Abstract
Background
Intravascular papillary endothelial hyperplasia (known also as Masson’s tumor) is a benign vascular lesion that commonly occurs in the skin and is rarely found in solid organs, especially in the kidney. In what follows, we will look into the first case of an unexpectedly diagnosed Masson’s tumor of the kidney presenting as a suspicious renal cyst.
Case presentation
A 61-year-old Arab man presented with a left renal cyst, incidentally revealed by ultrasonography. The laboratory values were unremarkable. Computed tomography and magnetic resonance imaging demonstrated a 38 mm left renal midportion Bosniak IV cyst. Our patient underwent a radical nephrectomy. Histopathology revealed the diagnosis of intravascular papillary endothelial hyperplasia. There was no recurrence detected after 9 years of follow-up.
Conclusions
Renal intravascular papillary endothelial hyperplasia is a rare benign tumor which can mimic a suspicious renal mass on radiological findings. Thus, this entity should be considered more often in the thick of the diagnostic possibilities in order to avoid unnecessary nephrectomies.
Electronic supplementary material
The online version of this article (10.1186/s13256-018-1898-2) contains supplementary material, which is available to authorized users.
Keywords: Bosniak classification, Intravascular papillary endothelial hyperplasia, Kidney, Masson’s tumor, Nephrectomy, Renal cyst
Background
Intravascular papillary endothelial hyperplasia (IPEH) is a benign vascular lesion commonly known as Masson’s tumor, which was first described in 1923 by Masson. IPEH is a reactive process of endothelial proliferation that takes place around thrombi in the setting of venous stasis.
This pathology occurs more often in the extremities of the body on cutaneous tissues. Only 12 cases of this tumor localized in the kidney have been described in the literature (Table 1). In this article, we report the first case in which this tumor presents as a suspicious renal cyst. We aimed to provide further insight about this rare entity to better characterize it, in order to avoid some unnecessary nephrectomies.
Table 1.
Clinical findings of renal Masson’s tumor in the literature
| Year | Source | Age (year) |
Sex | Presentation | Side | Size (mm) | Location | Radiologic findings | Preoperative diagnosis | Treatment | Follow-up (month) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1990 | Garber et al. [6] | 57 | M | Hematuria | R | 30 | Midportion | CT: renal mass MRI: high intensity enhancing |
Hypernephroma Vascular malformation |
Nephrectomy | 13/NED |
| 2 | 1996 | Steffee and Iskandar [12] | 63 | F | Acute renal failure | G | NA | Hilar | NA | Abnormal vascular flow | Transplant Nephrectomy | NA |
| 3 | 1997 | Johraku et al. [3] | 55 | F | Incidentally | R | 30 | Hilar | US: isoechoic mass CT/MRI: peripheral enhancement |
Renal hemangioma Renal neoplasm |
NSS | NA |
| 4 | 2000 | Kim et al. [4] | 7 | F | Hematuria | L | 18 | Midportion | US: hypoechoic mass CT: renal mass |
Wilms tumor Neuroblastoma |
Nephrectomy | NA |
| 5 | 2002 | Van den Bogaert et al. [13] | 64 | M | Incidentally | L | 26 | Hilar | US: hyperechoic lesion CT: heterogeneous enhancement MRI: T1 hypointense T2 hyperintense |
Renal neoplasm | Nephrectomy | NA |
| 6 | 2005 | Akthar et al. [14] | 40 | M | Hematuria | L | 24 | Midportion | US: hyperechoic lesion CT: soft tissue mass |
Renal neoplasm | Nephrectomy | NA |
| 7 | 2005 | Akhtar et al. [14] | 48 | M | Incidentally | L | 30 | Hilar | US/CT: renal mass | Renal neoplasm | Nephrectomy | NA |
| 8 | 2009 | Rizza et al. [7] | 49 | M | Massive retroperitoneal hemorrhage | L | 55 | Hilar | NA | NA | Nephrectomy | NA |
| 9 | 2011 | Pelosi et al. [8] | 30 | M | Incidentally | L | 12 | Hilar | CT: renal mass | Renal neoplasm | Nephrectomy | NA |
| 10 | 2012 | Mehta et al. [1] | 48 | M | NA | NA | 22 | NA | NA | Renal neoplasm | NSS | 132/NED |
| 11 | 2016 | Alkan et al. [10] | 50 | M | NA | L | 40 | Hilar | CT: renal mass with calcifications and internal homogeneous enhancement in the delayed phase MRI: T1 hypointense T2 hyperintense |
Renal neoplasm | NSS Nephrectomy |
3/LR 4/NED |
| 12 | 2017 | Moreillo-Vicente et al. [5] | 61 | M | Flank pain | R | 40 | Hilar | CT: heterogeneous mass with necrotic core MRI: T1 hypointense T2 hyperintense |
Adrenal neoplasm | Nephrectomy | 6/NED |
| 13 | 2018 | Our Case | 61 | M | Incidentally | L | 38 | Midportion | US: hypoechoic mass CT: peripheral enhancement MRI: peripheral enhancement |
Renal neoplasm | Nephrectomy | 108/NED |
CT computed tomography, F female, G graft, L left, LR local recurrence, M male, MRI magnetic resonance imaging, NA not assigned, NED no evidence of disease, NSS nephron-sparing surgery, R right, US ultrasonography
Case presentation
We describe a 61-year-old Arab man who retired from teaching 2 years ago. He did not smoke tobacco or consume alcohol. His past medical history included two surgical operations: a hydatid cyst of the liver operated on 6 years ago in a surgery department and a right ureteral lithiasis operated on in our urology department 4 years ago (at that time, he had only been explored by an intravenous pyelogram). He had been under alpha blocker for benign prostatic hyperplasia for 6 months. He was admitted for a suspicious renal cyst, incidentally found on renal and vesicoprostatic ultrasound. He had no complaints. His physical examination was unremarkable. His temperature was 37.2 °C, his blood pressure was 134/82 mmHg, and his pulse rate was regular at 74 beats per minute. On laboratory values, white blood cell count was 7.9 × 103/mL, red blood cell count 4.1 × 106/mL, hemoglobin 14.2 g/dL, platelets 396 × 103/mL, creatinine 1.04 mg/dL, sodium 138 mEq/L, potassium 4.1 mEq/L, and C-reactive protein 1 mg/L. Urines examination showed no leukocyturia or bacteriuria.
Renal and vesicoprostatic ultrasound found a non-vascularized cystic formation with a thickened and irregular wall on his left kidney.
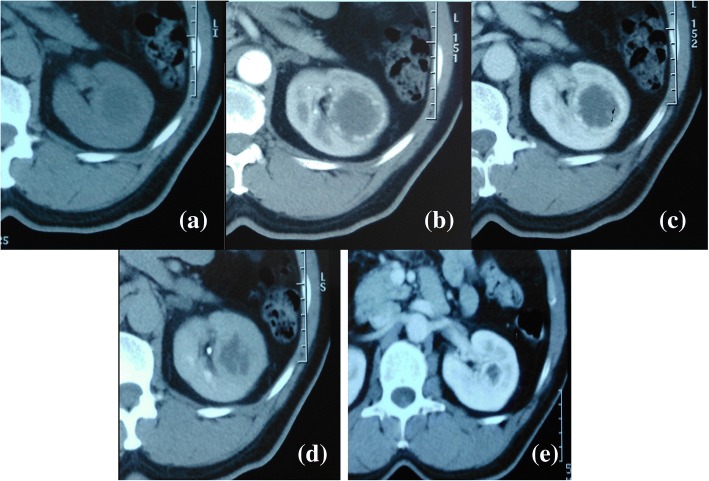
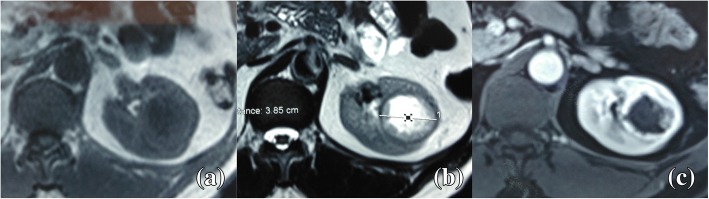
An abdominal computed tomography (CT) scan revealed a 38 mm left renal mid-pole lesion, isodense to the renal parenchyma. Dynamic CT showed an early intense and peripheral enhancement and nonenhanced central zone even in the delayed phase (Fig. 1). The renal artery and vein appeared normal. No metastases were demonstrated. We also recovered a CT realized 6 years ago in the surgery department, which illustrated the same lesion but 10 mm smaller (Fig. 1). For further characterization of the cyst, a magnetic resonance imaging (MRI) was performed. It revealed a lesion with thickened and irregular wall (from 3 to 10 mm) isointense on T1-weighted images and hypointense on T2- weighted images with intense enhancement. The central zone was hypointense on T1 and hyperintense on T2 with no enhancement (Fig. 2). Radiological findings concluded a Bosniak IV cyst. As this cyst type is considered clearly malignant, our patient was accordingly scheduled for surgery. A partial nephrectomy was considered technically difficult for this lesion, so he underwent an open left radical nephrectomy. His postoperative course was uneventful.
Fig. 1.
Computed tomography axial view of the tumor shows intense peripheral enhancement. a Non-enhanced computed tomography. b Arterial phase. c Portal phase. d Delayed phase. e The same lesion, 6 years ago
Fig. 2.
Magnetic resonance imaging axial view shows a hypointense mass on T1 (a) and hyperintense on T2 (b) with intense peripheral enhancement (c)
On gross examination, cut sections divulged a well-defined medio-renal hemorrhagic and brownish mass measuring 3 × 2.5 cm. Histological examination of the mass showed a mesenchymal proliferation arising from the wall of a large vessel and developing within its lumen. It was composed of hyalinized papillary and anastomosing channel-like structures that were lined by flat to plump endothelial cells with no atypia or mitotic activity (Fig. 3). An immunohistochemical study revealed diffuse staining of tumors cells for CD-31 and negativity for HMB-45 and cytokeratin (Fig. 3). The diagnosis of IPEH was retained. He was asymptomatic and no recurrence of the tumor has been detected during 9 years of regular clinical and radiological follow-up. Additional file 1 presents a timeline of the case.
Fig. 3.
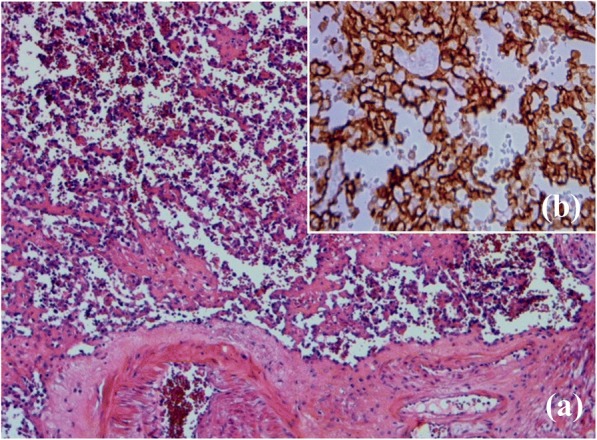
a The tumor composed of papillary and anastomosing channel-like structures (hematoxylin and eosin × 100). b Diffuse staining for CD31 (immunohistochemical × 200)
Discussion
Our case showed a Masson’s tumor of the kidney that presented as a suspicious cyst, with a slow growth over 6 years and 9 years of follow-up without recurrence. This is only the 12th renal case described in the literature (Table 1).
IPEH is a rare peculiar entity characterized by exuberant endothelial proliferation within the lumen of blood vessels [1]. Even though the precise etiology and physiopathology of IPEH remains undetermined and incompletely understood, many authors suggested that it could be linked to an alteration in the thrombosis process, due to an unusual thrombus organization [2]. Three types of IPEH have been defined: primary or pure type arising from dilated vessels; secondary or mixed type developing in pre-existing vascular lesions such as hemangioma; and third or extravascular type originating from hematomas [1]. In the kidney, IPEH can occur within vessels at many levels: the renal vein, the renal sinus, or the renal parenchyma per se [1]. IPEH has a frequent association with thrombus [2], but not in our case or in three others [1, 3, 4].
IPEH was mainly reported in the extremities skin and soft tissues. Solid organs have been rarely involved [5]. The first renal case was described by Garber et al. [6] in 1990 as a new renal lesion. IPEH generally occur at any age and most often in female patients [5]; however, a renal location seems to be involved more frequently in adult males than females (Table 1). Two cases were reported in patients with chronic kidney failure [7, 8]. The clinical manifestation of this tumor is not specific and varies widely. It can produce, as any other renal mass, flank pain, hematuria, or massive retroperitoneal hemorrhage or it can be asymptomatic and fortuitously diagnosed, as in our case. Lesion size ranges from 18 to 55 mm and is mostly localized in the left kidney.
In soft tissues, ultrasound shows typically a hypoechoic lesion and dynamic CT shows high peripheral enhancement of the lesion [9]. In the kidney, radiological features are non-specific to differentiate IPEH from other suspicious renal masses. However, in all cases, Masson’s tumor is located in the renal hilum or in the midportion of the kidney (Table 1), which should be considered in the diagnoses.
Preoperative diagnosis of renal IPEH was hard to carry out and this led to nephrectomy in all cases. It was managed by a partial nephrectomy as mentioned in three cases [1, 3, 10]. In other cases, radical nephrectomy was realized since tumors were located in the renal hilum.
Through Table 1, we can see that some features could evoke the diagnosis of renal IPEH. In that case, a lesion biopsy should be realized and the kidney could be spared. In our case, first, even though the slow growth of the tumor suggested a benign lesion, our decision to proceed in surgery was influenced by the radiological findings that indicated a Bosniak IV cyst, which is malignant in more than 90% of cases [11]. Second, we did not consider a biopsy because it was not recommended for cystic masses in the guidelines of that time [11].
Finally, neither metastases nor malignant degeneration has been reported with renal IPEH. There is only one case of recurrence, which occurred after a nephron-sparing surgery [10].
Conclusions
Masson’s tumor is a benign vascular degeneration. A renal localization for Masson’s tumor could barely be found in the literature. Preoperative diagnosis can be a real challenge. Nephrectomy was realized in all cases because of this entity’s non-specific radiological characteristics among suspicious renal masses.
Our case showed that Masson’s tumor can present as a suspicious renal cyst, an aspect that was not previously described in the few cases reporting this process in the kidney, and our literature review confirms that some features might evoke the diagnosis. Thus, this entity should be considered more often in the thick of the diagnostic possibilities in order to avoid unnecessary nephrectomies.
Additional file
Timeline of the case. (DOCX 14 kb)
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CT
Computed tomography
- IPEH
Intravascular papillary endothelial hyperplasia
- MRI
Magnetic resonance imaging
Authors’ contributions
MAE, conception and design of the study, analysis and interpretation of data, drafting the article, and revision and final approval of the version to be submitted. Abo, conception and design of the study, analysis and interpretation of data, drafting the article, and revision and final approval of the version to be submitted. ABl, histopathological analysis and interpretation of data, drafting the article, and revision and final approval of the version to be submitted. MG, acquisition of data, and revision and final approval of the version to be submitted. MCha, conception and design of the study, analysis and interpretation of data, and revision and final approval of the version to be submitted. ABM, radiological analysis and interpretation of data. HA conception and design of the study, analysis and interpretation of data, and revision and final approval of the version to be submitted. MCher, conception and design of the study, analysis and interpretation of data, and revision and final approval of the version to be submitted. MRBS, surgical performance, and revision and final approval of the version to be submitted. AD, conception and design of the study, analysis and interpretation of data, and revision and final approval of the version to be submitted. MCheb, revision and final approval of the version to be submitted. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical approval was not applicable for the case report. Written consent was obtained from the patient to participate in this case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamed Ali Essid, Phone: +216 20 966 145, Email: docdali86@gmail.com.
Abderrazak Bouzouita, Email: bouzouitabder@yahoo.fr.
Ahlem Blel, Email: blelahlem@gmail.com.
Maroua Gharbi, Email: marouagharb@gmail.com.
Marouen Chakroun, Email: marouenechakroun@gmail.com.
Aycha Ben Miled, Email: aycha.benmiled@gmail.com.
Haroun Ayed, Email: ayedharoun@yahoo.fr.
Mohamed Cherif, Email: drcherifmed@yahoo.fr.
Mohamed Riadh Ben Slama, Email: riadhbenslama@yahoo.fr.
Amine Derouiche, Email: amine_derouiche@yahoo.fr.
Mohamed Chebil, Email: mohamed.chebil.uro@gmail.com.
References
- 1.Mehta V, Ananthanarayanan V, Antic T, Krausz T, Milner J, Venkataraman G, et al. Primary benign vascular tumors and tumorlike lesions of the kidney: a clinicopathologic analysis of 15 cases. Virchows Arch. 2012;461(6):669–676. doi: 10.1007/s00428-012-1333-9. [DOI] [PubMed] [Google Scholar]
- 2.Akdur NC, Donmez M, Gozel S, Ustun H, Hucumenoglu S. Intravascular papillary endothelial hyperplasia: histomorphological and immunohistochemical features. Diagn Pathol. 2013;8:167. doi: 10.1186/1746-1596-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johraku A, Miyanaga N, Sekido N, Ikeda H, Michishita N, Saida Y, et al. A case of intravascular papillary endothelial hyperplasia (Masson’s tumor) arising from renal sinus. Jpn J Clin Oncol. 1997;27:433–436. doi: 10.1093/jjco/27.6.434. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, Park EJ, Lee JH, Nam JH, Lee MC, Park CS, et al. Intravascular papillary endothelial hyperplasia in the kidney of a child. Virchows Arch. 2000;436:398–400. doi: 10.1007/s004280050466. [DOI] [PubMed] [Google Scholar]
- 5.Moreillo-Vicente L, Gimenez-Bachs JM, Agusti-Martinez A, Salinas-Sanchez AS. Intravascular Papillary Endothelial Hyperplasia: Regarding a Case. Ann Clin Case Rep. 2017;2:1319. [Google Scholar]
- 6.Garber BB, Prestipino AJ, Pollack HM, Levine SR, Whitmore KE. Masson’s tumor of the kidney: a new renal lesion. J Urol. 1990;143:344–346. doi: 10.1016/S0022-5347(17)39956-1. [DOI] [PubMed] [Google Scholar]
- 7.Rizza V, Coletti G, Di Cocco P, Mazzotta C, Famulari A, Pisani F. Serious renal hemorrhage in Masson tumor. Transplant Proc. 2009;41(4):1402–1404. doi: 10.1016/j.transproceed.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Pelosi G, Sonzogni A, Viale G. Intravascular papillary endothelial hyperplasia of the renal vein. Int J Surg Pathol. 2011;19(4):518–520. doi: 10.1177/1066896909341800. [DOI] [PubMed] [Google Scholar]
- 9.Craig KA, Escobar E, Inwards CY, Kransdorf MJ. Imaging characteristics of intravascular papillary endothelial hyperplasia. Skelet Radiol. 2016;45(11):1467–1472. doi: 10.1007/s00256-016-2445-0. [DOI] [PubMed] [Google Scholar]
- 10.Alkan E, Sağlıcan Y, Özkanlı AO, Balbay MD. The first recurrent intravascular papillary endothelial hyperplasia (Masson's tumor) of the kidney. Turk J Urol. 2016;42(3):202–205. doi: 10.5152/tud.2016.43789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long JA, Neuzillet Y, Correas JM, de Fromont M, Lang H, Mejean A, et al. Atypical cysts and cystic tumours of the kidney: histological, radiological and surgical considerations. Conclusions of the AFU 2007 forum. Prog Urol. 2009;19(1):8–14. doi: 10.1016/j.purol.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Steffee C, Iskandar S. Intravascular papillary endothelial hyperplasia in a thrombosed renal allograft vein. Hum Pathol. 1996;27(9):986–989. doi: 10.1016/S0046-8177(96)90230-0. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bogaert S, Boel K, van Poppel H, Oyen R, Van Damme B. Masson’s tumour of the kidney. Cancer Imaging. 2002;2:116–119. [Google Scholar]
- 14.Akhtar M, Aslam M, Al-Mana H, Bamefleh H, Al-Khateeb SS, Lindstedt E. Intravascular papillary endothelial hyperplasia of renal vein: report of 2 cases. Arch Pathol Lab Med. 2005;129(4):516–519. doi: 10.5858/2005-129-516-IPEHOR. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Timeline of the case. (DOCX 14 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.