Abstract
As eusocial and wood-dwelling insects, termites have been shown to use vibrations to assess their food, to eavesdrop on competitors and predators and to warn nest-mates. Bioassay choice experiments used to determine food preferences in animals often consider single factors only but foraging decisions can be influenced by multiple factors such as the quantity and quality of the food and the wood as a medium for communication. A statistical analysis framework is developed here to design a single bioassay experiment to study multifactorial foraging choice (Pinus radiata) in the basal Australian termite species Coptotermes (C.) acinaciformis (Isoptera: Rhinotermitidae). By employing a correlation analysis, 17 measured physical properties of 1417 Pinus radiata veneer discs were reduced to five key material properties: density, moisture absorption, early wood content, first resonance frequency and damping. By applying a fuzzy c-means clustering technique, these veneer discs were optimally paired for treatment and control trials to study food preference by termites based on these five key material properties. A multifactorial analysis of variance was compared to a permutation analysis of the results indicating for the first time that C. acinaciformis takes into account multiple factors when making foraging decisions. C. acinaciformis prefer denser wood with large early wood content, preferably humid and highly damped. Results presented here have practical implications for food choice experiments and for studies concerned with communication in termites as well as their ecology and coevolution with trees as their major food source.
Keywords: bioassays, multifactorial analyses, statistics, permutation analysis, food choice, wood properties
1. Introduction
Preference experiments with animals are carried out to answer questions such as habitat and mate selection [1,2], group membership or predator recognition [3,4]. Food-choice bioassays usually consider a single property only [5–9]. However, plant-dwelling insects communicate multimodally and process complex, interrelated information, based on visual, tactile, magnetic, vibrational or chemical signals or cues [9–11].
As termites are significant economic pests, much research has been conducted to study their foraging and feeding preferences [7,12–14]. Termites are eusocial and live in large colonies of up to several million individuals; they are herbivores, and either plant-dwelling (one-piece termites), wood feeding, or soil-feeding and nest on, in or adjacent to the substrate they consume, often within trees or directly in the soil [13,15]. Termites use substrate-borne vibration signals (biotremology, cf. [16]) or cues to either assess the food for nest-mate recruitment [13,17] or to warn each other [18] or to eavesdrop on competitors [19], and predators [20].
An experimental design to determine feeding preference based on heterogeneous, natural materials is likely flawed if only a single property is considered, neglecting inter-sample palatability and factor interaction [9]. Also, termites may choose their food based on multiple factors including its quantity and quality as well as its suitability as a communication medium. However, multifactorial feeding preference to natural substrates and functionalities has never been systematically tested.
1.1. The substrate as food
Food quantity [13,21], wood fibre deterioration (dampwood termites, Termopsidae), and its chemical and energetic composition [12,22] are essential food-selection criteria in termites. Therefore, termite foraging behaviour was mostly studied by considering merely sequentially cut wood of equal shape and weight [13,14,23,24]; however, other factors are also important. Moist soil in direct contact with wood attracts termites and severe precipitation triggers termite attacks on building timbers [14,23]. Termites often prefer early wood, i.e. the wood that develops in spring with an abundance of rain and nutrients, which is generally softer and less dense, is preferably consumed [25,26]; and termites preferred uncompressed against compressed wood boards [25]. Other factors such as terpenes found in timbers of tree species with higher resin content or as found in some denser growth rings are repelling [27,28].
1.2. The substrate as communication medium
The substrate's function as a communication medium in biotremology is largely neglected [16]. Evans et al. [13] were the first to show that termites use vibrations to assess food size. For a given source, the substrate vibration response is determined by its natural frequencies and damping values. Here damping is the cause of the attenuation of vibration due to internal friction and is quantified by equation (2.1) in the next section.
By using wood–wood, wood–aluminium or wood–rubber beams of identical fundamental frequency or mass, Inta et al. [21] showed that Cryptotermes (Cr.) secundus preferred the wood–wood over wood–aluminium and wood–rubber choice; hence the fundamental frequency of a substrate or the mass is not the determining factor for food choice. However, the results for wood–rubber beam are ambiguous, suggesting damping could be important because rubber has a higher damping than aluminium or wood.
We hypothesized that termites use vibrations to make multifactorial foraging decisions and that multiple factors could originate from different physical properties related to two distinct functions of the substrate: nutritional aspects (feeding preference) and communication medium (signalling preference). To test the hypothesis, food-choice bioassays were conducted using Pinus radiata veneer discs. The objective of this research was to develop and apply a statistical analysis framework to identify key multiple influencing factors in termite foraging decisions using a single bioassay experiment.
2. Material and methods
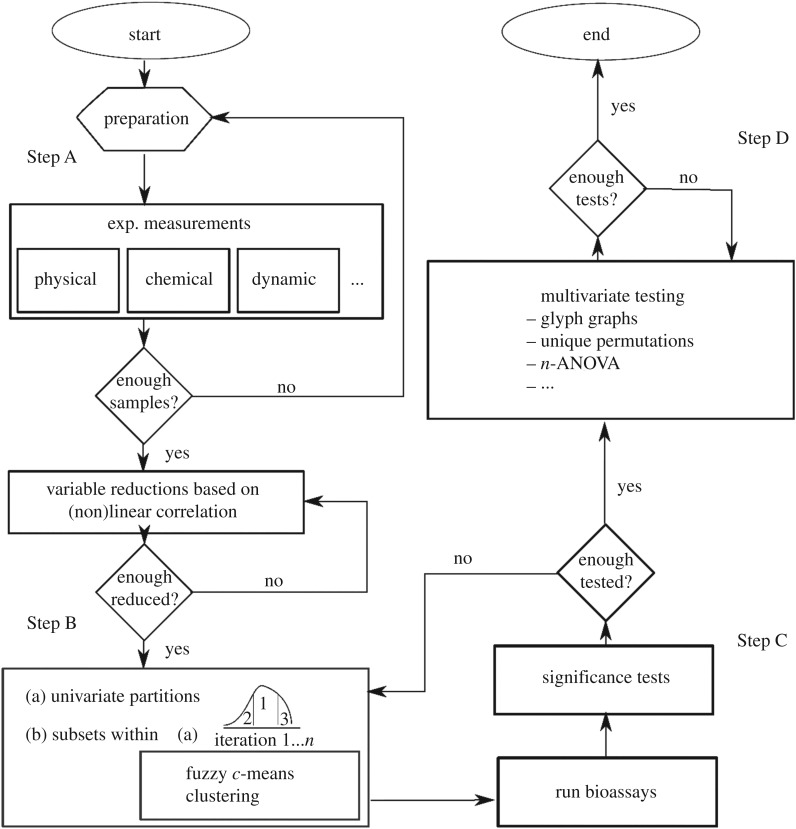
Figure 1 schematizes a flow chart of the systematic approach.
Figure 1.
Flow chart of the statistical approach. Step A: generation of populations of veneer discs, by experimentally identifying the maximum number of variables x1, x2, x3, …, xn, followed by a linear and a nonlinear correlation analysis to reduce the number of variables to a q-tupel of key material parameters; Step B: Generation of q univariate distributions, partitioned into lower bound (LB) and upper bound (UB) centre and extreme bands; application of the fuzzy c-means clustering algorithm [9]; Step C: Carrying out of bioassays and univariate analyses to determine main factors; Step D multivariate analyses.
Step A, the preparation stage, involves the manufacture of a large enough number of intact veneer discs and the formation of distributions of its physical properties (variables) as food and substrate material. To minimize the complexity of the bioassay, the variables are condensed to key material properties by determining their interrelations [29].
Using the naturally multivariate datasets, in Step B quasi-univariate subsets of key material properties are partitioned into extreme and centre bands. Optimal pairing of veneer discs is achieved by minimizing their differences in key material properties within each partitioned band using the fuzzy c-means clustering algorithm.
In Step C, the bioassays are carried out, followed by a one-way analysis of variance (ANOVA).
Step D involves multivariate analyses including a q-way analysis of variance (q-ANOVA or MANOVA), a unique permutation analysis and the calculation of canonical correlations to quantify those property interactions important in termite foraging.
2.1. Step A: measurements, statistical descriptors and reduction of variables
2.1.1. Producing samples
From 24 veneer sheets of P. radiata heartwood, we punched 1417 veneer discs of 60 ± 0.78 mm diameter using a die and a manual press (cf. Set B in [9]). We took 12 measurements of diameter and thickness every 30° along the circumference of the veneer disc (vernier calliper, Kincrome©, uncertainty ± 0.01 mm, electronic supplementary material, figure S1.1). We used only those sheets from which more than 20 intact veneer discs could be produced and we discarded cracked or fungal infested discs [30].
2.1.2. Extracting properties
For each veneer disc, we recorded 17 properties, namely its veneer sheet membership, its dry and moist weight, its moisture absorption, its thickness, surface area, density, the percentage of black background pixels and those related to wood taken from digital photography of the veneer discs, the mean intensity of pixels related to wood, the mode skewness of the light intensity distribution and from experimental vibration testing, each veneer disc's first and second natural frequency, its damping ratios and vibration amplitudes in resonance.
Each veneer disc was moistened three times within a computer-controlled environmental chamber set to 80 ± 3% relative humidity (RH) and 28°C for 8 h (ACS© Challenge DM600, Massa Martana, Italy, fluctuation from 0.1°C to ±0.3°C, and 1–3% RH; electronic supplementary material, figure S1.2a). The veneer discs were weighed using a high-precision scale (AEA 250 g, Adam Equipment Co. Ltd, Milton Keynes, UK, uncertainty 0.8 ± 0.5 mg; electronic supplementary material, figure S1.2b) and were oven-dried (12 h at 105°C, type XU490 France Etuves, 4 kW, 300°C max, fluctuation ± 0.2°C). To reduce moisture absorption of the discs from the air during cool down, a vacuum desiccator (TED Pella©, 6 bar line pressure, ca 10 600 cm3) filled with silicon dioxide SiO2 (200 g granules of Sigma-Aldrich© 13767, self-indicating orange) was used, cf. electronic supplementary material, figure S1.2c. The repeated thermal treatment of the veneer discs evaporated most of their α-/β-pinenes which were hence ignored [31–34]; we recorded the discs' average moisture absorption which resulted from moist and dry weight differences.
Termites prefer wood with a larger early wood proportion, which is usually brighter in colour than late wood [26,33]. Late wood is rarely consumed, owing to differences in its chemical composition, structure and lignin content [22,30,34,35]. We measured the coloration of veneer discs to quantify the early and the late wood content (electronic supplementary material, figures S2.1, S2.2) by using the mode skewness of the light intensity distribution by dividing the difference of the median and its mean intensity with its standard deviation [29] and shifted it to strictly positive values.
Pixel intensity distributions of digital photos were obtained using a camera (Olympus©, μ tough 12 MP, Tokyo, Japan 1660 × 1200 pixels) fixed on a miniature tripod (HAMA© Star 05, Monheim, Germany), cf. electronic supplementary material, figure S2.1a. The veneer discs were illuminated using three flashlights with white LEDs, wired up and connected to a 6 V DC adapter to avoid the 50 Hz flickering noise of the AC network. Pixel intensities (unit-less) ranged from zero to 255, and were calibrated relative to 15 758 mcd (luminance meter Vetus© SM208, 0.01–39 990 cd m–2). We assumed pixels below a cut-off intensity of 50 to be invalid and equal to noise or black background (electronic supplementary material, figure S2.2b), whereas we assigned pixel intensities from 50 to 125 and above to late wood and early wood, respectively (electronic supplementary material, figure S2.2). We calculated the median intensity and geometrical properties such as the disc's surface area.
We used a laser scanning vibrometer (Polytec© PSV-I-400, LR lenses, OFV-5000 controller, PSV-E-401 junction box) to measure the vibration response at 361 points distributed radially over each veneer disc resting on vibration isolating foam (Merford Regufoam® mixed cell polyurethane). A sweep signal (10 Hz to 7 kHz, n = 20 averages) from a Tektronix AFG dual channel arbitrary function generator [36,37] was used to drive a loudspeaker (Radioshack©, Realistic Minimus 7, 40 W max, 8 Ω), angled at 45° and positioned within 150 mm distance, to excite each disc for the determination of resonance frequencies fi, vibration response magnitudes Ai and damping ratios ζi:
| 2.1 |
The damping ratio ζi of the ith mode as shown in equation (2.1) is estimated using the half-power bandwidth criterion at Ai – 3 dB, with being the upper and lower bound frequency difference fi [36].
2.1.3. Correlation analysis
After the removal of outliers (median absolute deviation) and the formation of histograms, we calculated Pearson's correlation coefficient [29]. To also consider nonlinear interactions, we compared with the averaged cross-mutual information [38,39].
The cross-mutual information between two pieces of information (variables) is defined as the entropy of (its self-information) minus the entropy of this variable conditional on the knowledge the other variable, which expresses the average amount of decreasing information learned from about ,
| 2.2 |
Here, represents the joint probability density of measurements Xi and Yi; and are the unconditional probabilities of realizations and [40] so that if , i.e. for two independent random variables Xi and Yj, cf. equation (2.2). was then made scale-invariant through normalization by its absolute maximum. and indicated a weak, moderate and strong correlation for values within the intervals , and ; and , respectively [29]. Little correlated variables or ‘integrated’ quantities were eventually selected as q key material properties, e.g. volume was preferred over veneer thickness and diameter and density was preferred over volume and weight.
2.2. Step B: formation of univariate distributions
From each key material property, we calculated their normalized histograms and estimated their continuous distributions [40] (electronic supplementary material, S5). These continuous univariate distributions were partitioned into subsets of a lower bound (LB) and an upper bound (UB), i.e. extreme band partitions, which had values smaller (greater) than their distributions' mean (μ) minus (plus) their standard deviation (σ) (electronic supplementary material, figure S3.1). Those discs found in the extreme band partitions were used for the treatment trials, with those in the centre band partition used for control trials (electronic supplementary materials, S5).
We hypothesized that termites behaved indifferently towards property differences found in the centre band veneer discs and preferred either the LB or the UB side of each key material property. In each partition of each key material distribution, the fuzzy c-means clustering method was employed to minimize the property differences relative to the cluster centre [9] of paired veneer discs which were ranked to be used for successive trials (trial homogenization); the formation of ranks can be pictured by a spiralling sequence (conical helix) of discs in a q-dimensional key material property space. Within each cluster, the veneer discs had a variable cluster membership. The membership depended on the cluster properties such as partition size, number of material properties and clusters considered and the measured material property values.
The membership dependency shrank towards the outer trajectory/end of the conical helix, into a fuzzy region, so that veneer discs could also be members of another cluster. The veneer discs were then paired by taking successive samples from the LB and UB partition, starting from the cluster centre and spiralling outwards towards a lower cluster membership. The property differences of the pairs were crosschecked by visualization using glyph plots from multivariate data with spokes being proportional to the property value [40].
2.3. Step C: bioassays
A bioassay uses the subterranean termite species C. acinaciformis (Isoptera: Rhinotermitidae) as a test object to measure the response to a stimulus, here the physical properties of the veneer discs [41]. We conducted the bioassay in an environmental chamber 28°C and 80% RH. We trapped subcolonies of C. acinaciformis, from four different colonies, using specifically prepared trenches [42], set up in the Conapaira South and the Binya state forest (33.93° S, 146.17° E), Griffith, New South Wales, Australia. Contrary to other eusocial insects, termites keep foraging even in the absence of the royal pair so that it is sufficient to collect only mature soldiers and workers in their natural ratio, which was on average 1 : 9 [13,21,24].
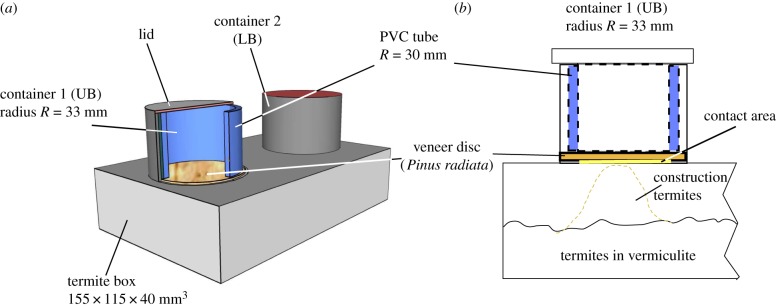
We used 100 termite nesting-boxes (figure 2a). The largest component, a polyethylene terephthalate (PET) box was filled with vermiculite and P. radiata sawdust (two teaspoons, 50 ml spring water), and served as the termites nesting area (figure 2b). PET cylinders were glued with hot-melt adhesive (HMA Bostik HG3) to a lid of the hexahedral termite box and housed the LB and UB veneer discs as choices with an airing hole to avoid the growth of fungus (electronic supplementary material, figure S4.1). The veneer discs were held in place by a piece of polyvinyl chloride (PVC) tubing on top of an annular carton ring (0.5 mm thick) as a compressible spacer pushed down by a lid. All glued parts were dried for 1 day, and then placed into a water bath to wash off residual odour.
Figure 2.
Schematics of the termite box design. (a) Three-dimensional view on the termite box showing the UB veneer disc through the cut open view of the PVC tubing; (b) two-dimensional schematic: within the PET cylinder the PVC tubing is pushed onto the veneer disc; the termites build up material underneath the LB and UB side to reach the contact zone (radius 27 mm). (Online version in colour.)
We added about 2040 termites, corresponding to about 10 g. The vermiculite/sawdust matrix stabilized the acclimatization process, suppressed the development of fungi and provided food for up to 4 days, enough time for the termites to acclimate in their new environment. The containers were initially equipped with two randomly chosen veneer discs of the centre band to promote building activity underneath the contact zone. The night prior to the start of the experiment, the optimal veneer disc pairs were fitted into the containers to avoid warping while adjusting their moisture content.
The bioassay was run in five successive trials (2 days each) for the extreme bands and the centre band for five key material properties using four colonies; colonies two and three had one extra trial so that in total 220 paired veneer discs were used over a period of 14 days. All termite boxes were wrapped with aluminium foil (electronic supplementary material, S4 and figure S4.1) to minimize the light exposure during the daily 10 min routine inspections. After each trial, the termite boxes were rotated by 90° within the shelving unit to increase statistical mixing. The lids were washed to remove pheromone traces and rinsed to remove residual detergent. We monitored the mean amount of wood eaten by the termites by dry weighing the veneer discs after the experiment (8 h, 105°C). Only those discs with more than 1% of the weight reduced in both the LB and UB were valid. Because sub-colonies of termites behave differently the amount of reduced wood in each container was normalized by the total wood reduced per termite box [13,20,21].
One-way ANOVA tests on groups of key material data were conducted with 5% significance thresholds (independent variable, always LB versus UB). We generated box plots of the normalized median wood reduction (Mood's median test [29]) to contrast results based on a normal distribution assumptions and N(0, σ2)-distributed errors relative to the median. A non-parametric Mood's test is a conservative choice and a safeguard against wrong conclusions of normality assumption [29].
2.4. Step D: multivariate analyses
Unbalanced q-way ANOVA (MANOVA) tests were conducted using the normalized reduced wood per trial. A linear additive model represents main preference factors (key material properties); their interacting effects used an additive model with multiplicative part, applying type III sum of squares [29,43]. However, the unbalanced q-way ANOVA does not determine the termites' sensitivities or the direction of preference [29,43] for which we used a statistical sport index bet model applied to the populations of the LB and the UB veneer discs [29] combined with a unique q-variate permutation analysis.
To conduct a q-variate permutation analysis, we merged the paired samples of the extreme bands with the centre band partitions. We counted how often a ‘home team’ was ‘victorious’, over the ‘road team’ (scoring of the permutation of either the LB or the UB side with regard to a certain characteristic) and calculated an odds ratio. This characteristic was either value of a key material property or the wood reduction (after the bioassay). Using the q key material properties provided |S| = card(S) = N unique permutations [29], with
| 2.3 |
By employing the Heaviside function on the absolute difference vector |x (UB) – y (LB)| relative to a variable threshold value with respect to a material property, we obtained binary vectors [29]
| 2.4 |
Using the above equation, we counted how often one property of the UB side veneer disc was greater than that of the LB side (relative frequency) and increased in increments of 1/1000 and determined differences between vector components with respect to a key material property as a the first scoring condition. A second application of equation (2.4), using the wood reduced, indicated the preference of the termites as a second scoring condition. By considering the sum of over each , relative frequencies over (here ) for each were formed
| 2.5 |
Owing to the complementarity of the LB side and the UB side, we assume without loss of generality that only those samples of veneer disc pairs were considered with more wood reduced on the UB than on the LB side. Plotting equation (2.5) against ordered in terms of permutations (from [0 0 … 0] to [1 1 … 1], i.e. all quantities of the UB are greater by than the LB) provided a contour plot of relative frequencies. By considering all , the contour plots provided a measure of ‘termite decision sensitivity’, i.e. how large the difference between the LB and UB must have been in order to evoke a change in the foraging behaviour. Applying equations (2.3)–(2.5), three different cases were considered.
The first case (i) simulates the natural distribution of key material properties based on all 1417 veneer disc samples by uniformly bootstrapping n = 10 000 samples and pairing them randomly to approximate the theoretical distribution from a small finite number of samples [29]. For case (ii), n = 188 veneer disc pairs of Step B were used. In the event that the distributions of (ii) looked similar to the ‘natural distribution’ of case (i), the subset of optimally paired veneer disc generated in Step B using the fuzzy c-means clustering without bootstrapping was a valid representative of the true distribution. Finally, the sample pairs of case (ii) were fed to termites to study multifactorial food preferences. For case (i) and (ii), only the first scoring condition, i.e. the difference between the LB and UB vectors with respect to a material property was considered; in case (iii), the termites' choice (second scoring condition) was applied.
Using cases (ii) and (iii), the relative preference π of acceptable food can be determined. For this purpose, the relative frequencies fic of the termites preferred wood pieces (electronic supplementary material, figure S10.1c) are subtracted from the relative frequencies fib of the available food (electronic supplementary material, figure S10.1b), integrated over relative differences δi, and normalized through strictly positive (using a constant c), logarithmically weighting with the median of the relative frequencies of preferred choice L;
| 2.6 |
An uncertainty corridor of around unity represents relative indifference to available acceptable food choices; permutations below that corridor indicate relative aversion of acceptable food choices, values above indicate a relative preference.
3. Software used
For all statistical analyses, we applied Matlab R 2013B (statistics and signal processing package) and R-Studio (v. 0.92). The mutual information was calculated using the nonlinear time-series analysis tool TISEAN. For the vibration measurements, the vibration analysis software PSV 8.7, Polytec© GmbH, Waldbronn, Germany) was used with Polytec Scan Viewer.
4. Results
4.1. Step A: measurements, statistical descriptors and variable reduction
The results of the correlation analysis between the 17 measured variables for P. radiata veneer discs (electronic supplementary material, table S5.1) were similar for both the Pearson correlation coefficient and the averaged cross-mutual information . The dry weight and the density were strongly and moderately correlated with = 0.83 and . We chose the density δ (μ ± σ: 474.58 ± 47.42 kg m−3) as a key material property ; because termites show an affinity to moisture [14], the ability of veneer discs to absorb moisture from the air (12.19 ± 0.88%), was chosen as the second key property which was only moderately nonlinearly correlated to the damping ratios (; ).
The veneer disc's mode skewness of light intensity distribution I (average −0.0851 ± 0.3392) as an indication of early or late wood was selected as the third key material property x3. The damping ratios and of the 1st and the 2nd vibration modes (electronic supplementary material, figure S6.1) were negatively correlated to their vibration resonance amplitudes ( = −0.97, and = −0.95, ). Also, their amplitudes were reasonably well correlated, hence only the 1st resonance frequency f1 (=x4), and its damping ratio (=x5) were selected as key material properties (more details on correlation analysis see the electronic supplementary materials, S5, figure S5.1 and table S5.1).
4.2. Step B: formation of univariate distributions and LB/UB partitions
Histograms and their continuous estimates for the five key material properties were generated (electronic supplementary material, figures S5.2, S5.3). Lilliefors tests indicated that all distributions belonged to the family of normal distributions (p < 0.001, [29]). The distribution of ‘density’ was Gaussian in shape; that of ‘moisture absorption’ was more platykurtic (less scattered) than the more leptokurtic ‘early wood content’. The distributions of the ‘damping’ and the ‘resonance frequency’ were positively and negatively skewed, respectively.
The minimum and maximum differences (ranges) between the LB and the UB sections in the extreme band partitions were: for (x1) ρ about 94.7 kg m−3 and 294.7 kg m−3; for (x2) mRH between 0.7% and 13.6%; for (x3) I between 0.69 and 1.85; for (x4) f1 between 284.4 Hz and 987.5 Hz; and for (x5) between 2.8% and 12.0%. Using the fuzzy c-means clustering algorithm, two or three clusters were formed within each partition, depending on whether the sample size was smaller, equal or larger than 30, respectively (electronic supplementary material, figure S7.1 [9]). This minimizes the differences of successive veneer discs in the extreme band partitions to 0.4 ± 0.6% (ρ), 0.3 ± 0.6% (mRH), 1.1 ± 6.4% (Ι), 1.4 ± 7.9% (f1) and 2.6 ± 11.2% () in the LB partitions; and to about 0.4 ± 1.0% (δ), 0.6 ± 4.3% (mRH), 4.4 ± 9.7% (Ι), 0.3 ± 0.4% (f1) and 2.5 ± 15.4% () in the UB partitions (mean ± standard deviation). The means and the standard deviations of the wood reduction for the extreme bands and the centre band partition are provided in table 1.
Table 1.
Mean ± s.d. values of wood reduction for the extreme bands and the centre band partition.
| n | mean ± s.d. (g) of LB | mean ± s.d. (g) of UB | mean ratio UB/LB | |
|---|---|---|---|---|
| extreme bands | ||||
| density, δ = x1 | 20 | 0.051 ± 0.06 | 0.220 ± 0.309 | 4.31 |
| moisture, mRH = x2 | 20 | 0.074 ± 0.162 | 0.051 ± 0.057 | 1.45 |
| early wood, I = x3 | 19 | 0.043 ± 0.041 | 0.113 ± 0.130 | 2.63 |
| frequency, f1 = x4 | 20 | 0.071 ± 0.088 | 0.109 ± 0.184 | 1.54 |
| damping, ξ1 = x5 | 20 | 0.039 ± 0.039 | 0.088 ± 0.137 | 2.26 |
| centre bands | ||||
| density, δ = x1 | 22 | 0.025 ± 0.027 | 0.072 ± 0.147 | 2.88 |
| moisture, mRH = x2 | 15 | 0.058 ± 0.056 | 0.077 ± 0.121 | 0.97 |
| early wood, I = x3 | 20 | 0.053 ± 0.059 | 0.074 ± 0.119 | 1.39 |
| frequency, f1 = x4 | 16 | 0.071 ± 0.066 | 0.086 ± 0.139 | 1.21 |
| damping, ξ1 = x5 | 16 | 0.052 ± 0.071 | 0.098 ± 0.088 | 1.37 |
4.3. Step C: bioassays
Termites showed statistically significant preferences only in the extreme band partitions and only for density and greater early wood content (table 2). In the centre-band, no significant absolute differences were observed.
Table 2.
Results of the one-way ANOVA test for the set of extreme bands samples and centre band samples paired to food-choice bioassays. d.f., degree of freedom.
| extreme bands |
centre bands |
|||||
|---|---|---|---|---|---|---|
| #d.f. | F-value | p-value | #d.f. | F-value | p-value | |
| density, δ = x1 | 39 | 5.39 | 0.026a | 43 | 0.1 | 0.753 |
| moisture, mRH = x2 | 39 | 1.41 | 0.242 | 29 | 2.27 | 0.144 |
| early wood, I = x3 | 37 | 4.42 | 0.042a | 39 | 2.76 | 0.106 |
| frequency, f1 = x4 | 39 | 0.02 | 0.877 | 29 | 1.74 | 0.198 |
| damping, ξ1 = x5 | 39 | 0.05 | 0.821 | 31 | 1.36 | 0.252 |
aStatistically significant.
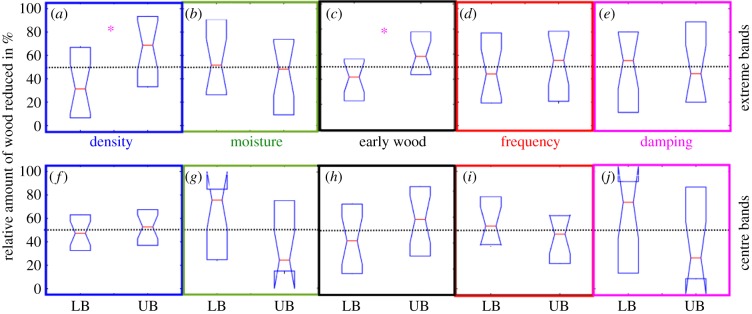
Box plots (with median-tests) of the relative amount of wood reduced in the extreme bands and the centre-band are depicted in figure 3 with results being in partial agreement with the one-way ANOVA tests (table 2). The median test, opposed to the one-way ANOVA, only indicates a preference (non-overlapping confidence intervals at the 5% level) to denser wood. Comparing the termite colonies, no significant behavioural differences were observed (electronic supplementary material, table S8.1).
Figure 3.
Results of univariate analysis. Significantly different outcomes as detected by non-overlapping notches (95% confidence intervals, median test cf. Spanos [29]) between the LB and UB; significant are highlighted by asterisks (*). For (a–e) of the extreme band partitions, only the density shows significant differences (n = {20, 20, 19, 20, 20}). In the centre band partition (f–j), termites are indifferent to any given choice (n = {22, 15, 20, 16, 16}). (Online version in colour.)
4.4. Step D: multivariate analyses
All statistically significant results of the five-way ANOVA test are listed in table 3 (for a complete list see electronic supplementary material, table S8.2). Termites preferred wood with higher density. The multivariate interactions between density, moisture absorption and early wood content as well as between density, moisture absorption and the damping ratio are also statistically significant.
Table 3.
Results of the five factorial ANOVA test for all samples to test significant preference for certain material property combinations; only significant outcomes are listed (complete list in electronic supplementary material, table S8.2).
| mean squared | F-value | p-value | |
|---|---|---|---|
| 1. Density × moisture × early wood | 2.4879 | 6.44 | 0.0122 |
| 2. Density | 1.6850 | 4.36 | 0.0385 |
| 3. Density × early wood × damping | 1.5541 | 4.02 | 0.0467 |
4.4.1. Multivariate permutation analysis
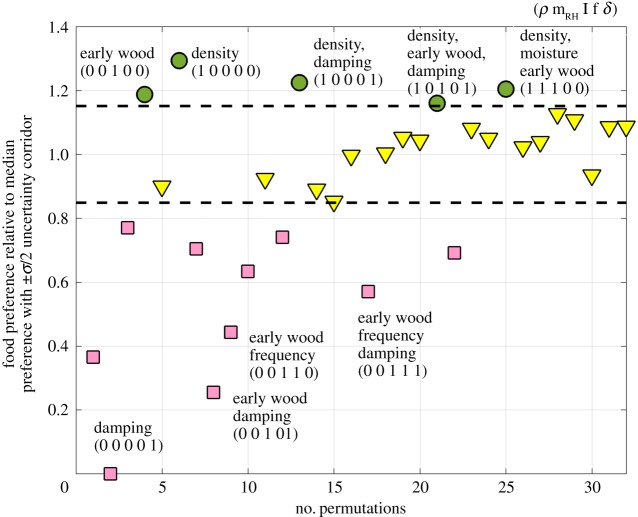
Figure 4 shows the results of equation (2.6), indicating indifference threshold corridor; relative preference or relative aversion towards various parameter permutations is found above and below this corridor, respectively. Since it is assumed that the food choices provided are acceptable (termites ate all the different choices provided), the terms positive and negative preference seem more appropriate. Additional graphs, which represent intermediate calculation results of equation (2.6), required to obtain results in figure 4, are presented in electronic supplementary material, S6.1. Different from the five-way ANOVA analysis, the permutation analysis allows a ranking of relative preference: dense wood is preferred over higher density and damping, over dense, moist and early wood, and over early wood (single factor) or higher density, early wood and damping.
Figure 4.
Results of permutation analysis. Preference for a certain permutation of wood properties; circles indicate a relative preference, a value of one indicates relative aversion, and a value of less than one indicates a relative aversion; triangle markers represent lie in a zone of indifference. (Online version in colour.)
5. Discussion
In total, 1417 veneer discs were produced and 17 physical properties were measured and by correlation analysis reduced to five key material properties: density, moisture, early wood content as well as the resonance frequency and damping. Applying the fuzzy c-means clustering technique to partitions of veneer distributions, the veneer discs were optimally paired for feeding termites in a bioassays choice experiment. Two functions of the substrate were tested: the veneer disc as food and the veneer disc as a communication medium. The suggested systematic approach enables both the main and the interaction effects of multifactorial foraging decisions in termites to natural substrates to be determined using a single experiment and standard statistical techniques.
Despite substantial research efforts in the past to understand the feeding preferences of termites, mostly only single factors were identified, namely the moisture content and the amount of wood [12,14,25,30]. Here, applying a conventional univariate one-way ANOVA and a median test to the dataset obtained from the bioassay experiment we identify that density rather than the amount of wood plays also a role; also a higher early wood content was preferred.
The five-way ANOVA shows that combinations of higher density, moisture absorption and early wood, or density, early wood and damping also seem important but no ranking of preference was possible and early wood as a single factor could not be identified. The permutation analysis based on a stochastic model, shows the combined results of the univariate and multivariate analysis and more. The permutation analysis indicates a clear ranking of preferences: density (single factor) is more important than higher density and damping, than higher density, moisture and early wood, than early wood (single factor) than density, early wood and damping. High damping values alone, without any other dominant properties, were not foraged on very much; the first fundamental frequency did not seem to play an important role.
Our results show that C. acinaciformis is very selective when it comes to foraging decisions even for only small amounts of wood, contrary to the widespread perception of termites being indiscriminating feeders [13,44]. Termites were attracted to higher density wood (range of 94.7 kg m−3)—possibly due to its higher energy content [33]. However, they were averse towards late wood [26], which is in fact very dense, but mechanically stronger, hard to machine and presumably associated with higher mandible wear rates [45]. Instead, they preferred the early wood. The absence of the combination of early wood and dense wood (with a certain amount of moisture or damping) indicates that there is a trade-off. Equivalent water content at 28°C and 80% RH is approx. 20%, only consisting of bound water. Although moisture content below fibre saturation point (28%) has effects on many physical properties of wood, whether termites can detect a small difference of hardness of wood needs to be studied further in light of miniscule moisture content changes below the fibre saturation point.
The literature suggests that C. acinaciformis likes moist wood. Wood can get humidified through the air, but also if in direct contact with moist soil or water. The exposure to moist soil and microbes might soften the wood fibres and enhance their machinability [46,47]. Higher moisture leads to higher damping; both were favoured by the termites according to the n-ANOVA and confirmed by the permutation analysis, even for very small differences of about 1.7% and 3.2%, respectively.
Contrary to that the fundamental frequency of the vibration response seems irrelevant. Rather than processing spectral information [13,17,19,21], termites could process vibrational cues by sensing time-dependent, dispersive and non-dispersive waves and their attenuation properties [36,48]. Reflected, scattered and dispersed waves and their amplitude provide important cues which might be used for foraging, including the location of tree knots, other insects (nest-mates, competitors, predators), the density of the wood or its moisture content or loading condition [15,20,49,50].
Moisture increases the damping of a substrate nonlinearly, reduces the veneer disc's resonance frequency and attenuates structural vibrations [33,36,49,50]. The average cross-mutual information indicates a nonlinear linkage between the moisture absorption and the damping ratio, which is likely to be associated with the visco-elastic properties within the fibres [33,49]. It is known that termites carry water in sacs (salivary reservoirs) to moisturize their food (the wood) by secreting this water to eat when the wood is dry. If wood had a higher moisture absorption capability, it is more likely to absorb water from various sources and thus would require a smaller amount of water to be transported to reach the ideal moisture content level [51], which would be advantageous for termites from an energy point of view. It is likely that substrate moisture levels are controlled by the termites to enhance building activities and foraging efficiency [51,52] but possibly also to manipulate vibration transmission, to either enhance communication or to impede eavesdropping by inquilines or predators on walking or feeding signals [20,33]. However, these behaviours need to be tested with another experiment; the research presented here was only able to exclude the frequency component explicitly as a decisive factor, which was believed before to play a role [13,21].
As a tree dwelling insect, C. acinaciformis may adapt its ‘vibrational calls’ (i.e. feeding activities, alarm [13,17,18]) or modify the response to other cues such as those related to walking or the alarm signal by considering the filter properties of the wood's transfer function [36,53]. Behavioural adaptation to substrate properties is known, e.g. in bees performing the waggle-dance on honeycombs with specific cell size to amplify the signal [54]. A similar behaviour could be useful in enhancing a soldier's alarm signal considering wood properties or termite clay constructions [52,55] or to monitor foraging sites for vibrational cues of intruders of competing colonies or predators such as spiders or ants [3,18,20,37,45]. As communication during foraging activities are situation-dependent, it can only be tested if the substrate properties are accurately known with a carefully designed bioassay and analysis method as suggested here.
Further, whether the structure of timber is related to a plant defence mechanism against the termites is not yet studied [56–59]; maybe the layers of higher density late wood act as a protective barrier against wood eating pests. Whether subordinate, coexisting termites species such as Cryptotermes (Cr.) secundus prefer the same food or whether the competition avoidance of Cr. secundus extends to choosing wood with different characteristics remains to be studied [17,19].
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
This research was supported under the Australian Research Council Discovery Projects funding scheme (project no. DP110102564) under a special research permit from the Forestry Corporation of NSW (FCNSW). The authors would like to thank T. K. Boyson, P. Wang and C. Oberst for their assistance in improving the clarity of the manuscript. The authors would like to acknowledge P. Gleeson's contribution in helping to set up the termite trenches.
Data accessibility
All data are provided as electronic supplementary materials or is derived from that data using the models described in the paper.
Authors' contributions
S.O. had the idea of the study, collected the termites, designed and conducted the experiments, developed the statistical framework and analysed, cleaned, interpreted the data, and wrote the paper. J.C.S.L. and T.A.E. provided funds, material and laboratory space for this research, and helped interpret the results. All authors revised the manuscript.
Competing interests
The authors declare to have no competing interests.
Funding
The research conducted was supported by the Australian Research Council under the Discovery Project funding scheme (project no. DP110102564).
References
- 1.Gilliam JF, Fraser DF. 1987. Habitat selection under predation hazard: test of a model with foraging minnows. Ecology 68, 1856–1862. ( 10.2307/1939877) [DOI] [PubMed] [Google Scholar]
- 2.Schwartz JJ, Hunce R, Lentine B, Powers K. 2016. Calling site choice and its impact on call degradation and call attractiveness in the gray treefrog, Hyla versicolor. Behav. Ecol. Soc. 70, 1–19. ( 10.1007/s00265-015-2016-8) [DOI] [Google Scholar]
- 3.Hoare DJ, Couzin ID, Godin J-GJ, Krause J. 2004. Context-dependent group size choice in fish. Anim. Behav. 67, 155–164. ( 10.1016/j.anbehav.2003.04.004) [DOI] [Google Scholar]
- 4.Rakotonirina H, Kappeler PM, Fichtel C. 2016. The role of acoustic signals for species recognition in redfronted lemurs (Eulemur rufifrons). BMC Evol. Biol. 16, 100 ( 10.1186/s12862-016-0677-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong C, Koehler P, Patterson R. 1993. Oral toxicity and repellency of borates to German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entom. 86, 1458–1463. ( 10.1093/jee/86.5.1458) [DOI] [PubMed] [Google Scholar]
- 6.Farrar R, Ridgway RL. 1994. Comparative studies of the effects of nutrient based phagostimulants. J. Econ. Entom. 87, 44–52. ( 10.1093/jee/87.1.44) [DOI] [Google Scholar]
- 7.Chen J, Henderson G. 1996. Determination of feeding preference of formosan subterranean termite (Coptotermes formosanus Shiraki) for some amino acid additives. J. Chem. Ecol. 22, 2359–2369. ( 10.1007/BF02029552) [DOI] [PubMed] [Google Scholar]
- 8.Rust M, Reierson D, Klotz J. 2004. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling argentine ants (Hymenoptera: Formicidae). J. Econ. Entom. 97, 1017–1024. ( 10.1093/jee/97.3.1017) [DOI] [PubMed] [Google Scholar]
- 9.Oberst S, Lai JCS, Evans TA. 2014. Novel method for pairing wood samples in choice tests. PLoS ONE 9, e88835 ( 10.1371/journal.pone.0088835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hölldobler B. 1999. Multimodal signals in ant communication. J. Comp. Phys. A 184, 129–141. [Google Scholar]
- 11.Detrain C, Deneubourg J-L. 2002. Complexity of environment and parsimony of decision rules in insect societies. Bio. Bulletin 202, 268–274. ( 10.2307/1543478) [DOI] [PubMed] [Google Scholar]
- 12.Peters BC, Fitzgerald CJ. 1997. Susceptibility of softwood blocks to damage by subterranean termites (Isoptera: Rhinotermitidae, Mastotermitidae). Mat. Organ. 31, 293–312. [Google Scholar]
- 13.Evans TA, Lai JCS, Toledano E, McDowall L, Rakotonarivo S, Lenz M. 2005. Termites assess wood size by using vibration signals. Proc. Natl Acad. Sci. USA 102, 3732–3737. ( 10.1073/pnas.0408649102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautam BK, Henderson G. 2011. Wood consumption by formosan subterranean termites (Isoptera: Rhinotermitidae) as affected by wood moisture content and temperature. Ann. Entom. Soc. Am. 104, 459–464. ( 10.1603/AN10190) [DOI] [Google Scholar]
- 15.Hadlington S, Staunton I. 2008. Australian termites. Sydney, Australia: University of New South Wales Press Ltd. [Google Scholar]
- 16.Hill P, Wessel A. 2016. Biotremology. Curr. Biol. 26, R187–R191. ( 10.1016/j.cub.2016.01.054) [DOI] [PubMed] [Google Scholar]
- 17.Evans TA, Inta R, Lai JCS, Lenz M. 2007. Foraging vibration signals attract foragers and identify food size in the drywood termite, Cryptotermes secundus. Insec. Soc. 54, 374–382. ( 10.1007/s00040-007-0958-1) [DOI] [Google Scholar]
- 18.Hager F, Kirchner WH. 2013. Vibrational long-distance communication in the termite Macrotermes natalensis and Odontotermes sp. J. Exp. Biol. 216, 3249–3256. ( 10.1242/jeb.086991) [DOI] [PubMed] [Google Scholar]
- 19.Evans TA, Inta R, Lai JCS, Prueger S, Foo NW, Fu EW, Lenz M. 2009. Termites eavesdrop to avoid competitors. Proc. R. Soc. B 276, 4035–4041. ( 10.1098/rspb.2009.1147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberst S, Bann G, Lai JCS, Evans TA. 2017. Cryptic termites avoid predatory ants by eavesdropping on vibrational cues from their footsteps. Ecol. Lett. 20, 212–221. ( 10.1111/ele.12727) [DOI] [PubMed] [Google Scholar]
- 21.Inta R, Lai JCS, Fu EW, Evans TA. 2007. Termites live in a material world: exploration of their ability to differentiate between food sources. J. R. Soc. Int. 4, 735–744. ( 10.1098/rsif.2007.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenz M, Hunt JH, Nalepa CA. 1994. Nourishment and evolution of insect societies: food resources, colony growth and caste development in wood-feeding termites. Boulder, CO: Westview Press. [Google Scholar]
- 23.Lenz M. 1985. Variability of vigour between colonies of Coptotermes acinaciformis (Froggatt) (Isoptera: Rhinotermitidae) and its implications for laboratory experimentation. Bull. Entom. Res. 75, 13–21. ( 10.1017/S0007485300014139) [DOI] [Google Scholar]
- 24.Korb J, Lenz M. 2004. Reproductive decision-making in the termite Cryptotermes secundus (Kalotermitidae) under variable food conditions. Behav. Ecol. 15, 390–395. ( 10.1093/beheco/arh033) [DOI] [Google Scholar]
- 25.Waller DA, Jones CG, La Fage JP. 1990. Measuring wood preference in termites. Entom. Exp. Appl. 56, 117–123. ( 10.1111/j.1570-7458.1990.tb01388.x) [DOI] [Google Scholar]
- 26.Bootle KR. 2010. Wood in Australia: types, properties and uses. Australia: McGraw-Hill Education-Europe. [Google Scholar]
- 27.Nicholas DD. (ed.). 1973. Wood deterioration and its prevention by preservative treatments, vol. 1 New York, NY: Syracuse University Press. [Google Scholar]
- 28.Zahid I, Grgurinovic C, Zaman T, De R. 2012. Assessment of technologies and dogs for detecting insect pests in timber and forest products. Scand. J. For. Res. 27, 492–502. ( 10.1080/02827581.2012.657801) [DOI] [Google Scholar]
- 29.Spanos A. 1999. Probability theory and statistical inference. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Scheffer TC, Cowling EB. 1996. Natural resistance of wood to microbial deterioration. Ann. Rev. Phytopathol. 4, 147–168. ( 10.1146/annurev.py.04.090166.001051) [DOI] [Google Scholar]
- 31.Peters BC, Fitzgerald CJ. 2004. Field exposure of Pinus heartwoods to subterranean termite damage (Isoptera: Rhinotermitidae, Mastotermitidae). Austr. Forestry 67, 7581 ( 10.1080/00049158.2004.10676210) [DOI] [Google Scholar]
- 32.Brito JO, Silva FG, Leao MM, Almeida G. 2008. Chemical composition changes in Eucalyptus and Pinus woods submitted to heat treatment. Biores. Tech. 99, 8545–8548. ( 10.1016/j.biortech.2008.03.069) [DOI] [PubMed] [Google Scholar]
- 33.Csanady E, Magoss E. 2013. Mechanics of wood machining. Berlin, Germany: Springer. [Google Scholar]
- 34.Bakar BFA, Hiziroglu S, Tahir PMD. 2013. Properties of some thermally modified wood species. Mat. Des. 43, 348–355. ( 10.1016/j.matdes.2012.06.054) [DOI] [Google Scholar]
- 35.Butler JHA, Buckerfield JC. 1979. Digestion of lignin by termites. Soil Bio. Biochem. 11, 507–513. ( 10.1016/0038-0717(79)90010-5) [DOI] [Google Scholar]
- 36.Fletcher NH, Rossing TD. 2012. The physics of musical instruments. New York, NY: Springer. [Google Scholar]
- 37.Oberst S, Nava-Baro E, Lai JCS, Evans TA. 2014. Quantifying ant activity using vibration measurements. PLoS ONE 9, e90902 ( 10.1371/journal.pone.0090902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marwan N, Romano MC, Thiel M, Kurths J. 2007. Recurrence plots for the analysis of complex systems. Phys. Rep. 438, 237–329. ( 10.1016/j.physrep.2006.11.001) [DOI] [Google Scholar]
- 39.Oberst S, Lai JCS. 2015. A statistical approach to estimate the Lyapunov spectrum in disc brake squeal. J. Sound Vib. 334, 120–135. ( 10.1016/j.jsv.2014.06.025) [DOI] [Google Scholar]
- 40.Bowman AW, Azzalini A. 1997. Applied smoothing techniques for data analysis. New York, NY: Oxford University Press. [Google Scholar]
- 41.Robertson JL, Russell RM, Preisler HK, Savin NE. 2007. Bioassays with arthropods. Boca Raton, FL: CRC Press. [Google Scholar]
- 42.Evans TA, Gleeson PV. 2006. The effect of bait design on bait consumption in termites (Isoptera: Rhinotermitdae). Bull. Entom. Res. 96, 85–90. ( 10.1079/BER2005397) [DOI] [PubMed] [Google Scholar]
- 43.Seber GAF. 1984. Multivariate observations. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- 44.Sylvain TBC, Senan S, Lucie YN, Yao T, Souleymane K. 2014. Termites impact on different age of cocoa (Theobroma cocoa L.) plantations with different fertilizer treatments in semi-deciduous forest zone (Oume, Ivory Coast). Herald J. Agri. Food Sci. Res. 4, 21–27. [Google Scholar]
- 45.Matsuoka H, Fujii Y, Okumura S, Imamura Y, Yoshimura T. 1996. Relationship between the type of feeding behavior of termites and the acoustic emission (AE) generation. Departmental Bulletin Paper Wood Research Institute, Kyoto University 2008-04-24T10:19:37Z.
- 46.Joquet P, Dauber J, Lagerlöf J, Lavelle P, Lepage M. 2006. Soil invertebrates as ecosystem engineers: intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 32, 153–164. ( 10.1016/j.apsoil.2005.07.004) [DOI] [Google Scholar]
- 47.Ayayee PA, Jones SC, Sabree ZL. 2015. Can 13C stable isotope analysis uncover essential amino acid provisioning by termite-associated gut microbes? PeerJ 3, e1218 ( 10.7717/peerj.1218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casas J, Magal C, Sueur J. 2007. Dispersive and non-dispersive waves through plants: implications for arthropod vibratory communication. Proc. R. Soc. B 274, 1087–1092. ( 10.1098/rspb.2006.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wegst UGK. 2006. Wood for sound. Am. J. Bot. 93, 1439–1448. ( 10.3732/ajb.93.10.1439) [DOI] [PubMed] [Google Scholar]
- 50.Cocroft RB, Gogala M, Hill PSM, Wessel A. 2014. Studying vibrational communication. Berlin, Germany: Springer. [Google Scholar]
- 51.Tomoe NT, Yoshimura IY. 2005. Feeding activities of Coptotermes formosanus Shiraki and Reticulitermes speratus (Kolbe) as affected by moisture content of wood. J. Wood Sci. 51, 60–65. ( 10.1007/s10086-003-0612-0) [DOI] [Google Scholar]
- 52.Oberst S, Lai JCS, Evans TA. 2016. Termites utilise clay to build structural supports and so increase foraging resources. Sci. Rep. 6, 20990 ( 10.1038/srep20990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young SL, Chyasnavichyus M, Erko M, Barth FG, Fratzl P, Zlotnikov I, Politi Y, Tsukruk VV. 2015. A spider's biological vibration filter: micromechanical characteristics of a biomaterial surface. Acta Biomat. 10, 4832 ( 10.1016/j.actbio.2014.07.023) [DOI] [PubMed] [Google Scholar]
- 54.Sandemann DC, Tautz J, Lindauer M. 1996. Transmission of vibration across honeycombs and its detection by bee leg receptors. J. Exp. Biol. 199, 2585–2594. [DOI] [PubMed] [Google Scholar]
- 55.Kandasami RK, Renee MB, Tejas GM. 2016. Effect of biocementation on the strength and stability of termite mounds. Environ. Geotech. 3, 99–113. ( 10.1680/jenge.15.00036) [DOI] [Google Scholar]
- 56.Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE. 1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 11, 41–65. ( 10.1146/annurev.es.11.110180.000353) [DOI] [Google Scholar]
- 57.Rosenthal GA, Berenbaum MR. 1992. Herbivores: their interactions with secondary plant metabolites. San Diego, CA: Academic Press. [Google Scholar]
- 58.Rausher MD. 2001. Co-evolution and plant resistance to natural enemies. Nature 411, 857–864. ( 10.1038/35081193) [DOI] [PubMed] [Google Scholar]
- 59.Iqbal N, Ali Khan HA, Saeed S. 2015. Response of Microtermes mycophagus (Isoptera: Termitidae) to twenty one wood species. PeerJ 3, e1132 ( 10.7717/peerj.1132) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided as electronic supplementary materials or is derived from that data using the models described in the paper.