference group.
All statistical analyses were performed by SPSS (V.20.0) and SAS (V.9.2, SAS institute). The significance level was at two-tailed probability <0.05.
Results
General and sleep characteristics of participants
The general characteristics of included participants are presented in table 1. Among the 10 740 participants, 1789 were indicated as cognitive impairment by MMSE and the prevalence of cognitive impairment was 16.7% (95% CI 16.0 to 17.4). The mean age of participants was 69.5 years, with a higher mean age (73.6 years) in those suffering cognitive impairment (p<0.01). Besides age, the majority of general characteristics was significantly associated with cognitive function, including gender, race, education level, marital status, family income, smoking, alcohol drinking, BMI, physical activity, hypertension, PHQ-9 scores and ADL scores, so these variables were considered as potential confounders and were adjusted in further analysis. Meanwhile, the distributions of diabetes and CHD showed no difference between participants with and without cognitive impairment.
Table 1.
General characteristics of 10 740 included participants
| Characteristics | Overall (n=10 740) | Cognitive impairment | P value | |
| Yes (n=1789) | No (n=8951) | |||
| Age (years, mean, SD) | 69.5 (7.6) | 73.6 (8.6) | 68.7 (7.1) | <0.01 |
| Gender (N, %) | <0.01 | |||
| Man | 5010 (46.6) | 680 (38.0) | 4330 (48.4) | |
| Women | 5730 (53.4) | 1109 (62.0) | 4621 (51.6) | |
| Race (N, %) | <0.01 | |||
| Han ethnicity | 10 351 (96.6) | 1699 (95.1) | 8652 (96.9) | |
| Minority | 362 (3.4) | 87 (4.9) | 275 (3.1) | |
| Education level (N, %) | <0.01 | |||
| <Primary | 5165 (48.2) | 1058 (59.2) | 4107 (46.0) | |
| Primary | 4573 (42.7) | 578 (32.4) | 3995 (44.8) | |
| Junior middle | 831 (7.8) | 132 (7.4) | 699 (7.8) | |
| Senior middle | 122 (1.1) | 16 (0.9) | 106 (1.2) | |
| College and higher | 18 (0.2) | 2 (0.1) | 16 (0.2) | |
| Marital status (N, %) | <0.01 | |||
| Single | 176 (1.6) | 39 (2.2) | 137 (1.5) | |
| Married | 7988 (74.4) | 1066 (59.6) | 6922 (77.4) | |
| Divorced/widowed | 2571 (23.9) | 683 (38.2) | 1888 (21.1) | |
| Family income (N, %) | ||||
| ≤10 000 CNY | 1303 (12.1) | 397 (22.2) | 906 (10.1) | <0.01 |
| 10,001–20 000 CNY | 2032 (18.9) | 398 (22.3) | 1634 (18.3) | |
| 20,001–50 000 CNY | 3899 (36.3) | 542 (30.3) | 3357 (37.5) | |
| 50,001–100 000 CNY | 1955 (18.2) | 272 (15.2) | 1683 (18.8) | |
| >100 000 CNY | 1548 (14.4) | 179 (10.0) | 1369 (15.3) | |
| Smoking (N, %) | <0.01 | |||
| Never | 7765 (72.3) | 1438 (80.4) | 6327 (70.7) | |
| Past | 2046 (19.1) | 242 (13.5) | 1804 (20.2) | |
| Current | 929 (8.6) | 109 (6.1) | 820 (9.2) | |
| Alcohol drinking (N, %) | <0.01 | |||
| Never | 7445 (69.3) | 1337 (74.7) | 6108 (68.2) | |
| Current | 2692 (25.1) | 345 (19.3) | 2347 (26.2) | |
| Past | 602 (5.6) | 107 (6.0) | 495 (5.5) | |
| BMI (kg/m2, mean, SD) | 23.3 (3.3) | 23.0 (3.5) | 23.4 (3.2) | <0.01 |
| Physical activity (yes, N, %) | 2476 (23.1) | 327 (18.3) | 2149 (24.0) | <0.01 |
| Hypertension (presence, N, %) | 4741 (44.1) | 855 (47.8) | 3886 (43.4) | <0.01 |
| Diabetes (presence, N, %) | 977 (9.1) | 162 (9.1) | 815 (9.1) | 0.95 |
| CHD (presence, N, %) | 340 (3.2) | 64 (3.6) | 276 (3.1) | 0.28 |
| PHQ-9 scores (mean, SD) | 1.5 (2.7) | 2.3 (3.6) | 1.4 (2.5) | <0.01 |
| ADL scores (mean, SD) | 99.2 (5.4) | 97.3 (11.2) | 99.6 (3.1) | <0.01 |
Data for the following are missing: one for age, 27 for race, 31 for education, five for marital status, three for family income, one for smoking, one for alcohol drinking, two for BMI, one for physical activity, one for diabetes, five for PHQ-9 scores.
ADL, activities of daily living scale; BMI, body mass index; CHD, coronary heart disease; CNY, Chinese Yuan; PHQ-9, patient health questionnaire-9 scale;.
Descriptive statistics for sleep characteristics of included participants were shown in table 2. Among all participants, 46.2% (n=4960) reported no postlunch napping, and the proportion was similar between groups with 1–30, 31–60 and>60 min on postlunch napping. Most of participants (80.6%) spend 5–6.9 or 7–8.9 hours on night-time sleep. Only 6.4% (n=686) reported sleeping more than 9 hours at night.
Table 2.
Sleep characteristics among 10 740 included participants.
| Sleep characteristics | Overall (n=10 740) | Cognitive impairment | |
| Yes (n=1789) | No (n=8951) | ||
| Postlunch napping duration (N, %) | |||
| 0 min | 4960 (46.2) | 865 (48.4) | 4095 (45.7) |
| 1–30 min | 1933 (18.0) | 269 (15.0) | 1664 (18.6) |
| 31–60 min | 2017 (18.8) | 327 (18.3) | 1690 (18.9) |
| >60 min | 1830 (17.0) | 328 (18.3) | 1502 (16.8) |
| Night-time sleep duration (N, %) | |||
| <5 hours | 1397 (13.0) | 290 (16.2) | 1107 (12.4) |
| 5–6.9 hours | 4169 (38.8) | 710 (39.7) | 3459 (38.6) |
| 7–8.9 hours | 4488 (41.8) | 646 (36.1) | 3842 (42.9) |
| ≥9 hours | 686 (6.4) | 143 (8.0) | 543 (6.1) |
Association between sleep characteristics and cognitive impairment
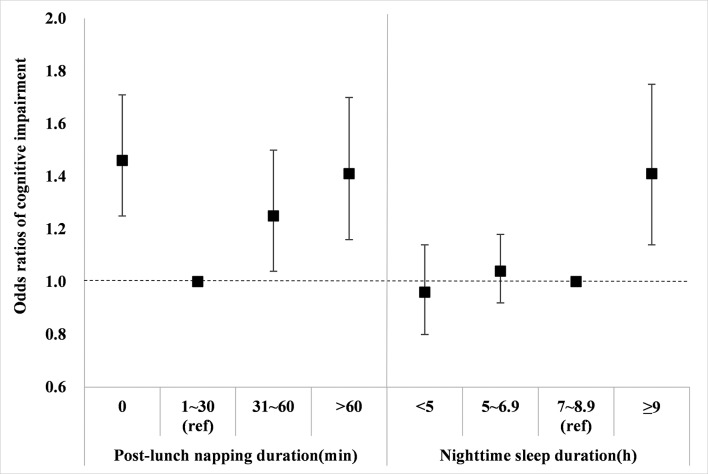
We explored the association of sleep characteristics with cognitive impairment by logistic regression, controlled for all potential confounders identified above, and the results are shown in table 3 and figure 1. In the preliminary model (model 1), relative to participants with 1–30 min of postlunch napping, those who did not nap (OR=1.47, 95% CI 1.25 to 1.72, p<0.01), napped 31–60 min (OR=1.25, 95% CI 1.04 to 1.51, p=0.02) and napped >60 min (OR=1.42, 95% CI 1.18 to 1.72, Pp<0.01) had significantly higher risks for cognitive impairment. These associations were relatively steady after additional adjustment for night-time sleep duration in advanced logistic regression model (model 2). In addition, no significant interaction between postlunch napping duration and potential confounders was found in any models (all p for interaction >0.05).
Table 3.
Association of sleep characteristics with cognitive impairment
| Sleep characteristics | N cases /N total |
Model 1 | Model 2 | ||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Postlunch napping duration* | |||||
| 0 min | 865/4960 | 1.47 (1.25 to 1.72) | <0.01 | 1.46 (1.25 to 1.71) | <0.01 |
| 1–30 min | 269/1933 | 1 (reference) | — | 1 (reference) | — |
| 31–60 min | 327/2017 | 1.25 (1.04 to 1.51) | 0.02 | 1.25 (1.04 to 1.50) | 0.02 |
| >60 min | 328/1830 | 1.42 (1.18 to 1.72) | <0.01 | 1.41 (1.16 to 1.70) | <0.01 |
| Night-time sleep duration† | |||||
| <5 hours | 290/1397 | 0.96 (0.81 to 1.14) | 0.64 | 0.96 (0.80 to 1.14) | 0.60 |
| 5–6.9 hours | 710/4169 | 1.04 (0.92 to 1.18) | 0.51 | 1.04 (0.92 to 1.18) | 0.51 |
| 7–8.9 hours | 646/4488 | 1 (reference) | — | 1 (reference) | — |
| ≥9 hours | 143/686 | 1.40 (1.13 to 1.75) | <0.01 | 1.41 (1.14 to 1.75) | <0.01 |
Potential confounders included age, gender, race, education level, marital status, family income, smoking, alcohol drinking, body mass index, physical activity, hypertension, patient health questionnaire-9 scale scores and activities of daily living scale scores.
Sample size of analyses was 10 691, due to the missing values of potential confounders.
*All potential confounders were adjusted in model 1, and night-time sleep duration (continuous variable) was further adjusted in model 2.
†All potential confounders were adjusted in model 1, and postlunch napping duration was further adjusted in model 2.
Figure 1.
ORs for the association of sleep characteristics with cognitive impairment. The blocks represent the ORs, and error bars represent the corresponding 95% CIs. The ORs of postlunch napping duration were adjusted for all potential confounders and night-time sleep duration (continuous variable). The ORs of night-time sleep duration were adjusted for all potential confounders and post-lunch napping duration. Potential confounders included age, gender, race, education level, marital status, family income, smoking, alcohol drinking, body mass index, physical activity, hypertension, patient health questionnaire-9 scale scores and activities of daily living scale scores. Abbreviations: min, minutes; h, hours; ref, reference group.
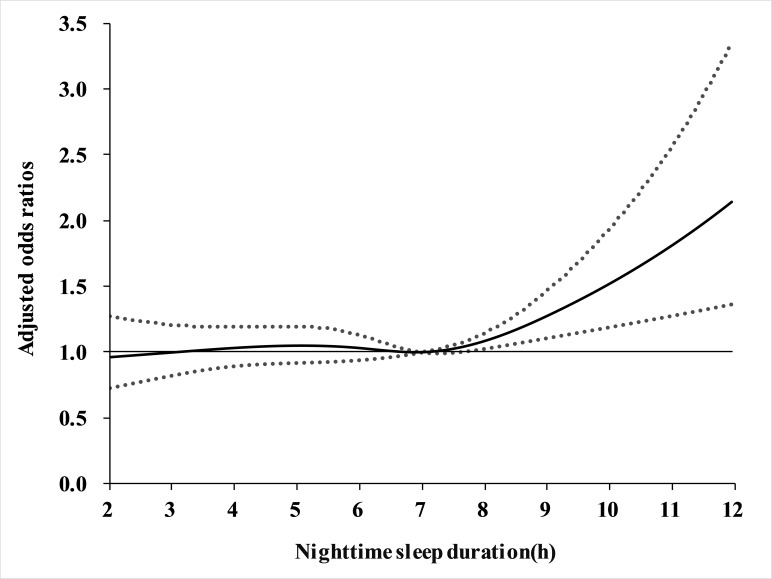
For night-time sleep duration, long sleepers (≥9 hours) were significant associated with a higher risk of cognitive impairment in model 1 (OR=1.40, 95% CI 1.13 to 1.75, p<0.01) compared with moderate sleepers (7–8.9 hours), whereas no identifiable association was found in very short sleepers (<5 hours) and short sleepers (5–6.9 hours). Similarly, further adjustment for postlunch napping duration (model 2) did not materially alter the results (table 3 and figure 1). Moreover, we did not find any significant interaction of night-time sleep duration with these covariates (all p for interaction >0.05). The non-linear association of night-time sleep duration with cognitive impairment risk was further illustrated in figure 2. The restricted cubic spline regression confirmed that the risk of cognitive impairment was higher with prolonged night sleep duration.
Figure 2.
Trend of cognitive impairment risk through the range of night-time sleep duration. The solid line indicates the point estimation for adjusted ORs of cognitive impairment, and the dotted lines represent 95% CIs. All potential confounders as well as postlunch napping duration were adjusted. Potential confounders included age, gender, race, education level, marital status, family income, smoking, alcohol drinking, body mass index, physical activity, hypertension, patient health questionnaire-9 scale scores and activities of daily living scale scores. Abbreviation: h, hours.
We also ran an unadjusted model and a model adjusted only for demographics, and the results were presented in online supplementary table S1.
bmjopen-2018-023188supp001.pdf (167KB, pdf)
We then examined the combined effects of postlunch napping duration and night-time sleep duration on the cognitive performance, and the results are presented in table 4. Based on the results mentioned above, we subsequently set normal night-time sleep duration (7–8.9 hours) and appropriate postlunch napping duration (1–30 min) as a reference group. Compared with the reference group, participants with both longer night-time sleep duration (≥9 hours) and longer postlunch napping duration (>60 min) (OR=2.01, 95% CI 1.30 to 3.13, p<0.01) as well as those with both longer night-time sleep duration (≥9 hours) and appropriate postlunch napping duration (1–30 min) (OR=2.01, 95% CI 1.20 to 3.38, p<0.01) showed significantly higher odds of cognitive impairment. Meanwhile, we observed a statistically significant, almost a 34% increase in odds of cognitive impairment in participants with both shorter night-time sleep duration (5–6.9 hours) and no napping (OR=1.34, 95% CI 1.06 to 1.79, p=0.02). Numbers of cases and total participants (N cases/N total) in each combination were shown in table 5.
Table 4.
Combined effects (ORs with 95% CIs) of postlunch napping duration and night-time sleep duration on risk of cognitive impairment
| Postlunch napping duration | Night-time sleep duration | |||
| <5 hours | 5–6.9 hours | 7–8.9 hours | ≥9 hours | |
| 0 min | 1.03 (0.76, 1.40) | 1.34 (1.06, 1.79)* | 1.17 (0.90, 1.52) | 1.21 (0.81, 1.81) |
| 1–30 min | 0.87 (0.58, 1.31) | 0.80 (0.64, 1.03) | 1 (reference) | 2.01 (1.20, 3.38)** |
| 31–60 min | 1.05 (0.69, 1.60) | 1.03 (0.77, 1.39) | 1.02 (0.74, 1.40) | 1.14 (0.65, 2.01) |
| >60 min | 1.08 (0.70, 1.65) | 1.36 (0.99, 1.87) | 0.94 (0.69, 1.28) | 2.01 (1.30, 3.13) ** |
ORs and corresponding 95% CIs were generated with adjustment for age, gender, race, education level, marital status, family income, smoking, alcohol drinking, body mass index, physical activity, hypertension, patient health questionnaire-9 scale scores, and activities of daily living scale scores.
*P<0.05; **P<0.01.
Table 5.
Numbers of cases and total participants (N cases/N total) in each combination of postlunch napping duration and night-time sleep duration
| Postlunch napping duration | Night-time sleep duration | |||
| <5 hours | 5–6.9 hours | 7–8.9 hours | ≥9 hours | |
| 0 min | 140/704 | 335/1780 | 341/2189 | 49/287 |
| 1–30 min | 53/258 | 99/886 | 90/679 | 27/110 |
| 31–60 min | 49/223 | 158/902 | 101/776 | 19/116 |
| >60 min | 48/212 | 118/601 | 114/844 | 48/173 |
Discussion
In this cross-sectional analysis, we explored the independent and combined association of postlunch napping duration and night-time sleep duration with cognitive impairment in a large population-representative sample of Chinese elderly. The results of our study suggested that napping for 1–30 min after lunch was associated with a lower risk of cognitive impairment. Furthermore, longer duration of night-time sleep (≥9 hours) increased the risk of cognitive impairment. In addition, there were combined effects of postlunch napping duration and night-time sleep duration on risk of cognitive impairment.
To our best knowledge, this is the first study simultaneously exploring the independent and combined association of postlunch napping duration and night-time sleep duration on risk of cognitive impairment. Several population-based studies have investigated the independent relationship between night-time sleep duration and cognitive impairment.5 19 We found that longer night-time sleep duration was independently associated with a significantly worse cognitive function (MMSE), in agreement with the majority of previous studies.7 20–22 Biological mechanisms mediating the association of night-time sleep duration with cognitive impairment remain unclear. However, there are several plausible explanations. Longer night-time sleep duration can result in inflammatory reactions by increase the level of C-reactive protein and interleukin-6,23 while inflammation is recognised as a contributor to the occurrence of cognitive impairment and pathogenesis of AD.24 Meanwhile, longer duration of recumbent position possibly increases the period with high intracranial pressure, and may subsequently alter the cerebrospinal/interstitial fluid dynamic and compromise the b-amyloid clearance during sleep,25 which is implicated in the pathophysiology of cognitive dysfunction in AD.26
Likewise, our analysis discovered that postlunch napping was independently related to lower risk of cognitive impairment, partially in accordance with an analysis of China Health and Retirement Longitudinal Study12 indicating the protective role of postlunch napping. Nonetheless, it was demonstrated that elderly with moderate postlunch napping duration (30–90 min) had better cognitive performance in that study,12 whereas shorter duration (1–30 min) was suggested in our study. It needs to note that distinction of cognitive assessment (MMSE vs telephone interview of cognitive status, word recall and figure drawing) and cut points for moderate napping group (30–60 vs 30–90 min) may contribute to different results. Although less is known about the mechanisms responsible for benefits of nap on cognition, there is increasing evidence supporting the neuroprotective effect of short nap. Napping has been shown to improve hippocampus-dependent associative memories for older adults,27 which may delay the onset of cognitive impairment or dementia.28 Moreover, napping may slow the neurodegenerative pathologies through reduction in oxidative stress,29 while oxidative damage occurred early in the pathogenesis of AD.30 Specifically, researchers confirmed that brief naps (<30 min) can ameliorate alertness, decrease sleepiness and fatigue, in addition to improving accuracy and speed on a number of cognitive tasks in older individuals.10 Furthermore, longer naps (>30 min) can produce cognitive impairment from sleep inertia for a short period after waking.31 Unlike longer naps (>30 min), brief naps (<30 min) are associated with shorter periods of sleep inertia, and in some instances, no sleep inertia.31 This may help to explain our findings with regard to optimal duration of postlunch napping for cognition.
Interestingly, we observed significant combined effects of postlunch napping duration and night-time sleep duration on risk of cognitive impairment, which highlighted that both longer night-time sleep duration (≥9 hours) and longer postlunch napping duration (>60 min), as well as longer night-time sleep duration (≥9 hours) and appropriate postlunch napping duration (1–30 min) were associated with approximately 101% increase in odds of cognitive impairment. That emphasised the independent and combined detrimental effects of longer night-time sleep duration (≥9 hours) on cognitive function. However, night-time sleeping ≥9 hours combined with postlunch napping 31–60 min showed no association. The reason of partly inconsistent pattern was unclear, possibly due to the limited sample size in this combination. Meanwhile, poorer cognitive function was detected among elderly with both shorter night-time sleep duration (5–6.9 hours) and no napping. Some earlier studies reported that short sleepers had a higher risk of cognitive impairment,8 32 33 while others generally supported null association.7 20 34 35 It is likely that napping duration, which was not collected or not jointly considered in those epidemiological studies, may differ in effects of short night-time sleep on cognition. The combination of night-time sleeping <5 hours and no napping, which is supposed to show an equivalent (if not greater) OR, actually did not. That remains to be explained. Similarly, a study including 2947 community-dwelling adults aged ≥65 years in Hong Kong also found that mean MMSE score of participants sleeping <5 hours was higher than that of participants sleeping 5–5.9 hours22. In addition, several previous studies assessing sleep duration over a 24-hour period (included both night-time sleep and daytime napping) indicated that longer total sleep time was related to poorer cognitive function,36–38 in accordance with our findings. Overall, our results simultaneously provide appropriate duration of night-time sleep and postlunch napping. These findings need verification in future studies.
There are several methodological issues and limitations. First, information on the duration of postlunch napping and night-time sleep was obtained from participants’ subjective report, so the information bias cannot be excluded. Although a previous validation study found that participants self-reported usual sleep duration well compared with sleep durations derived from sleep diaries,39 objective measurement is needed in future studies on this topic. Second, due to the cross-sectional data, we cannot conclude whether duration of postlunch napping and night-time sleep altered the risk of cognitive impairment or different cognitive performance resulted in variation of postlunch napping duration and night-time sleep duration. For instance, the error rate of recalling sleep information could be higher in participants with more severe forms of cognitive impairment. If more of them were wrong to report longer night-time sleep duration and postlunch napping duration, it would result in the association being in the opposite direction. In the next stage of our programme, a prospective analysis will be conducted to prove the relation. Third, some particular sleep disturbance unmeasured in this study might influence sleep duration and risk of cognitive impairment,40 41 which might confound the results. It was suggested that both longer durations of sleep and cognitive impairment could be attributable to sleep-disordered breath (characterised by recurrent arousals from sleep and intermittent hypoxaemia) in older women.12 42 43 Fourth, the napping variable did not include any information about frequency of naps, so it is not possible to know if the naps occur monthly, weekly or daily. Different frequency of naps may have different causes. Meanwhile, the reasons for napping were not assessed in the study. According to the nap question asked in this study, it was assumed that the postlunch naps were intentional naps and were taken routinely. Finally, we did not take genetic factors into consideration, such as apolipoprotein E carrier status.44
Despite these limitations, this study has a number of strengths. (1) The limited number of studies based on Chinese population, even Asian population, makes our study a valuable extension on previous work. (2) Our study was based on a large community-based sample of the elderly, and the results are relatively robust and are potentially correlated with the broader population of elderly people in Zhejiang province. (3) A wide range of potential confounders was taken into account in our analyses, and adjusted ORs were estimated in logistic model to control several well-known and biologically important risk factors for cognitive impairment. (4) As far as we know, this is the first study to examine the independent effects of postlunch napping duration, night-time sleep duration and their combined effects on risk of cognitive impairment simultaneously. Our findings may provide some new insight for prevention of cognitive impairment and AD.
Supplementary Material
Acknowledgments
We wish to thank the investigators from seven selected counties for their investigation and data collection.
Footnotes
J-FL, F-DL and X-GC contributed equally.
Contributors: F-DL and J-FL conducted study, analysed data, interpreted results and wrote the paper. MY and J-FL designed study and critically revised the manuscript. X-GC and X-QP conducted study and acquired subjects and data. FH, Y-JZ, X-YW and TZ conducted study and interpreted results. MY supervised the study.
Funding: This research was supported by Provincial Medical Research Fund of Zhejiang (2015RCB011, 2017KY285) and Zhejiang Provincial Natural Science Foundation of China (LQ19H260001).
Competing interests: Not required.
Patient consent: Obtained.
Ethics approval: The Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are not publicly available due to local ethical restrictions.
References
- 1. Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 2. Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement 2014;10:1–9. 10.1016/j.jalz.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 3. Williams JW, Plassman BL, Burke J, et al. Preventing Alzheimer’s disease and cognitive decline. Evid Rep Technol Assess 2010(193):1–727. [PMC free article] [PubMed] [Google Scholar]
- 4. Dolgin E. Deprivation: a wake-up call. Nature 2013;497:S6–S7. 10.1038/497S6a [DOI] [PubMed] [Google Scholar]
- 5. Kim HB, Myung SK, Lee SM, et al. Longer duration of sleep and risk of cognitive decline: a meta-analysis of observational studies. Neuroepidemiology 2016;47:171–80. 10.1159/000454737 [DOI] [PubMed] [Google Scholar]
- 6. Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2006;61:405–10. 10.1093/gerona/61.4.405 [DOI] [PubMed] [Google Scholar]
- 7. Loerbroks A, Debling D, Amelang M, et al. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry 2010;25:100–9. 10.1002/gps.2305 [DOI] [PubMed] [Google Scholar]
- 8. Keage HA, Banks S, Yang KL, et al. What sleep characteristics predict cognitive decline in the elderly? Sleep Med 2012;13:886–92. 10.1016/j.sleep.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 9. Whinnery J, Jackson N, Rattanaumpawan P, et al. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep 2014;37:601–11. 10.5665/sleep.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res 2009;18:272–81. 10.1111/j.1365-2869.2008.00718.x [DOI] [PubMed] [Google Scholar]
- 11. Lan TY, Lan TH, Wen CP, et al. Nighttime sleep, chinese afternoon nap, and mortality in the elderly. Sleep 2007;30:1105–10. 10.1093/sleep/30.9.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Cacchione PZ, Hodgson N, et al. Afternoon Napping and Cognition in Chinese Older Adults: Findings from the China Health and Retirement Longitudinal Study Baseline Assessment. J Am Geriatr Soc 2017;65:373–80. 10.1111/jgs.14368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han X, Liu B, Wang J, et al. Long sleep duration and afternoon napping are associated with higher risk of incident diabetes in middle-aged and older Chinese: the dongfeng-tongji cohort study. Ann Med 2016;48:216–23. 10.3109/07853890.2016.1155229 [DOI] [PubMed] [Google Scholar]
- 14. Yang L, Yang H, He M, et al. Longer sleep duration and midday napping are associated with a higher risk of chd incidence in middle-aged and older chinese: the dongfeng-tongji cohort study. Sleep 2016;39:645–52. 10.5665/sleep.5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y. The rating scales for neurology. 1st ed Beijing: China Friendship Publishing Company, 2005. [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamaki M, Shirota A, Hayashi M, et al. Restorative effects of a short afternoon nap (<30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online 2000;3:131–9. [PubMed] [Google Scholar]
- 18. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:n/a–57. 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 19. Lo JC, Groeger JA, Cheng GH, et al. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med 2016;17:87–98. 10.1016/j.sleep.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 20. Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the mini-mental score: the northern manhattan study. J Clin Sleep Med 2013;9:669–73. 10.5664/jcsm.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 2011;34:1347–56. 10.5665/SLEEP.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Auyeung TW, Lee JS, Leung J, et al. Cognitive deficit is associated with phase advance of sleep-wake rhythm, daily napping, and prolonged sleep duration--a cross-sectional study in 2,947 community-dwelling older adults. Age 2013;35:479–86. 10.1007/s11357-011-9366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep 2009;32:200–4. 10.1093/sleep/32.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calsolaro V, Edison P. Neuroinflammation in alzheimer’s disease: current evidence and future directions. Alzheimers Dement 2016;12:719–32. 10.1016/j.jalz.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 25. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–7. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 2010;13:812–8. 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Studte S, Bridger E, Mecklinger A. Nap sleep preserves associative but not item memory performance. Neurobiol Learn Mem 2015;120:84–93. 10.1016/j.nlm.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 28. Campuzano MTG, Shams E, Virues J, et al. The effect of associative memory exercises in older adults. Procedia - Social and Behavioral Sciences 2013;82:707–12. 10.1016/j.sbspro.2013.06.333 [DOI] [Google Scholar]
- 29. Faraut B, Bayon V, Léger D. Neuroendocrine, immune and oxidative stress in shift workers. Sleep Med Rev 2013;17:433–44. 10.1016/j.smrv.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 30. Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res 2007;85:3036–40. 10.1002/jnr.21346 [DOI] [PubMed] [Google Scholar]
- 31. Lovato N, Lack L. The effects of napping on cognitive functioning. Prog Brain Res 2010;185:155–66. 10.1016/B978-0-444-53702-7.00009-9 [DOI] [PubMed] [Google Scholar]
- 32. Tworoger SS, Lee S, Schernhammer ES, et al. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord 2006;20:41–8. 10.1097/01.wad.0000201850.52707.80 [DOI] [PubMed] [Google Scholar]
- 33. Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement 2016;12:21–33. 10.1016/j.jalz.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johar H, Kawan R, Emeny RT, et al. Impaired sleep predicts cognitive decline in old people: findings from the prospective KORA age study. Sleep 2016;39:217–26. 10.5665/sleep.5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blackwell T, Yaffe K, Laffan A, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep 2014;37:655–63. 10.5665/sleep.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benito-León J, Louis ED, Bermejo-Pareja F. Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES). J Psychiatr Res 2013;47:1998–2003. 10.1016/j.jpsychires.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Devore EE, Grodstein F, Duffy JF, et al. Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc 2014;62:1073–81. 10.1111/jgs.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Virta JJ, Heikkilä K, Perola M, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep 2013;36:1533–41. 10.5665/sleep.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep 2004;27:440–4. 10.1093/sleep/27.3.440 [DOI] [PubMed] [Google Scholar]
- 40. Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev 2018;40:4–16. 10.1016/j.smrv.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 41. de Almondes KM, Costa MV, Malloy-Diniz LF, et al. Insomnia and risk of dementia in older adults: Systematic review and meta-analysis. J Psychiatr Res 2016;77:109–15. 10.1016/j.jpsychires.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 42. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–9. 10.1001/jama.2011.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leng Y, McEvoy CT, Allen IE, et al. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol 2017;74:1237–45. 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu CC, Liu CC, Kanekiyo T, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106–18. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023188supp001.pdf (167KB, pdf)