Abstract
Spatial and temporal heterogeneity are fundamental mechanisms structuring home ranges. Under optimality, an individual should structure their space use economically to maximize fitness. We evaluated support for three hypotheses related to range optimality in American black bears (Ursus americanus), predicting (a) range location on a landscape will correspond with high vegetation productivity, (b) increasing forest fragmentation will result in larger ranges, and (c) increasing proportion of forest and/or mean vegetation productivity will result in smaller ranges. We used black bear radio telemetry data from Michigan (2009–2015), Missouri (2010–2016), and Mississippi (2008–2017), USA. Annual space use excluded winter, and we separated seasonal space use into spring, summer, and fall. We collected data from 143 bears (80 females, 63 males), resulting in 97 annual and 538 seasonal ranges. We used generalized linear mixed models to evaluate productivity (estimated through Normalized Difference Vegetation Index [NDVI]) selection, and range size (km2) variation between individuals. At the annual scale, black bears consistently selected areas with greater vegetation productivity than the surrounding landscape; yet selection weakened and was more variable seasonally. Opposite to our prediction, we found that increasing fragmentation consistently resulted in smaller ranges; non‐forested land covers and forest edges might provide greater abundance or more diverse foods for bears. Ranges with a greater proportion of forest were smaller, likely reflecting an increase in food and cover which could reduce movements, yet there was no support for more productive ranges also being smaller as expected from an area minimizing strategy. Black bears displayed a scale‐dependent space use strategy: at larger spatial and temporal scales, productivity acted as the strongest limiting factor and energy maximizing was the dominant strategy, while an area minimizing strategy was exhibited seasonally. We revealed consistent, scale‐dependent responses by black bears to environmental conditions, demonstrating the intrinsic plasticity of this adaptable omnivore.
Keywords: carnivore, forest, fragmentation, movement, North America, productivity, space use, ursid
1. INTRODUCTION
Movement is a key factor for the survival of most animals and is subject to strong selective pressures (Nathan, 2008; Powell & Mitchell, 2012). Consequently, natural selection should favor movement strategies that maximize fitness, which may manifest as maximized rates of resource acquisition or production of offspring (Austin, Bowen, & McMillan, 2004). Home ranges are the result of animals moving in relation to the distribution of resources (Börger, Dalziel, & Fryxell, 2008; Van Moorter, Rolandsen, Basille, & Gaillard, 2016) at multiple scales (Johnson, 1980 ) and are limited by species traits and evolution (McLoughlin & Ferguson, 2000). Under the framework of optimality, home range location and size should be the result of an individual attempting to structure their space use economically to maximize fitness (Mitchell & Powell, 2004). Mitchel and Powell (2007) proposed two main strategies for an individual optimally selecting spatially heterogeneous resource patches: area minimizing or energy maximizing (similar to time minimizing and rate maximizing; Krebs & Kacelnik, 1991).
The structure and location of mammalian and avian home ranges have been found to be influenced by the spatial distribution of resources (Eide, Jepsen, & Prestrud, 2004; Johnson, Kays, Blackwell, & Macdonald, 2002; Marable, Belant, Godwin, & Wang, 2012; McClintic, Taylor, Jones, Singleton, & Wang, 2014). In particular, home range location (second‐level habitat selection; Johnson, 1980) can be influenced by features such as vegetation type, land use, plant productivity, and risk avoidance (Marchand et al., 2015). Within populations, food availability is likely the main determinant of home range size (McLoughlin & Ferguson, 2000), and size should decrease when high predictability of resource distribution across the landscape allows animals to locate their home range in areas of higher than average quality (Mitchell & Powell, 2004). As productivity increases, individuals following the area minimizing strategy should need a smaller area to fulfill their energetic needs, therefore, moving shorter distances and displaying smaller home ranges (Barraquand & Murrell, 2012; Dahle & Swenson, 2003; McNab, 1963). In addition, the patchiness of resources on the landscape can further structure home ranges areas. The resource dispersion hypothesis, originally a hypothesis of mammalian gregariousness (Carr & Macdonald, 1986), states that as spatial variability of resources increases, individuals must use a larger area to acquire sufficient resources (Macdonald & Johnson, 2015). For an individual following an area minimizing strategy, increased dispersion of resources should result in a larger home range. Resource dispersion can occur naturally in heterogeneous landscapes, or be a result of anthropogenic habitat fragmentation; mammals of all sizes have experienced habitat loss and fragmentation around the world (Crooks, 2002; Crooks et al., 2017), affecting their space use and behavior (Tucker et al., 2018; Wolf & Ripple, 2017).
Studying how species modify their space use depending on landscape structure is vital for managing species such as American black bears (Ursus americanus), which are currently recolonizing parts of North America after major past range contractions (Scheick & McCown, 2014) and displaying increased conflict in human‐modified areas (Baruch‐Mordo, Breck, Wilson, & Theobald, 2008; McFadden–Hiller, Beyer, & Belant, 2016). Our objective was to evaluate black bear optimality regarding annual and seasonal home range location and size (Table 1). Black bears are very suitable for testing hypotheses of home range optimization because they display site fidelity, use heterogeneous habitats, and their food resources are mostly fixed in space (i.e., vegetation; Mitchell & Powell, 2007). We analyzed if home range location on the landscape is influenced by vegetation productivity, and examined the influence of extrinsic factors on home range size (Table 1). Within this conceptual framework, bears behaving optimally should display home ranges that are, on average, more productive than the study area, and seasonal home ranges should be more productive compared to the area around them, reflecting economically driven behavior. In addition, bears are forest obligate species (Herrero, 1972; Pelton, 2003) and individuals following an area minimizing strategy should display smaller home ranges as vegetation productivity and forest proportion increase, and spatial variability of resources (fragmentation) decreases.
Table 1.
Hypotheses evaluating optimality in black bear home range location and size, together with associated factors, predictions, and support
| Hypothesis | Predictions |
|---|---|
| Food selection | Home ranges will be more productive than surrounding areas |
| Fragmentation | Greater edge density results in larger home ranges |
| Productivity | Greater forest proportion results in smaller home ranges |
| Greater productivity results in smaller home ranges |
2. METHODS
2.1. Study areas
We used data from black bear (Figure 1) studies in Michigan (MI, data from 2009 to 2011 and 2013 to 2015), Missouri (MO, data from 2010 to 2016), and Mississippi (MS, data from 2008 to 2017), USA (Figure 2). In MS, topography is generally flat with elevations from 0 to 247 m above sea level. Vegetation is primarily agricultural land with forested areas along the Mississippi River. Agricultural and urban lands comprise about 45,000 and 2,400 km2 of the state, respectively (Mississippi Automated Resource Information System 2014). Bear density throughout MS was estimated at <1/100 km2 (R. Rummell, Mississippi Department of Wildlife, Fisheries, and Parks [MDWFP], pers. comm.). Black bears were captured in the Delta region of western MS, where most black bear sightings occur (Simek, Belant, Young, Shropshire, & Leopold, 2012). In MO, data collection was conducted in the Ozark Highlands. This region contains karst topography with elevations from about 70 to 280 m and has a humid warm continental climate. Dominant land covers include forest, crop and pasture, grassland, and human developed areas (Karstensen, 2010), and black bear density was 1.7/100 km2 (Wilton et al., 2014). In MI, data collection was conducted in the Upper Peninsula. This area has flat topography with elevations ranging approximately from 160 to 240 m and a humid cold continental climate. Predominant vegetation includes upland and lowland hardwoods, lowland conifer swamps, upland conifers, aspen (Populus spp.) stands, row‐crop, and livestock agriculture, and some herbaceous wetlands (Duquette, Belant, Svoboda, Beyer, & Lederle, 2014). Black bear density is 14–19/100 km2 (J. L. Belant, unpublished data). Black bears in MS and MO are not harvested, and in MI they are harvested annually during September and October; only males and females without dependent young are legal for harvest (Belant, Etter, Mayhew, Visser, & Friedrich, 2011).
Figure 1.

Collared American black bear (Ursus americanus) in Michigan, USA
Figure 2.

Location of the three black bear study areas (dashed polygons) located primarily in Michigan (top), Missouri (middle), and Mississippi (bottom), USA
2.2. Capture and marking
Black bears on each study area were captured using modified Aldrich foot snares (Johnson & Pelton, 1980) and culvert traps. Captured individuals were immobilized with tiletamine and zolazepam at a dosage of 4–7 mg/kg of estimated body weight (Telazol; A. H. Robins Company, Richmond, Virginia, USA), administered with a syringe pole or dart syringe fired from a CO2‐powered pistol or rifle. Each bear received a GPS radiocollar: MI (Lotek Wireless, Newmarket, Ontario, Canada), MO (Northstar RASSL Globalstar, King George, Virginia, USA; Advanced Telemetry Systems M2610B, Isanti, Minnesota, USA; Lotek Wireless 7000MU, Newmarket, Ontario, Canada), MS (Telonics Inc., Mesa, Arizona, USA; Lotek Iridium Collars, Lotek Wireless Inc., Newmarket, Ontario, Canada). All collars had leather breakaway links (Garshelis & McLaughlin, 1998). All capturing and handling of bears follow the American Society of Mammalogists guidelines (Sikes & Gannon, 2011) and was approved by the Mississippi State University Institutional Animal Care and Use Committee (MS protocol: 14‐098, MO: 13‐094, MI: 15‐013). We located dens using aerial and ground‐based telemetry, and we recovered data from GPS collars during recaptures, UHF data downloads during flights, and den visits.
2.3. Data Analysis
For home range (hereafter range) analyses we randomly subsampled location estimates such that no individual had >1 location per day, reducing temporal autocorrelation and standardizing relocation intervals among data sets (Hiller, Belant, & Beringer, 2015). To describe annual space use, we separated data by year and excluded data collected during the denning period. Annual activity includes locations from 15 March to 30 November for MS and MO and 15 April to 31 October for MI. To describe seasonal space use, we separated data into three seasons: spring (den emergence; 15 March to 31 May in MS and MO, 15 April to 15 June in MI), summer (mating and dispersal; 1 June to 31 August in MS and MO, 15 June to 31 August in MI), and fall (hyperphagia; 1 September to 30 November in MS and MO, 1 September to 31 October in MI), (Benson & Chamberlain, 2006, Hiller et al., 2015, J. L. Belant unpublished data). To estimate space use, we used fixed‐kernel techniques with plug‐in bandwidths (Gitzen, Millspaugh, & Kernohan, 2006) to determine the area of the 95% utilization distribution (UD) within a given range (Kernohan, Gitzen, & Millspaugh, 2001) using the rhr package in program R (R Core Team, 2013). The plug‐in method minimizes oversmoothing in resulting kernels generated from GPS data (Kertson & Marzluff, 2011). Cell size for kernel smoothing was kept constant among all home range calculations to allow for direct comparisons of range size. We considered data adequate for range modeling when locations spanned at least 75% of the time period being analyzed (e.g., ≥68 of 90 days covered), and exceeded 40 and 25 relocations for annual and seasonal range estimation (Börger et al., 2006; Haines, Hernandez, Henke, & Bingham, 2009; Powell, 2000). For individuals with data for >1 year or >1 season, each seasonal or annual range from each bear was considered a sampling unit.
Black bears throughout their range have an opportunistic omnivorous diet dominated by plants and insects (Pelton, 2003, Costello et al. 2016). To assess if range location within the study area was influenced by environmental productivity, we used the Normalized Difference Vegetation Index (NDVI) to measure vegetation greenness as an index of plant productivity. The relationship between NDVI and average energy availability is well established (Pettorelli et al., 2006; Wiegand, Naves, Garbulsky, & Fernández, 2008), and has been employed for taxa including herbivores (Garel et al., 2006) and brown bears (Bojarska & Selva, 2012). We used the 16‐day composite NDVI data from the eMODIS server (250‐m resolution; United States Geological Survey). We rescaled raw NDVI values by multiplying them by a factor of 0.0001. We calculated the average NDVI of the annual range for each bear in each study site and each year and calculated the average NDVI for each study area and each year. The study area was determined as the minimum convex polygon that included all ranges in each state. To assess seasonal selection, we calculated the mean NDVI of each seasonal range for each bear and year (spring, summer, fall), and compared it to the NDVI for that same time period for a buffer surrounding the seasonal range, representing potential movement in a 2–3 month period. To obtain the buffer distance, we calculated the average seasonal range size for females and males separately and used the respective radius to create the buffer around ranges.
To assess spatial configuration of forested land covers, we used 30‐m resolution data from the U.S. Geological Survey (Homer et al., 2015). We designated all forested land covers and woody wetlands (NLCD 41, 42, 43, 90) as potential black bear habitat (Sollmann, Gardner, Belant, Wilton, & Beringer, 2016), and calculated proportion of forest and forest patch edge density for each range using the package SDMTools in R v.3.3.2 (R Development Core Team, 2013).
We used generalized linear mixed models (GLMM) with a Gaussian distribution to assess variation in productivity (NDVI) and area (km2) of annual or seasonal ranges of individual bears in relation to individual, site, temporal, and environmental covariates. We chose four analyses to evaluate our hypotheses (Table 2), selecting ecologically relevant factors and interactions. We included bear ID and year as potential random effects in all analyses. We used Pearson's correlation coefficient (r) to test for multicollinearity among independent variables. If |r| < 0.70 for any pair of independent variables (Dormann et al., 2013), we assumed multicollinearity did not compromise models results. If multicollinearity existed for a pair of variables, they were not included in the same model. To assess whether data were normally distributed, we examined residuals for indication of systematic lack of fit using the global model and full data set. When the response variable had a skewed distribution (i.e., area), we transformed the data (log10) to increase homogeneity of the variance. Factors were scaled before analysis to allow comparisons of effects. We used package lme4 in R (R Development Core Team, 2013) to run the GLMMs. We used Akaike Information Criterion adjusted for small samples (AICc) to rank models based on model complexity and fit (Burnham & Anderson, 2002). For each analysis, (Table 2) we first performed all combinations of random factors (plus all fixed effects) to find the best fitting random structure (lowest AICc score) for the data. We then performed all combinations of selected fixed factors and interactions (Table 2), always including the chosen random structure. All models within an AICc difference of <2 from the top‐ranked model were considered top models. To avoid overparameterization, we chose the simplest model (lowest value for degrees of freedom) within top models as the “best fit model”: a compromise between simplicity and explanatory power (Richards, Whittingham, & Stephens, 2011). We also considered R 2 values to describe performance and compare between top models. We calculated both conditional R 2 (all variance explained) and marginal R 2 (variance explained only by fixed factors) via R Package MuMin (Barton, 2013) based on Nakagawa and Schielzeth (2013). For the best fit model, we estimated parameter coefficients, standard errors, and 95% confidence intervals. We used R v.3.3.2 (R Development Core Team, 2013) for all statistical analyses.
Table 2.
Selected factors and interactions for analyses of (a) annual (n = 97), and (b) seasonal (n = 538) productivity selection and (c) annual (n = 97) and (d) seasonal (n = 538) home range size variation for black bears
| Fixed factors | Interactions | Response | |
|---|---|---|---|
| (a) | State | Sex*State | Normalized Difference Vegetation Index (NDVI) difference between annual home range and study area |
| Sex | |||
| (b) | Sex | Season*Sex | NDVI difference between seasonal home range and seasonal buffer |
| Season | Season*State | ||
| State | Sex*State | ||
| (c) | Prop forest | Sex*State | Size of annual home range |
| Edge density | |||
| Mean NDVI | |||
| State | |||
| Sex | |||
| (d) | Prop forest | Season*Sex | Size of seasonal home range |
| Edge density | Season*State | ||
| Mean NDVI | Sex*State | ||
| Season | |||
| State | |||
| Sex |
3. RESULTS
We collected data adequate for range modeling from 143 bears; 43 from MI (19 F, 24 M), 73 from MO (45 F, 28 M), and 27 from MS (16 F, 11 M). Range sizes are presented as median values as data were non‐normally distributed. The median annual range area was 18.7 km2 for females and 89.9 km2 for males (Supporting information Appendix S1 Table A). Male annual ranges were 5.8 times larger than females in MI, 5.3 times larger in MO, and 3.8 times larger in MS. The median seasonal range area for all bears was 15.4 km2 for females and 59.3 km2 for males (Supporting information Appendix S1 Table A). The median proportion of forest within seasonal ranges was 0.85 in MI (0.87 F, 0.83 M), 0.92 in MO (0.93 F, 0.89 M), and 0.85 in MS (0.89 F, 0.67 M). Seasonal proportion of forest ranged from 0.85 to 0.96 for females, and from 0.67 to 0.92 for males (Supporting information Appendix S1 Table A). Within each of our study areas, the most abundant land covers not considered black bear habitat were cultivated crops in MI and MS, and pasture/hay in MO.
3.1. Productivity selection
The selected random structure for productivity selection at the annual scale was bear ID and year. One model best described the annual NDVI difference (Table 2, analysis A), which contained all fixed factors and one interaction (Table 3), with an AICc weight of 0.99. Conditional R 2 was 0.93 and marginal R 2 was 0.86. There were no competing models. Bears in all states selected areas with greater NDVI than the study areas (Table 3, Figure 3). There was no difference in selection between MI and MO, and bears in MS showed stronger selection than the other two states. Males in MS selected areas of lower NDVI than the female–male difference in MI and MO.
Table 3.
Parameter estimates and standard deviations (SD) for annual home range productivity selection (Normalized Difference Vegetation Index difference) for black bears (2008–2017) in Michigan (MI), Missouri (MO), and Mississippi (MS), USA
| Parameter | Estimate | SD |
|---|---|---|
| Intercept | 0.31 | 0.09* |
| State MO | 0.10 | 0.09 |
| State MS | 1.67 | 0.09* |
| Sex M | −0.01 | 0.12 |
| State MO: sex M | 0.02 | 0.18 |
| State MS: sex M | −0.63 | 0.18* |
p < 0.05.
Figure 3.
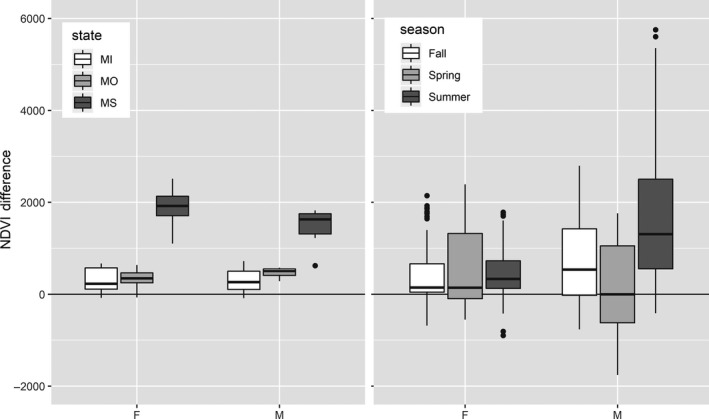
Normalized Difference Vegetation Index (NDVI) difference (rescaled by a factor of 0.0001) for female and male black bear (a) annual home ranges and study area (n = 97) and (b) seasonal home ranges and seasonal buffers (n = 538), in Michigan (MI), Missouri (MO), and Mississippi (MS), USA. Positive values indicate selection and negative values avoidance
The selected random structure for productivity selection at the seasonal scale was bear ID and year. One model best described the NDVI difference between seasonal ranges and seasonal buffers (Table 2, analysis B) which consisted of all fixed factors and interactions (Table 4), with an AICc weight of 0.99. Conditional R 2 was 0.64 and marginal R 2 was 0.42. There were no competing models. There was selection for greater productivity in summer ranges by both sexes (Table 4, Figure 3). Several interactions revealed more detailed patterns, for example, male bears use areas less productive than surrounding buffers during spring and areas more productive during summer, and we quantified interesting variations in selection for each study area by season and sex (Table 4).
Table 4.
Parameter estimates and standard deviations (SD) for seasonal home range productivity selection (Normalized Difference Vegetation Index difference) for black bears (2008–2017) in Michigan (MI), Missouri (MO), and Mississippi (MS), USA
| Parameter | Estimate | SD |
|---|---|---|
| Intercept | 0.03 | 0.19 |
| Sex: Male | −0.11 | 0.21 |
| Season Spring | 0.24 | 0.19 |
| Season Summer | 0.38 | 0.17* |
| State MO | 0.13 | 0.20 |
| State MS | 0.99 | 0.22* |
| Sex Male: season Spring | −0.53 | 0.17* |
| Sex Male: season Summer | 1.06 | 0.14* |
| Sex Male: state MO | 1.02 | 0.22* |
| Sex Male: state MS | −0.93 | 0.27* |
| Season Spring: state MO | −0.49 | 0.21* |
| Season Summer: state MO | −0.23 | 0.18 |
| Season Spring: state MS | 0.24 | 0.22 |
| Season Summer: state MS | −0.52 | 0.19* |
p < 0.05.
3.2. Home range size variation
The selected random structure for annual size variation was bear ID. Three competing models (Supporting information Appendix S1 Table B) best‐described size variation in annual ranges; the best fit model (Table 5) included the effects of sex and forest edge density, with no interactions. This model had a conditional R 2 of 0.85 and marginal R 2 was 0.49. Males had larger ranges than females, and forest edge density had a negative influence on annual range area (Table 5, Figure 4).
Table 5.
Parameter estimates and standard deviations (SD) for annual home range size variation for black bears (2008–2017) in Michigan (MI), Missouri (MO), and Mississippi (MS), USA
| Parameter | Estimate | SD |
|---|---|---|
| Intercept | 1.37 | 0.04* |
| Sex: Male | 0.67 | 0.08* |
| Forest edge density | −0.10 | 0.02* |
p < 0.05.
Figure 4.
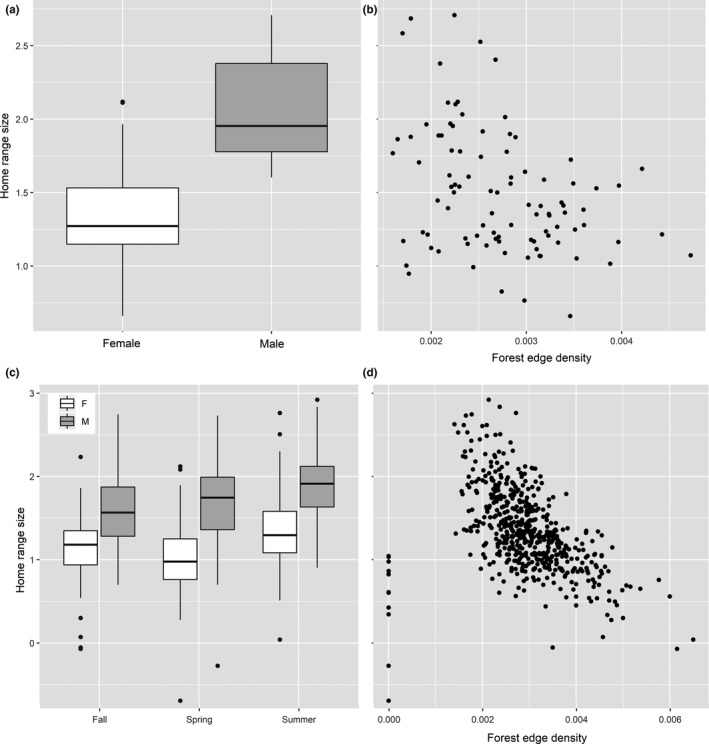
Black bear annual home range sizes (log10 transformed, n = 97) in relation to (a) sex, and (b) forest edge density. Seasonal home range sizes (log10 transformed, n = 538) in relation to (c) sex and season, and (d) forest edge density
The selected random structure for seasonal size variation was bear ID. Two competing models (Supporting information Appendix S1 Table C) best described the size variation in seasonal ranges; the best fit model (Table 6) included sex, state, season, edge density, proportion of forest, and two interactions. This model had a conditional R 2 of 0.68 and marginal R 2 of 0.55. Overall, males displayed larger seasonal ranges than females, and summer ranges were largest for both sexes (Table 6, Figure 4). Forest edge density and proportion of forest had a negative influence on range area for all bears, but edge density was three times more influential. Males in the spring and summer had larger ranges than in the fall. Bears in MO had larger ranges, and in MS smaller ranges (spring and summer only), than MI (Table 6, Figure 4).
Table 6.
Parameter estimates and standard deviations (SD) for seasonal home range size variation for black bears (2008–2017) in Michigan (MI), Missouri (MO), and Mississippi (MS), USA
| Parameter | Estimate | Std. |
|---|---|---|
| (Intercept) | 1.11 | 0.08* |
| Sex Male | 0.35 | 0.06* |
| Season Spring | −0.02 | 0.09 |
| Season Summer | 0.21 | 0.08* |
| State MO | 0.17 | 0.08* |
| State MS | −0.01 | 0.09 |
| Forest edge density | −0.18 | 0.02* |
| Proportion of forest | −0.06 | 0.02* |
| Season Spring: sex Male | 0.20 | 0.08* |
| Season Summer: sex Male | 0.14 | 0.07* |
| Season Spring: state MO | 0.09 | 0.10 |
| Season Summer: state MO | −0.03 | 0.09 |
| Season Spring: state MS | −0.37 | 0.10* |
| Season Summer: state MS | −0.22 | 0.09* |
p < 0.05.
4. DISCUSSION
As expected for a behaviorally flexible species with a large geographic range, black bears demonstrated high individual variability in spatial selection and range size; nevertheless, we observed broad patterns. Annually, black bears consistently selected areas with greater vegetation productivity than the surrounding landscape; yet selection for productivity weakened and became more variable seasonally. Opposite to our prediction, increasing fragmentation of forest patches consistently resulted in smaller annual and seasonal ranges in all areas. In contrast, our results supported our prediction that ranges with proportionately more forest would be smaller, but found no support for more productive ranges also being smaller as we would expect with an area minimizing strategy.
Under the framework of optimality, home range location should reflect a fitness‐maximizing strategy (Mitchell & Powell, 2004, 2007 ) while achieving nutritional security (Macdonald & Johnson, 2015). Different limiting factors can act at different scales (Rettie & Messier, 2000), and our results suggest vegetation productivity can influence spatial selection at coarser spatial and temporal scales. Different mammalian taxa, such as ungulates (Stillfried et al., 2017), primates (Zinner, Pelaez, & Torkler, 2002), and carnivores (Duquette et al., 2017; Mitchell & Powell, 2007), have been found to use areas of greater quality than the surrounding landscape, yet the limiting effect of vegetation productivity in black bear space use becomes weaker at finer scales reflecting a shift in limiting factors. Similarly, NDVI had little to no explanatory power related to fine scale (3rd or 4th scale; Johnson, 1980) spatial selection for wolves (Milakovic et al., 2011) or black bears (Duquette et al., 2017). Alternatively, NDVI may be limited to serve as an adequate proxy for omnivore food resources at finer scales. NDVI mirrors climatic conditions and can function as a good coarse proxy for overall vegetation productivity and food availability, however, at smaller scales it can fail to reflect the abundance of some food items like fruits, insects, or mast (Bojarska & Selva, 2012; Zhou et al., 2011). In addition, the finest resolution for NDVI layers (250 m) might not provide enough variability for smaller scale analyses if individuals are already selecting areas more productive at larger scales; detecting selection for homogeneous or common resources at certain scales is problematic (Kertson & Marzluff, 2011), that is, detecting selection within selection.
Increasing forest edge density had a consistent negative relationship with black bear range size both annually and seasonally. This relationship was surprising as we predicted forest fragmentation would cause bears to increase movement to obtain enough food resources located within forests. An alternative explanation is that non‐forested land covers and forest edges themselves can be a source of food resources for bears. For example, forest edges often facilitate the growth of a diversity of early successional vegetation that opportunistic omnivores can consume (Litvaitis 2001). Black bears in other areas have been associated with diverse land covers (Carter, Brown, Etter, & Visser, 2010), and reduce their ranges in areas with forest clearcuts (Brodeur, Ouellet, Courtois, & Fortin, 2008). Forest edge density could potentially act as a superior proxy than NDVI for black bear food resources at a fine scale; if so, then the prediction of smaller ranges when more food is available, under an area minimizing approach, would be supported. Human‐derived landscape fragmentation can result in smaller ranges by allowing resource generalists to take advantage of resources in areas surrounding their ranges (Andren, 1994; Tigas, Vuren, & Sauvajot, 2002). Black bears that have regular access to human‐derived food are larger, move less, and use less natural food (Massé, Dussault, Dussault, & Ibarzabal, 2014) and a recent global assessment found that mammals in human‐modified areas have decreased movement rates (Tucker et al., 2018). But the negative relationship between fragmentation and range area can also occur when little to no anthropogenic disturbances occur; for example, brown bears in Alaska used smaller areas as the landscape became more heterogeneous (Mangipane et al., 2018) and larger home range sizes were linked to areas of lower habitat diversity for black bears in Arkansas (Smith and Pelton 1990). In contrast, Karelus, McCown, Scheick, Kerk, and Oli (2016) suggested that fragmentation caused black bears in Florida to use larger areas, and a black bear study in Missouri suggested that increasing land cover diversity resulted in larger range areas (Hiller et al., 2015). Detecting and measuring the effect of landscape fragmentation on species is dependent on the vegetation community being fragmented and what is considered “non‐habitat”; human‐modified land covers can be hostile to some species, or populations within species, while providing attractive resources to others (Crooks, 2002).
Proportion of forest had a negative influence on seasonal range sizes, supporting our prediction of an area minimizing strategy (Mitchell & Powell, 2004, 2007 ). More available forested areas likely reflect an increase in food, water, and shelter which would result in reduced movements. Though black bears are a forest obligate species (Herrero, 1972; Pelton, 2003), they can use non‐forested areas (e.g., agriculture or low‐density human areas) to supplement feeding. In Missouri, black bear density declined with increasing forest cover (Sollmann et al., 2016), and when sufficient forest is available, human‐modified areas can provide attractive food resources (Beckmann & Berger, 2003; Ditmer et al., 2015; Merkle, Robinson, Krausman, & Alaback, 2013). Male bears in this study had lower proportion of forest within their ranges than female conspecifics, possibly reflecting risky food‐seeking behavior, mate seeking, or exploratory movements (Beckmann & Berger, 2003; Merkle et al., 2013). Though we expected more productive ranges (mean NDVI) to be smaller than less productive ranges following an area minimizing strategy, that prediction was not supported; ranges overall had high NDVI values possibly suggesting an energy maximizing approach. A negative relationship between productivity and range size has been observed in other carnivores (Bengsen et al., 2016; Ferguson, Currit, & Weckerly, 2009; Herfindal, Linnell, Odden, Nilsen, & Andersen, 2005), though the form of these relationships has been inconsistent among species (Nilsen, Herfindal, & Linnell, 2005).
In addition to our main predictions, we found that differences in range sizes existed between males and females annually and during all seasons; males displayed ranges from two to six times larger than females. In a polygynous mating system, adult males are expected to structure their space use to maximize mating opportunities (Sandell, 1989) and male range sizes should be greater than required for metabolic requirements (Dahle & Swenson, 2003; Liberg, Sandell, Pontier, & Natoli, 2000; Sandell, 1989); our results support these predictions. In other solitary polygynous carnivores (e.g., bobcats), male range areas are partially determined by female range areas (Ferguson et al., 2009). In agreement, we found that male ranges were largest where female ranges were also the largest (Missouri). Both female and male bears had the largest ranges during the mating and dispersal period (June‐July), consistent with previous studies (e.g., Costello, Creel, Kalinowski, Vu, & Quigley, 2009; Massé et al., 2014). Some of the smallest black bear ranges have been reported in the highly productive areas in the Mississippi Delta (Benson & Chamberlain, 2007; Oli, Jacobson, & Leopold, 2002), which is consistent with our results. We also found that bears in Mississippi had the greatest productivity selection (Table 3) which might be partially influenced by landscape structure; the Mississippi Delta includes highly productive hardwood forests constrained by less productive agriculture. Notably, ranges for males in Michigan during fall were smaller than for males in other areas, possibly related to seasonal risk avoidance in relation to black bear harvest (Stillfried, Belant, Svoboda, Beyer, & Kramer‐Schadt, 2015); while baiting may facilitate those limited movements by providing clustered high energy resources. Finally, increased population density should result in overall smaller ranges on average when compared to less dense populations (Kjellander et al., 2004), yet we did not find a pattern of increasing population density resulting in increasingly smaller ranges for our three study areas.
Black bears did not display a clearly defined scale‐independent strategy for structuring ranges (energy maximizing or area minimizing), consistent with a previous study (Mitchell & Powell, 2007). The high productivity of ranges of all sizes suggests energy maximizing, while the negative relationship between range size and both fragmentation and forest proportion suggests area minimizing. More limiting factors act at larger scales (Rettie & Messier, 2000), which would suggest productivity is the strongest limiting factor and energy maximizing is the dominant strategy while plasticity allows for seasonal area minimizing. The life history of black bears points to them being energy maximizers; species whose potential reproductive success is related to their energy gain (McLoughlin, Ferguson, & Messier, 2000). For many ursids, body mass and body fat have been found to influence reproductive success of males (Costello et al., 2009) and females (Atkinson & Ramsay, 1995; Belant, Kielland, Follmann, & Adams, 2006; López‐Alfaro, Robbins, Zedrosser, & Nielsen, 2013; Robbins, Ben‐David, Fortin, & Nelson, 2012), and their typical weight fluctuations during the year (i.e., hyperphagia, hibernation, den emergence; Hellgren, 1998; Farley & Robbins, 1995) should be facilitated by an energy maximizing strategy. In addition, the usual lack of territoriality (Mitchell & Powell, 2007) would facilitate an “expansionist” or energy maximizer behavior (Macdonald & Johnson, 2015) and dietary studies on captive and wild black bears suggest they fit an energy maximizing strategy (Costello et al. 2016).
Spatial and temporal heterogeneity are fundamental mechanisms structuring ranges (Börger et al., 2008; Macdonald & Johnson, 2015; Mitchell & Powell, 2007) and will become increasingly important as human modification of the landscape continues to influence species’ movements (Tucker et al., 2018). Black bears optimally locate their annual ranges to maximize access to areas of high vegetation productivity while adapting their space use to the amount of forest available and the degree of fragmentation, displaying scale‐dependent energy maximizing and area minimizing strategies. By quantifying black bear space use across different areas, over time, and among and within individuals, we revealed consistent large‐scale responses to environmental conditions while highlighting the intrinsic plasticity of this flexible omnivore.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
MG and JB conceptualized and designed the study. MG, DB, and JB acquired the data. MG, GW, and JB analyzed and interpreted the data. MG and JB drafted the manuscript. MG, JB, GW, and DB provided critical revisions.
DATA ACCESSIBILITY
Data used in this manuscript is accessible in Dryad, https://doi.org/10.5061/dryad.3vc108b.
Supporting information
ACKNOWLEDGMENTS
This study was funded by the Michigan Department of Natural Resources; Mississippi Department of Wildlife, Fisheries, and Parks; and the Missouri Department of Conservation through the Federal Aid in Wildlife Restoration Act, and the Forest and Wildlife Research Center at Mississippi State University. We thank participating landowners for land access and technicians for field support, and F. Bled and B. Strickland for valuable comments and suggestions. The authors declare no conflict of interest.
Gantchoff M, Wang G, Beyer D, Belant J. Scale‐dependent home range optimality for a solitary omnivore. Ecol Evol. 2018;8:12271–12282. 10.1002/ece3.4690
REFERENCES
- Andren, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos, 71, 355–366. [Google Scholar]
- Atkinson, S. N. , & Ramsay, M. A. (1995). The effects of prolonged fasting of the body composition and reproductive success of female polar bears (Ursus maritimus). Functional Ecology, 9, 559–567. [Google Scholar]
- Austin, D. , Bowen, W. D. , & McMillan, J. I. (2004). Intraspecific variation in movement patterns: Modeling individual behaviour in a large predator. Oikos, 105, 15–30. [Google Scholar]
- Barraquand, F. , & Murrell, J. D. (2012). Evolutionarily stable consumer home range size in relation to resource demography and consumer spatial organization. Theoretical Ecology, 5, 567–589. [Google Scholar]
- Barton, K. (2013). MuMIn: Multi‐model inference. R Package Version, 1.15. Retrieved from https://CRAN.R-project.org/package=MuMIn. [Google Scholar]
- Baruch‐Mordo, S. , Breck, S. W. , Wilson, K. R. , & Theobald, D. M. (2008). Spatiotemporal distribution of black bear‐human conflicts in Colorado, USA. Journal of Wildlife Management, 72, 1853–1862. [Google Scholar]
- Beckmann, J. P. , & Berger, J. (2003). Rapid ecological and behavioural changes in carnivores: The responses of black bears (Ursus americanus) to altered food. Journal of Zoology, 261, 207–212. 10.1017/S0952836903004126 [DOI] [Google Scholar]
- Belant, J. L. , Etter, D. R. , Mayhew, S. L. , Visser, L. G. , & Friedrich, P. D. (2011). Improving large scale mark–recapture estimates for American black bear populations. Ursus, 22, 9–23. [Google Scholar]
- Belant, J. L. , Kielland, K. , Follmann, E. H. , & Adams, L. G. (2006). Interspecific resource partitioning in sympatric ursids. Ecological Applications, 16, 2333–2343. [DOI] [PubMed] [Google Scholar]
- Bengsen, A. J. , Algar, D. , Ballard, G. , Buckmaster, T. , Comer, S. , Fleming, P. J. S. , … Zewe, F. (2016). Feral cat home–range size varies predictably with landscape productivity and population density. Journal of Zoology, 298, 112–120. [Google Scholar]
- Benson, J. F. , & Chamberlain, M. J. (2006). Food habits of Louisiana black bears (Ursus americanus luteolus) in two subpopulations of the Tensas River Basin. American Midland Naturalist, 156, 118–127. [Google Scholar]
- Benson, J. F. , & Chamberlain, M. J. (2007). Space use and habitat selection by female Louisiana black bears in the Tensas River Basin of Louisiana. Journal of Wildlife Management, 71, 117–126. 10.2193/2005-580 [DOI] [Google Scholar]
- Bojarska, K. , & Selva, N. (2012). Spatial patterns in brown bear Ursus arctos diet: The role of geographical and environmental factors. Mammal Review, 42, 120–143. [Google Scholar]
- Börger, L. , Dalziel, B. D. , & Fryxell, J. M. (2008). Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecology Letters, 11, 637–650. [DOI] [PubMed] [Google Scholar]
- Börger, L. , Franconi, N. , De Michele, G. , Gantz, A. , Meschi, F. , Manica, A. , … Coulson, T. (2006). Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology, 75, 1393–1405. [DOI] [PubMed] [Google Scholar]
- Brodeur, V. , Ouellet, J.‐P. , Courtois, R. , & Fortin, D. (2008). Habitat selection by black bears in an intensively logged boreal forest. Canadian Journal of Zoology, 86, 1307–1316. [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information–theoretic approach (2nd ed.). New York, NY: Springer. [Google Scholar]
- Carr, G. M. , & Macdonald, D. W. (1986). The sociality of solitary foragers: A model based on resource dispersion. Animal Behaviour, 34, 1540–1549. [Google Scholar]
- Carter, N. H. , Brown, D. G. , Etter, D. R. , & Visser, L. G. (2010). American black bear habitat selection in northern Lower Peninsula, Michigan, USA, using discrete–choice modeling. Ursus, 21, 57–71. [Google Scholar]
- Core Team, R. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/. [Google Scholar]
- Costello, C. M. , Creel, S. R. , Kalinowski, S. T. , Vu, N. V. , & Quigley, H. B. (2009). Determinants of male reproductive success in American black bears. Behavioral Ecology and Sociobiology, 64, 125. [Google Scholar]
- Costello, C. M. , Cain, S. L. , Pils, S. , Frattaroli, L. , Haroldson, M. A. , & van Manen, F. T. , (2016). Diet and macronutrient optimization in wild ursids: a comparison of grizzly bears with sympatric and allopatric black bears. PLoS one, 11, e0153702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks, K. R. (2002). Relative sensitivities of mammalian carnivores to habitat fragmentation. Conservation Biology, 16, 488–502. [Google Scholar]
- Crooks, K. R. , Burdett, C. L. , Theobald, D. M. , King, S. R. , Di Marco, M. , Rondinini, C. , & Boitani, L. (2017). Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proceedings of the National Academy of Sciences of the United States of America, 114, 7635–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle, B. , & Swenson, J. (2003). Home ranges in adult Scandinavian brown bears Ursus arctos: Effect of population density, mass, sex, reproductive status and habitat type. Journal of Zoology, 260, 329–335. [Google Scholar]
- Ditmer, M. A. , Garshelis, D. L. , Noyce, K. V. , Laske, T. G. , Iaizzo, P. A. , Burk, T. E. , … Fieberg, J. R. (2015). Behavioral and physiological responses of American black bears to landscape features within an agricultural region. Ecosphere, 6, 28. [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carre, G. , … Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36, 27–46. [Google Scholar]
- Duquette, J. F. , Belant, J. L. , Svoboda, N. J. , Beyer, D. E. Jr , & Lederle, P. E. (2014). Effects of maternal nutrition, resource use and multi–predator risk on neonatal white–tailed deer survival. PLoS ONE, 9, e10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette, J. F. , Belant, J. L. , Wilton, C. M. , Fowler, N. , Waller, B. W. , Beyer, D. E. , … Beringer, J. (2017). Black bear (Ursus americanus) functional resource selection relative to intraspecific competition and human risk. Canadian Journal of Zoology, 95, 203–212. [Google Scholar]
- Eide, N. E. , Jepsen, J. U. , & Prestrud, P. (2004). Spatial organization of reproductive Arctic foxes Alopex lagopus: Responses to spatial and temporal availability of prey resources. Journal of Animal Ecology, 73, 1056–1068. [Google Scholar]
- Farley, S. D. , & Robbins, C. T. (1995). Lactation, hibernation, and mass dynamics of American black bears and grizzly bears. Canadian Journal of Zoology, 73, 2216–2222. [Google Scholar]
- Ferguson, A. W. , Currit, N. A. , & Weckerly, F. W. (2009). Isometric scaling in home–range size of male and female bobcats (Lynx rufus). Canadian Journal of Zoology, 87, 1052–1060. [Google Scholar]
- Garel, M. , Solberg, E. J. , SÆther, B. E., Herfindal, I., … K. A. (2006). The length of growing season and adult sex ratio affect sexual size dimorphism in moose. Ecology, 87, 745–758. [DOI] [PubMed] [Google Scholar]
- Garshelis, D. L. , & McLaughlin, C. R. (1998). Review and evaluation of breakaway devices for bear radiocollars. Ursus, 10, 459–465. [Google Scholar]
- Gitzen, R. A. , Millspaugh, J. J. , & Kernohan, B. J. (2006). Bandwidth selection for fixed–kernel analysis of animal utilization distributions. Journal of Wildlife Management, 70, 1334–1344. [Google Scholar]
- Haines, A. M. , Hernandez, F. , Henke, S. E. , & Bingham, R. L. (2009). A method for determining asymptotes of home–range area curves. National Quail Symposium Proceedings, 6, Article 51. [Google Scholar]
- Hellgren, E. C. (1998). Physiology of hibernation in bears. Ursus, 10, 467–477. [Google Scholar]
- Herfindal, I. , Linnell, J. D. , Odden, J. , Nilsen, E. B. , & Andersen, R. (2005). Prey density, environmental productivity and home–range size in the Eurasian lynx (Lynx lynx). Journal of Zoology, 265, 63–71. [Google Scholar]
- Herrero, S. (1972). Aspects of evolution and adaptation in American black bears (Ursus americanus Pallas) and brown and grizzly bears (U. arctos Linne.) of North America In Herrero S. (Ed.), Bears: Their biology and management (pp. 221–231). Morges, Switzerland: International Union for the Conservation of Nature. [Google Scholar]
- Hiller, T. L. , Belant, J. L. , & Beringer, J. (2015). Sexual size dimorphism mediates effects of resource dispersion on American black bear space use. Journal of Zoology, 296, 200–207. [Google Scholar]
- Homer, C. G. , Dewitz, J. A. , Yang, L. , Jin, S. , Danielson, P. , Xian, G. , … Megown, K. (2015). Completion of the 2011 National Land Cover Database for the conterminous United States‐Representing a decade of land cover change information. Photogrammetric Engineering and Remote Sensing, 81, 345–354. [Google Scholar]
- Johnson, D. (1980). The comparison of usage and availability measurements for evaluating resource preference. Ecology, 61, 65–71. [Google Scholar]
- Johnson, D. D. P. , Kays, R. , Blackwell, P. G. , & Macdonald, D. W. (2002). Does the resource dispersion hypothesis explain group living? Trends in Ecology and Evolution, 17, 563–570. 10.1016/S0169-5347(02)02619-8 [DOI] [Google Scholar]
- Johnson, K. G. , & Pelton, M. R. (1980). Environmental relationships and the denning period of black bears in Tennessee. Journal of Mammalogy, 61, 653–660. [Google Scholar]
- Karelus, D. L. , McCown, J. W. , Scheick, B. K. , Kerk, M. V. D. , & Oli, M. K. (2016). Home ranges and habitat selection by black bears in a newly colonized population in Florida. Southeastern Naturalist, 15, 346–364. [Google Scholar]
- Karstensen, K. A. (2010). Land cover change in the Ozark Highlands, 1973–2000. Open‐File Report 2010–1198. Reston, VA: United States Geological Survey. [Google Scholar]
- Kernohan, B. J. , Gitzen, R. A. , & Millspaugh, J. J. (2001). Analysis of animal space use and movements. In Millspaugh J. J. & Marzluff J. M. (Eds.), Radio tracking and animal populations. San Diego, CA: Academic Press. [Google Scholar]
- Kertson, B. N. , & Marzluff, J. M. (2011). Improving studies of resource selection by understanding resource use. Environmental Conservation, 38, 18–27. [Google Scholar]
- Kjellander, P. , Hewison, A. J. M. , Liberg, O. , Angibault, J. M. , Bideau, E. , & Cargnelutti, B. (2004). Experimental evidence for density–dependence of home–range size in roe deer (Capreolus capreolus): A comparison of two long–term studies. Oecologia, 139, 478–485. [DOI] [PubMed] [Google Scholar]
- Krebs, J. R. , & Kacelnik, A. (1991). Decision‐making In Krebs J. R. & N. B. Davies (Eds.), Behavioural ecology: An evolutionary approach. London: Blackwell Scientific. [Google Scholar]
- Liberg, O. , Sandell, M. , Pontier, D. , & Natoli, E. (2000). Density, spatial organisation and reproductive tactics in the domestic cat and other felids The domestic cat: The biology of its behaviour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Litvaitis, J. A. (2001). Importance of early‐successional habitats to mammals in eastern forests. Wildlife Society Bulletin, 29, 466–473. [Google Scholar]
- López‐Alfaro, C. , Robbins, C. T. , Zedrosser, A. , & Nielsen, S. E. (2013). Energetics of hibernation and reproductive trade–offs in brown bears. Ecological Modelling, 270, 1–10. [Google Scholar]
- Macdonald, D. W. , & Johnson, D. D. P. (2015). Patchwork planet: The resource dispersion hypothesis, society, and the ecology of life. Journal of Zoology, 295, 75–107. [Google Scholar]
- Mangipane, L. S. , Belant, J. L. , Hiller, T. L. , Colvin, M. E. , Gustine, D. D. , Mangipane, B. A. , & Hilderbrand, G. V. (2018). Influences of landscape heterogeneity on home–range sizes of brown bears. Mammalian Biology, 88, 1–7. [Google Scholar]
- Marable, M. K. , Belant, J. L. , Godwin, D. , & Wang, G. (2012). Effects of resource dispersion and site familiarity on movements of translocated wild turkeys on fragmented landscapes. Behavioural Processes, 91, 119–124. [DOI] [PubMed] [Google Scholar]
- Marchand, P. , Garel, M. , Bourgoin, G. , Dubray, D. , Maillard, D. , & Loison, A. (2015). Coupling scale–specific habitat selection and activity reveals sex–specific food/cover trade–offs in a large herbivore. Animal Behavior, 102, 169–187. [Google Scholar]
- Massé, S. , Dussault, C. , Dussault, C. , & Ibarzabal, J. (2014). How artificial feeding for tourism–watching modifies black bear space use and habitat selection. Journal of Wildlife Management, 78, 1228–1238. [Google Scholar]
- McClintic, L. F. , Taylor, J. D. , Jones, J. C. , Singleton, R. D. , & Wang, G. (2014). Effects of spatiotemporal resource heterogeneity on home range size of American beaver. Journal of Zoology, 293, 134–141. [Google Scholar]
- McFadden–Hiller, J. E. , Beyer, D. E. Jr , Belant, J. L. (2016). Spatial distribution of black bear incident reports in Michigan. PLoS ONE, 11, e0154474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin, P. D. , & Ferguson, S. H. (2000). A hierarchical pattern of limiting factors helps explain variation in home range size. Écoscience, 7, 123–130. [Google Scholar]
- McLoughlin, P. , Ferguson, S. , & Messier, F. (2000). Intraspecific variation in home range overlap with habitat quality: A comparison among brown bear populations. Evolutionary Ecology, 14, 39–60. [Google Scholar]
- McNab, B. K. (1963). Bioenergetics and the determination of home range size. The American Naturalist, 97, 133–140. [Google Scholar]
- Merkle, J. A. , Robinson, H. S. , Krausman, P. R. , & Alaback, P. (2013). Food availability and foraging near human developments by black bears. Journal of Mammalogy, 94, 378–385. [Google Scholar]
- Milakovic, B. , Parker, K. L. , Gustine, D. D. , Lay, R. J. , Walker, A. B. , & Gillingham, M. P. (2011). Habitat selection by a focal predator (Canis lupus) in a multiprey ecosystem of the northern Rockies. Journal of Mammalogy, 92, 568–582. [Google Scholar]
- Mississippi Automated Resource Information System (2014). Available at www.maris.state.ms.us.
- Mitchell, M. S. , & Powell, R. A. (2004). A mechanistic home range model for optimal use of spatially distributed resources. Ecological Modeling, 177, 209–232. [Google Scholar]
- Mitchell, M. S. , & Powell, R. A. (2007). Optimal use of resources structures home ranges and spatial distribution of black bears. Animal Behavior, 74, 219–230. [Google Scholar]
- Nakagawa, S. , & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution, 4, 133–142. [Google Scholar]
- Nathan, R. (2008). An emerging movement ecology paradigm. Proceedings of the National Academy of Sciences of the United States of America, 105, 19050–19051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen, E. B. , Herfindal, I. , & Linnell, J. D. C. (2005). Can intra–specific variation in carnivore home–range size be explained using remote–sensing estimates of environmental productivity? Ecoscience, 12, 68–75. 10.2980/i1195-6860-12-1-68.1 [DOI] [Google Scholar]
- Oli, M. K. , Jacobson, H. A. , & Leopold, B. D. (2002). Pattern of space use by female black bears in the White River National Wildlife Refuge, Arkansas, USA. Journal for Nature Conservation, 10, 87–93. [Google Scholar]
- Pelton, M. R. (2003). Black bear In Feldhamer G. A. & Chapman J. A. (Eds.), Wild mammals of North America. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Pettorelli, N. , Gaillard, J. M. , Mysterud, A. , Duncan, P. , Delorme, D. , Van Laere, G. , … Klein, F. (2006). Using a proxy of plant productivity (NDVI) to find key periods for animal performance: The case of roe deer. Oikos, 112, 565–572. [Google Scholar]
- Powell, R. A. (2000). Animal home ranges and territories and home range estimators In Boitani L. & Fuller T. K. (Eds.), Research techniques in animal ecology controversies and consequences. New York, NY: Columbia University Press. [Google Scholar]
- Powell, R. A. , & Mitchell, M. S. (2012). What is a home range? Journal of Mammalogy, 93, 948–958. [Google Scholar]
- Rettie, W. J. , & Messier, F. (2000). Hierarchical habitat selection by woodland caribou: Its relationship to limiting factors. Ecography, 23, 466–478. [Google Scholar]
- Richards, S. A. , Whittingham, M. J. , & Stephens, P. A. (2011). Model selection and model averaging in behavioural ecology: The utility of the IT‐AIC framework. Behavioral Ecology and Sociobiology, 65, 77–89. 10.1007/s00265-010-1035-8 [DOI] [Google Scholar]
- Robbins, C. T. , Ben‐David, M. , Fortin, J. K. , & Nelson, O. L. (2012). Maternal condition determines birth date and growth of newborn bear cubs. Journal of Mammalogy, 93, 540–546. [Google Scholar]
- Sandell, M. (1989). The mating tactics and spacing patterns of solitary carnivores Carnivore behavior, ecology, and evolution. Boston, MA: Springer. [Google Scholar]
- Scheick, B. K. , & McCown, W. (2014). Geographic distribution of American black bears in North America. Ursus, 25, 24–33. [Google Scholar]
- Sikes, R. S. , Gannon, W. L. , Animal Care and Use Committee of the American Society of Mammalogists (2011). Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy, 92, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek, S. L. , Belant, J. L. , Young, B. W. , Shropshire, C. , & Leopold, B. D. (2012). History and status of the American black bear in Mississippi. Ursus, 23, 159–167. [Google Scholar]
- Sollmann, R. , Gardner, B. , Belant, J. L. , Wilton, C. M. , & Beringer, J. (2016). Habitat associations in a recolonizing, low‐density black bear population. Ecosphere, 7, 8. [Google Scholar]
- Stillfried, M. , Belant, J. L. , Svoboda, N. J. , Beyer, D. E. , & Kramer‐Schadt, S. (2015). When top predators become prey: Black bears alter movement behaviour in response to hunting pressure. Behavioural Processes, 120, 30–39. 10.1016/j.beproc.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Stillfried, M. , Gras, P. , Boerner, K. , Goeritz, F. , Painer, J. , Roellig, K. , … Kramer‐Schadt, S. (2017). Secrets of success in a landscape of fear: Urban wild boar adjust risk perception and tolerate disturbance. Frontiers in Ecology and Evolution, 5, 157 10.3389/fevo.2017.00157 [DOI] [Google Scholar]
- Tigas, L. A. , Van Vuren, D. H. , & Sauvajot, R. M. (2002). Behavioral responses of bobcats and coyotes to habitat fragmentation and corridors in an urban environment. Biological Conservation, 108, 299–306. [Google Scholar]
- Tucker, M. A. , Böhning‐Gaese, K. , Fagan, W. F. , Fryxell, J. M. , Van Moorter, B. , Alberts, S. C. , … Bartlam‐Brooks, H. (2018). Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science, 359, 466–469. [DOI] [PubMed] [Google Scholar]
- Van Moorter, B. , Rolandsen, C. M. , Basille, M. , & Gaillard, J. M. (2016). Movement is the glue connecting home ranges and habitat selection. Journal of Animal Ecology, 85, 21–31. [DOI] [PubMed] [Google Scholar]
- Wiegand, T. , Naves, J. , Garbulsky, M. F. , & Fernández, N. (2008). Animal habitat quality and ecosystem functioning: Exploring seasonal patterns using NDVI. Ecological Monographs, 78, 87–103. [Google Scholar]
- Wilton, C. M. , Puckett, E. E. , Beringer, J. , Gardner, B. , Eggert, L. S. , & Belant, J. L. (2014). Trap array configuration influences estimates and precision of black bear density and abundance. PLoS ONE, 9, e111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, C. , & Ripple, W. J. (2017). Range contractions of the world’s large carnivores. Royal Society Open Science, 4, 170052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. B. , Newman, C. , Xu, W. T. , Buesching, C. D. , Zalewski, A. , Kaneko, Y. , … Xie, Z. Q. (2011). Biogeographical variation in the diet of Holarctic martens (genus Martes, Mammalia: Carnivora: Mustelidae): Adaptive foraging in generalists. Journal of Biogeography, 38, 137–147. [Google Scholar]
- Zinner, D. , Pelaez, F. , & Torkler, F. (2002). Distribution and habitat of grivet monkeys (Cercopithecus aethiops aethiops) in eastern and central Eritrea. African Journal of Ecology, 40, 151–158. 10.1046/j.1365-2028.2002.00360.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this manuscript is accessible in Dryad, https://doi.org/10.5061/dryad.3vc108b.


