Abstract
Blue and near-ultraviolet structural colours have often been reported in understorey plants living in deep shade. While this intense blue coloration is very catchy to the eye of a human observer, there are cases in which structural colours can be hidden either by the scattered light interacting with pigments or because they are found in unexpected positions in the plants. Here, we show that the fronds of Microsorum thailandicum produce structural coloration on both the adaxial and abaxial epidermal surface. While cellulose helicoidal structures are responsible for this coloration in both epidermal layers, the reflected colours are consistently different: an intense blue reflection is found in the adaxial epidermis while red-shifted and less intense colours are observed in the abaxial epidermis, possibly suggesting photo-adaptation of the plant to the light environment. By comparing the optical properties of the fern with its anatomy we computed the theoretical reflection accounting for the presence of disorder in the cellulose helicoidal architecture.
Keywords: structural colour, plant cell wall, cellulose helicoidal architecture, Microsorumthailandicum, circular polarization, iridescence
1. Background
Structural colours are extremely widespread in nature [1–4]. They do not derive from pigments, but rely on constructive interference of light scattered from nanostructures, with dimensions of the order of the wavelength of visible radiation, 400–700 nm [1]. Often, structural coloration can be more intense than coloration by pigments, and can be dependent on the angle of observation. This effect of the reflected wavelength being angle-dependent is called iridescence [3,5]. From flowers [6,7] to fruits [8–10] to leaves [11,12], such brilliant colorations are observed in several plant tissues with different biological functions [13,14], and using several morphologies [1,15]. A common architecture to produce structural colour that is found in several plant tissues consists of cellulose microfibrils assembled into helicoidal architectures in the cell wall [16]. The inherent birefringence of cellulose microfibrils and their chiral spatial organization provide a circularly polarized light reflection in a range of wavelengths which are determined by the dimensionality (referred to as pitch) of the helicoid [17]. In more detail, these helicoidal architectures are composed of different layers or pseudolayers of cellulose microfibrils oriented parallel to each other. These layers are stacked up, with a small rotation angle between them. After every 180° rotation, the microfibrils have the same orientation. The distance between two equally orientated layers is defined as the pitch p, and it is related to the reflection maximum λ via , where n is the average refractive index of the medium.
Colour-generating cellulose-based structures are found in the cell wall of many different plant tissues like leaves and fruits [2,18,19], and similar architectures made of chitin have been found in beetles [16,20–22]. In plants, they have been observed via transmission electron microscopy (TEM) imaging in the juvenile fronds of the fern Danaea nodosa [12], via spectroscopy and electron microscopy in the leaves of the Malaysian rain forest understory plants Lindsaea lucida and Diplazium tomentosum [23], and in the leaves of the tropical rainforest understorey sedge Mapania caudata [11]. In fruits, helicoidal cellulose microfibril architectures have been described in the secondary cell wall of the pericarp of the monocot Pollia condensata [8], and in the secondary cell wall of the endocarp of the dicot Margaritaria nobilis [9].
However, in leaves, these helicoidal structures have so far only been observed in the adaxial epidermal cell walls and their colour is prevalently in the blue and near UV spectral region. Here, we studied the optical properties and anatomy of Microsorum thailandicum, a member of the Microsorum punctatum complex of the large fern genus Microsorum [24–26]. Microsorum thailandicum was described as iridescent by Boonkerd & Nooteboom [24], but the origin of the structural colour was still unclear. In our investigation, we observed that both the adaxial and abaxial epidermal cells contain helicoidal cell walls reflecting blue and green-to-red circularly polarized light, respectively. Finally, by performing electron microscopy and (micro-)spectrophotometry on the same area, we quantitatively correlated the measured reflectivity with the anatomy of the structures, providing an understanding on how the structural disorder affects the optical properties of the fern.
2. Methods
2.1. Plants
Plants were either grown in an office environment or in a growth cabinet set to 25°C and lowest light option (approx. 2000 lux illuminance) during the day, for 16 h, and 20°C and darkness during the night (Panasonic Versatile Environment Test Chamber MLR-352-PE, Panasonic Corporation, Japan). They were watered from below once a week and misted daily.
2.2. Photography
Photos of the fern and its fronds were taken with a Nikon D3200 camera (18-55 VR II kit, AF-S DX Nikkor 18–55 mm f/3.5-5.6G VR II, Nikon, Japan), in macro mode, and automatic focusing, using a tripod. A linear polarizer was added to reduce gloss from the cuticle (Hoya CIR-PL slim, Hoya Corporation, Japan).
2.3. Optical microscopy
2.3.1. Cross section of fronds
A TEM block was made via high-pressure freezing and freeze substitution (§2.4) and sliced into semi-thin cross sections with a Leica Ultracut E ultramicrotome (Leica Microsystem GmbH, Austria). It was stained with Richardson’s stain and observed in transmission microscopy on a Zeiss microscope and 5× objective to investigate the ultrastructure of the frond.
Thickness of fronds. The thickness of the same fronds used for the gradient investigation (comparison of reflection intensity between fronds, see §3.3) and integrating sphere measurements (for total transmission and reflection, see electronic supplementary material) was determined by cutting two thin slices in the same tip, middle and base area with a razor blade. Per cross section, six thicknesses were measured, three on each side of the frond. They were measured at equal spacing across the area where the frond surface was horizontal (since spectra were also generally taken from that area), aligned with the shortest distance at this cross-sectional point. Images of freshly cut cross sections of fronds to obtain their thickness were recorded with a Zeiss stereoscope and processed with ImageJ [27,28]; see §3.3 and electronic supplementary material.
2.3.2. Polarized optical microscopy and micro-spectroscopy
Optical microscopy was carried out on a customized Zeiss microscope equipped with epi-illumination and a 5×, 20× and 50× objective. Different configurations were used. For polarized optical imaging, the sample was illuminated with an unpolarized halogen lamp, and a polarizer and a quarter-waveplate were mounted into the collection optical path, and left and right channel configuration was obtained by independent motors. For images of cells, the 20× objective was used. Spectra were collected via a 100 µm optical fibre mounted in confocal configuration to the focal plane of the objective and connected to a spectrometer (AvaSpec-HS2048 spectrophotometer, spectral range 350–800 nm and resolution of 5 nm). Investigation on the single-cell level was achieved with the 50× objective and the 100 µm optical fibre for collecting spectra. In-house software controlling a motorized stage was employed to scan a single cell with high spatial resolution.
Three fronds from one plant were investigated for the statistics of optical response study, to guarantee equivalent growth conditions.
Three fronds from another plant were investigated with respect to the gradient of structural colour observed, and whether both the adaxial and abaxial surfaces are always both coloured. Three spectra from the tip, the middle and the base of each frond, adaxial and abaxial surface, were taken.
All fronds were imaged while still on the plant, or alternatively the entire frond was cut off and imaged straight away without making any further cuts on it. Generally, upon being cut off and cut into pieces, the fronds will dry out, the coloration decreases and finally disappears within a few hours.
2.4. Electron microscopy
2.4.1. Transmission electron microscopy
Sample embedding by high-pressure freezing and freeze substitution. 3 mm circular frond samples were cut, placed within brass specimen carriers and loaded into a Leica EM ICE high-pressure freezer, followed by immersion in liquid nitrogen and freeze substitution (Leica AFS2, Leica Microsystems GmbH, Germany). The frozen samples still in their carriers were placed inside 2 ml capped cryovials with 1 ml of acetone at the surface of a bath of liquid nitrogen to avoid warming of the samples. The tips of the tweezers were similarly cooled before use. Samples were brought up to room temperature over 4 days after which they were transferred to 100% ethanol, followed by a resin series (alcohol : medium grade LR White resin ratios of 3 : 1, 1 : 1, 1 : 3 and 100% LR White resin). The resin was changed daily over 4 days after which the specimens were placed in gelatin capsules and polymerized in a Fistreem vacuum oven (digital, Fistreem International Limited, UK) at 60°C and 440 mmHg for 22 h. TEM blocks produced via high-pressure freezing and freeze substitution were only used for ultrastructure investigations by optical microscopy.
Sample embedding by chemical fixation. Small pieces of native, hydrated plant tissue were cut and entirely immersed in a buffered fixative solution containing glutaraldehyde (2 wt%) and formaldehyde (2 wt%) for 16 h at 4°C. The specimens were then rinsed with deionized water and fixed for 2 h at 4°C in a buffered OsO4 solution. The specimens were rinsed again in deionized water and successively dehydrated in graded ethanol aqueous solutions (30–100 wt%) and then dry acetonitrile. They were incubated for 16 h in a 50 : 50 (v/v) mixture of acetonitrile and Quetol 651 epoxy resin, and subsequently immersed in Quetol resin for two weeks, allowing the resin to infiltrate into the specimens. The specimens were placed in a silicon mould with Quetol resin and cured for 48 h at 65°C. Finally, ultrathin sections were prepared using an ultramicrotome (Ultracut UCT, Leica Microsystem GmbH, Austria) equipped with a 35° diamond knife (Diatom, USA) and mounted on continuous carbon coated copper grids. The sections were then post-stained with 1 wt% uranyl acetate aqueous solution and Reynolds lead citrate solution. TEM observations were carried out with a Philips CM-200 ‘Cryo’ electron microscope operated at 200 kV (Thermo Fisher Scientific Inc., USA). All TEM imaging was carried out on blocks made via chemical fixation.
2.4.2. Cryogenic scanning electron microscopy
Cryogenic scanning electron microscopy (cryo-SEM) observation was performed using a field-emission scanning electron microscope (Verios 460, Thermo-Fisher Scientific Inc., USA) equipped with a cryo-preparation system (PP3010T, Quorum, UK). The frond was cut into a small strip and mounted upright on a specimen holder using a colloidal graphite suspension. The specimen was quench-frozen in liquid ethane and transferred into the cryo-preparation chamber, where it was freeze-fractured, sublimed and subsequently sputter-coated with platinum. SEM imaging was carried out at an acceleration voltage of 2 kV and a working distance of approx. 4 mm.
Before cryo-SEM, a few millimetre wide section of the frond was marked off and polarized optical microscopy was carried out on it, so that the optical response could be correlated to the same section of the frond that the cryo-SEM measurements are from. This way, the modelling approach is based on the same small area of the frond.
2.4.3. Block-face scanning electron microscopy
A smooth surface of resin embedded specimen was prepared using an ultramicrotome (Ultracut UCT, Leica Microsystem GmbH, Austria) for block-face SEM observation. SEM imaging was carried out using a concentric backscatter detector on a field emission scanning electron microscope (Quanta 250, Thermo-Fisher Scientific Inc., USA) operated at 4 kV with a working distance of 7 mm.
2.5. Data and spectra processing
Matlab was used for all data and spectra processing.
Spectra were always referenced to a white diffuser (USRS-99-010, Labsphere, USA), except for the ones used for modelling, which were referenced to a silver mirror (PF10-03-P01, Thorlabs, USA). When referencing to a white diffuser, it is not unusual to obtain reflection intensities higher than unity, in the case that the reflection of the sample is strong and more directional than the white diffuser. Referencing to a silver mirror gives an absolute measure of reflectivity, since all incident light is captured within the numerical aperture of the optical fibre. This is the same referencing method as the one assumed for the modelling.
For the gradient analysis, the spectra of three cells at the tip, the middle and the base of each frond were averaged, for both adaxial and abaxial surface, and a high, a medium and a low structurally coloured frond, respectively.
To obtain histograms of the peak maxima, the inbuilt Matlab ‘maxvalue’ function was used. To obtain the full width at half maximum (FWHM), we used the Matlab function ‘findpeaks’. The distribution of peak positions was then fitted with a Gaussian distribution, and the distribution of peak widths (FWHM) with a lognormal distribution. For obtaining the average spectra, all spectra of the same surface were averaged.
For the analysis of pitches from the cryo-SEM and block-face SEM images, the ImageJ greyscale function was used to count layers. Three lines were measured per cell (corresponding to one SEM image), on the left, middle and right, and these values were averaged, to obtain the average and standard deviation (plotted as error bars) per cell.
Finally, individual spectra are plotted for the modelling section, to demonstrate how much heterogeneity and how many different spectral features are found for different cells.
2.6. Modelling
The freely available Python implementation of Berreman 4×4 (21 August 2016 on github [29]) was used for all simulations, using Python v. 3.6. We tried out different combinations of parameter fitting of twist defects, pitches and normal and extraordinary refractive indices in order to match the experimental spectra. The total number of half pitches was fixed to 80 to resemble a total cell wall height of approx. 10–15 µm. We used the discrete sum of absolute differences between spectrum and simulation of the main peak as the fitting objective (also called the L1 norm). We fitted only to the main peak since we could not reproduce all spectral features using the Berreman model. It is important to keep in mind that this approach influences the results, but if the main peak is caused by a regular part—potentially located in a more complex structure—then we still obtain a reasonable indication of refractive indices. Since the optimization problem was expected to have many local minima, we tried the different global optimization algorithms implemented in the Python library SciPy 0.19. We found that basinhopping worked best for this case and gave most consistent results throughout multiple runs. Convergent results were normally observed within a few minutes, and the optimizer was therefore manually terminated. We found that refractive index parameters of no = 1.528 + 0.0075i and ne = 1.474 + 0.0075i gave a peak height and width that most closely fitted the shape of the main peak of the measurements. Since we do not have enough information to predict the dispersion, and it is expected to be low [30], none was assumed. The refractive indices are close to reported parameters for other helicoidal systems [30,31]. In trying to fit to more complex spectral shapes, we did not obtain any better fit (with realistic parameters) that resembled the shape of the recorded spectra.
3. Results
3.1. Optical response of frond
The optical response of the fronds of M. thailandicum was studied using a customized microscopy setup which allows one to simultaneously image the epidermis of the frond in different polarization configurations and to collect spectra in the corresponding imaged area.
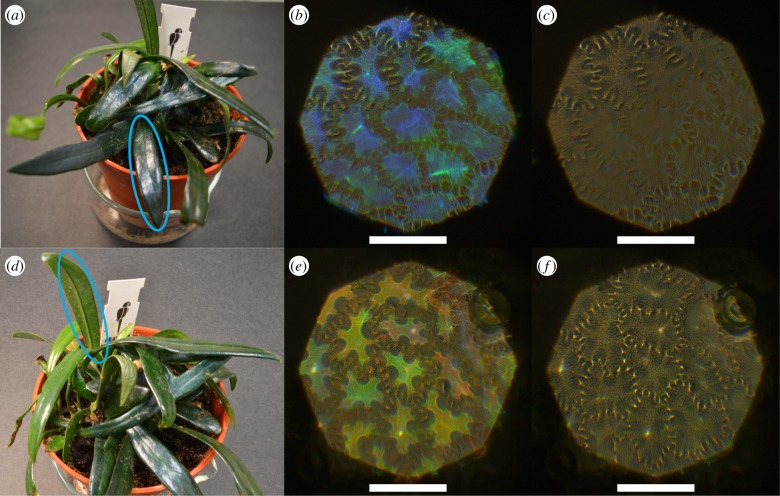
Figure 1a shows a photo of the plant; there are several fronds with an intense blue coloration. Figure 1b,c depicts epi-illumation microscope images of the same area of the adaxial epidermis of a blue frond in the left and right circular polarization channels (LCP and RCP), respectively. The blue colour, corresponding to the position of the cell in the epidermal layer, is visible only in the LCP, but not in the RCP (figure 1b,c), indicating the presence of a helicoidal structure in the cell wall of the outer epidermal cell. Interestingly, the abaxial epidermis displays similar properties: a clear LCP reflection but no RCP reflection is observed from the cells in the green and red spectral region, see figure 1e,f, even though the abaxial surface appears non-iridescent by naked eye, as seen on the encircled area in figure 1d.
Figure 1.
(a) Photo of M. thailandicum; adaxial surface encircled in blue was studied. (b,c) Optical micrographs of reflection in left-handed (LCP) and right-handed circular polarized light channel (RCP) of adaxial surface. (d) Photo of M. thailandicum; abaxial surface encircled in blue was studied. (e,f) Optical micrographs of reflection in LCP and RCP of abaxial surface. Scale bar is 100 µm.
The overall coloration is a combination of structural and pigment colour. The main pigment is chlorophyll and reflects in the green. Since most of the structural colour of the abaxial surface is in a similar range, it is not easily visible by eye.
3.2. Anatomy of frond
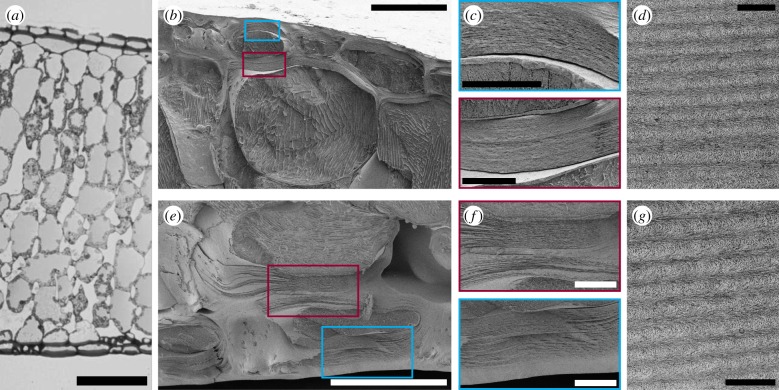
Owing to the interesting optical appearance, the ultrastructure of the frond was investigated to locate and confirm the helicoidal architecture. Figure 2a depicts an optical transmission image of a semi-thin cross section of an embedded frond. Both on the adaxial and abaxial surface, the upper or lower epidermis are clearly visible, the cell walls of these cells look thickened, and the epidermis is covered with the cuticle. The mesophyll consists of the palisade tissue towards the adaxial surface, and the spongy mesophyll towards the abaxial surface.
Figure 2.
(a) Optical transmission micrograph of semi-thin cross section of embedded frond. Scale bar is 200 µm. (b,e) Cryo-SEM images of adaxial and abaxial epidermal cells of the frond, respectively. Scale bar is 50 µm. (c,f) Zoom of epidermal cell walls in the blue/red boxes for adaxial and abaxial epidermis, respectively. Scale bar is 10 µm. (d,g) TEM images of adaxial and abaxial cell wall, respectively, showing the Bouligand arcs characteristic for the helicoidal arrangement of cellulose microfibrils. Scale bar is 500 nm.
Zooms of the adaxial and abaxial epidermal cells via cryo-SEM are shown in figure 2b,c,e,f, respectively. By increasing the magnification, it is possible to observe a thickened cell wall with a layered structure for the outermost layer of cells in the two epidermises. Furthermore, the same thickening with layered structure can also be observed in the surface-facing side of the cell walls of the second epidermal layer; see red boxes (b,c and e,f). Although ice crystal artifacts are visible in the cell (figure 2b,e), the ultrastructure of the epidermal cell walls is considered to be preserved during the sample preparation for cryo-SEM measurements. This is because the growth of ice crystals is likely minimal in the observed area as the secondary cell wall is generally less hydrated than other parts of plant cells, and the epidermal cells are located in the outermost layer of the specimen [32]. TEM imaging was used to further investigate these regions, see figure 2d,g, where the Bouligand arcs characteristic for the helicoidal arrangement of cellulose microfibrils in the cell wall are observed [22,33].
3.3. Variation between fronds
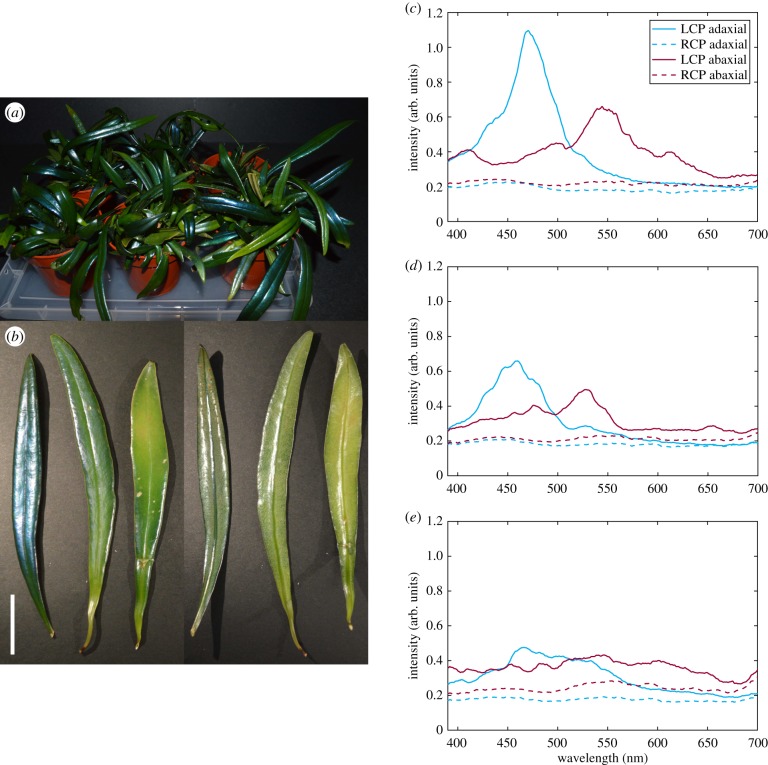
When looking at a number of plants (figure 3a), we noticed that, even though the macroscopic appearance of a single frond is fairly homogeneous, there is a large amount of variation in intensity of structural coloration between different plants, and even between different fronds on the same plant. Whether and how intensely fronds develop structural coloration probably depends on a variety of factors, like temperature, light and humidity, which we were not able to fully control over a long enough period of time. To investigate this variation, we studied the intensity and spectral variation of the structural colour in representative fronds from the same plant. The fronds were chosen to have one frond show very intense blue structural coloration on the adaxial surface, one that almost did not show any, and one in between the two extremes. To account for the age of the frond, we collected fronds with comparable stiffness, length and thickness. In more detail, the collected fronds had a thickness of (0.92 ± 0.20) mm for the intensely structurally coloured one, the medium one of (1.00 ± 0.20) mm, and the low coloured one (1.07 ± 0.27) mm.
Figure 3.
(a) Photo of a variety of specimens. (b) Photo of the adaxial (left) and abaxial (right) surface of three fronds showing high, medium and low structural coloration, from left to right. Scale bar is 2 cm. (c–e) Average reflection spectra in LCP and RCP of frond, adaxial and abaxial surface. (c) Spectra of the most intensely structurally coloured frond. (d) Spectra of the medium structurally coloured frond. (e) Spectra of the low structurally coloured frond. All spectra are averaged from measurements of nine cells each across the frond.
In particular, we investigated the gradient of structural colour observed, and whether both the adaxial and abaxial surfaces are always both coloured with the same intensity (figure 3b). As visible in the photograph, the spectra of the most intensely coloured frond showed the highest intensity, the medium coloured frond medium intensity, and the low coloured frond the least intensity of reflection. This trend was observed for both the adaxial and the abaxial surface and structural coloration for both epidermises correlate in intensity (figure 3c–e).
Additionally, integrating sphere measurements of areas of a few millimetres from very strongly and very low structurally coloured fronds were also performed to estimate the total transmission through and reflection of the fronds and the adaxial and abaxial epidermis separately; see the electronic supplementary material.
3.4. Variation of the optical response within the same frond
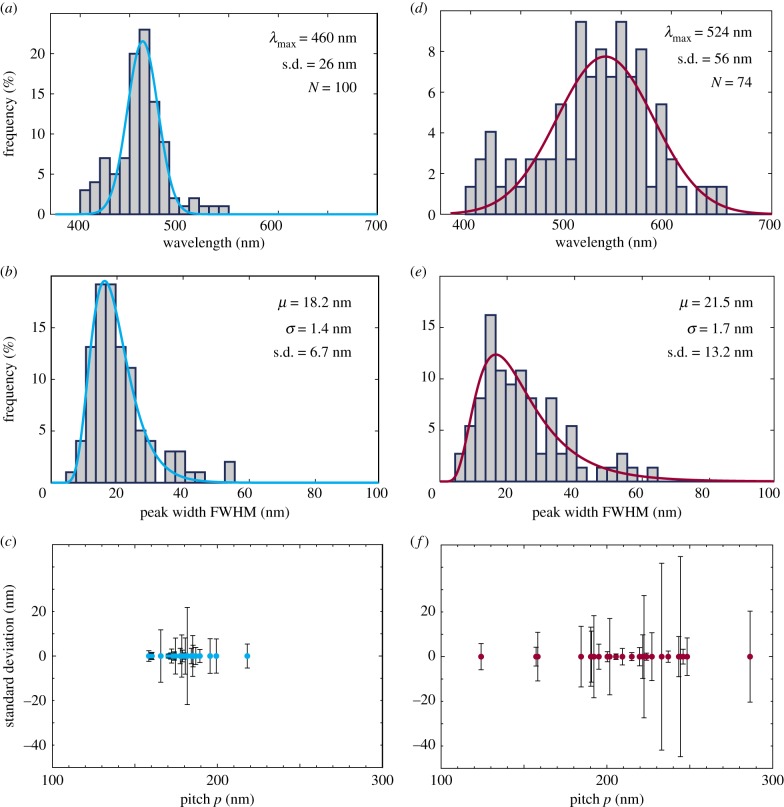
Additionally to the variation of macroscopic appearance of the fronds, variation between the individual cells on each frond is revealed by optical microscopy (figure 1b,e). To characterize this variation, we statistically analysed the optical response of 100 cells of the adaxial surface from the strongly structurally coloured frond shown in figure 1a, and 74 cells of the abaxial surface from the strongly structurally coloured frond shown in figure 1d. Firstly, the maximum of each LCP reflection was determined for both adaxial and abaxial epidermal cells, and these values were plotted in a histogram, shown in figure 4a,d, respectively. The distribution of the maximum reflection wavelength of the peaks is well approximated with a Gaussian distribution, while the distribution of the FWHM of the peaks shown in figure 4b,e is approximated with a lognormal distribution.
Figure 4.
(a–c) Adaxial surface. (a) Distribution of peak wavelengths from structurally coloured cells of frond. (b) Distribution of peak widths as determined by finding the FWHM. (c) Distribution of pitches p and their standard deviation measured from the layering visible in the uppermost helicoidal cell wall measured from block-face SEM and cryo-SEM images. (d–f) Abaxial surface. Same as for adaxial surface. (Online version in colour.)
Interestingly, the variation of reflected colours is much narrower for the adaxial surface than for the abaxial surface. For the adaxial surface, all reflection maxima are found between 400 and 550 nm. The Gaussian distribution gives a mean value of 460 nm, and a standard deviation of ±26 nm. This corresponds very closely with the averaged spectrum of all 100 cells, which has a maximum reflection at 461 nm, see the electronic supplementary material. Furthermore, the lognormal fitting of the FWHM histogram yields the values 18.2 nm and 1.4 nm for the mean μ and for σ, giving a standard deviation of ±6.7 nm. On the other hand, the reflection of the abaxial epidermis varies much more, all the reflection maxima are found between 400 and 650 nm; so almost over the entire visible spectrum. When sorting the reflection maxima into a histogram, the approximated Gaussian distribution yields a mean value of 524 nm and a standard deviation of ±56 nm. Correspondingly, the shape of the average abaxial reflection spectrum is very wide, and the reflection maximum lies at 511 nm; see the electronic supplementary material. Moreover, μ, σ and the standard deviation for the lognormal distribution of peak widths are 21.5, 1.7 and ±13.2 nm, respectively, also showing greater variation than for the adaxial epidermis.
In order to compare these observations to the anatomy of the fronds, we investigated the helicoidal architecture on an individual cell level by electron microscopy. Representative block-face SEM images of the adaxial and abaxial outermost thickened epidermal cell wall used to measure the pitch p are shown in the electronic supplementary material. The pitch p is the height of the helicoidal axis within which the cellulose microfibrils complete a 180° rotation.
The pitch p lies between 150 and 220 nm for the adaxial outermost thickened epidermal cell wall, with most values between 160 and 200 nm, with the standard deviation within each cell below ±22 nm, typically below ±10 nm (figure 4c; from 24 cells). For the abaxial outermost thickened epidermal cell wall, the pitch p is spread out over a much bigger range, between 120 and 290 nm with most values between 150 and 250 nm, and the standard deviation within each cell is bigger as well, up to ±42 nm (from 25 cells), as depicted in figure 4f .
The average refractive index n of the medium can be approximated to 1.50, by considering that the typical value of the refractive index for pure crystalline cellulose is 1.55 [17], and an average value of 1.45 from the other cell wall components, namely cellulose microfibrils with disordered surface, hemicellulose, lignin, water, small amounts of protein, etc. [34].
Even if the calculated reflection maxima from the pitch data are higher for both adaxial and abaxial cell walls, which is unusual since one may expect shrinkage of structures during the chemical TEM sample processing [35,36], we observed the same trends for both surfaces, using both techniques (cryo-SEM and block-face SEM). We can account for this discrepancy by considering that we are overestimating the pitch by approximately 10%. We believe that the main reason that leads to the measurement of a larger pitch, both with cryo-SEM and block-face SEM, is due to the not perfectly perpendicular cuts with respect to the direction of the helicoidal axis and due to the not perfectly perpendicular measurement direction when processing the images. If the measurement direction is perfectly aligned with the helicoidal axis, the value of the pitch is correct, but any deviation from perfect alignment will always lead to an overestimation of the pitch [37]. While utmost care was taken during measurements and during image processing in ImageJ, contributions from this issue cannot be fully excluded, also since the axis of the cell and therefore the helicoids is different from cell to cell and can also change slightly within the cell.
3.5. Variation within single cell
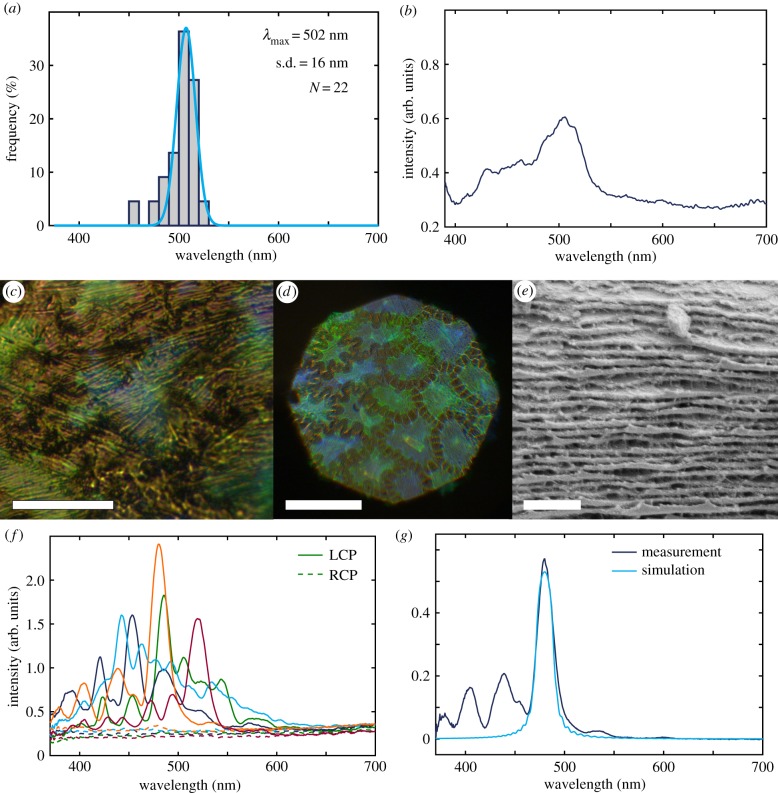
To investigate the disorder in the distribution of the pitch, a single cell of the adaxial epidermis was scanned with larger spatial resolution (see figure 5c). Figure 5a,b reports the statistical analysis of the measured reflection spectra (22 in total) as processed in the same way as in §3.4 and in the electronic supplementary material, reporting the reflection maxima in a histogram and the average of all spectra. The average of all 22 spectra of this cell gives a maximum of 506 nm, again corresponding closely with the histogram. Furthermore, all the obtained reflection spectra fall well within the range observed for the previous measurement of 100 adaxial cells across a frond. This means that the variation is the same on a frond, between different cells, as it is within a single cell.
Figure 5.
(a) Reflection maxima distribution of single cell. (b) Average spectrum of all 22 LCP spectra from single cell. (c) Optical micrograph of reflection in LCP of single cell of adaxial surface. Scale bar is 20 µm. (d) Optical micrograph of reflection in LCP of adaxial surface, specific part of frond for cryo-SEM. Scale bar is 100 µm. (e) Cryo-SEM micrograph zoom of the helicoidal layering, adaxial surface. Scale bar is 1 µm. (f) Individual reflection spectra in LCP and RCP of five cells of a specific part of frond taken for cryo-SEM, adaxial. (g) Measurement taken from a single cell and simulation based on the Berreman 4×4 method (referenced to silver mirror).
3.6. Modelling the optical response
Next, a very small area of a frond was marked off and subjected both to polarized optical microscopy and then cryo-SEM analyses. A representative optical micrograph of the LCP is shown in figure 5d, and example spectra from that area are shown in figure 5f, while a cryo-SEM image of the layered cell wall of the same area is shown in figure 5e. The variation of the pitch p measured from this frond area was then used to model the optical response (figure 5g).
We modelled the circularly polarized spectral reflection using an open source Python implementation of Berreman’s 4×4 matrix method that simulates stratified (layered) anisotropic media [29,38]. This is the most common approach for simulating helicoidally arranged cellulose microfibrils [30]. In figure 5g, an ideal helicoidal arrangement of cellulose microfibrils with 40 half pitches of 159.9 nm and refractive indices of no = 1.528 + 0.0075i and ne = 1.474 + 0.0075i was simulated. The figure shows that the main reflection peak position, height and width can be roughly captured, but also that the recorded spectra are much more complex. This is probably due to significant deviations in the helicoidal twist of the cellulose layers from an ideal helicoidal structure (twist defects, varying pitch, etc.) and other geometrical artifacts (curvature of cells, non-planar cellulose layers, etc.). To obtain more information on the arrangement of the cellulose stack, we furthermore tried to use the classifications proposed by Carter et al. for a non-ideal helicoidal reflector in beetles. The best matching classification is ‘Spectra with diminishing oscillations’, but such spectra are not well described by a few local defects or pitch changes, as described in their supplementary information [39]. Furthermore, looking at individual spectra from several cells (figure 5f), we also found that no single classification fitted them all. We therefore conclude that the spectral features indicate a large degree of disorder distributed throughout the cell wall and are not localized to a few defect sites or abrupt pitch changes. This inference corresponds well with the observations made from electron microscopy imaging (e.g. figure 5e).
4. Discussion
4.1. Colour variation and plant cell wall biosynthesis
Our systematic statistical investigations allow us to conclude that, despite the variation in the reflection response from different fronds in the plant, the reflection maxima of the adaxial epidermis are much narrower with less variation between cells and within cells, compared to the abaxial epidermis. These observations have interesting implications for the biosynthesis of the adaxial versus the abaxial epidermal cell walls. For the abaxial cells, there is much more variation between the different cells, and the range of reflected colours is larger, hence the biosynthesis of the plant cell wall is presumably less orderly regulated than for the adaxial surface. Furthermore, when the reflection wavelength lies at the green or red end of the visible spectrum, it means that the cellulose microfibrils in the helicoidal cell wall architecture have to be spaced further apart than when reflecting in the blue range. We do not know what exactly is used as a spacer between the layers of parallel cellulose microfibrils, but there are at least three different options. (i) Hemicellulose and lignin content: we think it is most likely that hemicellulose or lignin or both act as a spacer between the cellulose microfibrils. This would mean that in green and red cells, more material, like hemicellulose or lignin is deposited. Quantifying the hemicellulose and lignin content (ideally at a single-cell level, but at least for adaxial and abaxial epidermis separately) could give some insights on whether they act as a spacer, in which case their biosynthetic pathways should be investigated further. (ii) Rotation angle: another option is that the rotation angle between the cellulose microfibrils is smaller in the abaxial epidermal cell walls. In this case, more layers of cellulose microfibrils would be layered up and would thus increase the pitch, resulting in a shift of reflection to longer wavelengths. If there is a smaller rotation angle between cellulose microfibrils, a higher cellulose content in the abaxial epidermis could be observed. To investigate this hypothesis, the cellulose content should be investigated for the two epidermises separately. (iii) Water content: the third option is a difference in water content. The abaxial epidermis does seem more hydrated than the adaxial when preparing TEM specimens, and dehydration does affect the structural coloration of the fronds, making higher water content in the abaxial cell walls an option.
4.2. Influence of disorder on optical response
The model adopted so far to systematically analyse the optical response by helicoidal structures does not encompass all factors contributing to the spectral response. By trying to fit our spectra to the model developed to take into account defects and irregularities in spectra of helicoidal beetle cuticles [39], we were not able to reproduce all the measured spectral features. Therefore, we conclude that a few discrete defect sites do not dominate the reflection spectrum of the plant cell walls. Rather, we expect the reflection to be caused by a more complex and distributed disorder in the cell walls. Looking at spectra from several cells, we also found that no single classification from Carter et al. fitted them all, suggesting that the spectral features indicate a large degree of disorder distributed throughout the cell wall and not localized to a few defect sites or abrupt pitch changes. This conclusion is also supported by our electron microscopy imaging, where a lot of small irregularities in the layering can be observed. Furthermore, in our statistical analysis of the variation of reflections on the same frond, we found that the peak widths (FWHM) follow a lognormal distribution, rather than a Gaussian. This hints to the concept that many small defects are found increasing the peak width, and they add up in a logarithmic way.
4.3. Photosynthesis and light harvesting
We speculate that the transmitted light through the adaxial epidermis and mesophyll (including chlorophyll) could then be reflected back into the mesophyll by the abaxial epidermis. So while the mainly blue reflection of the adaxial epidermis could protect the frond from photo-damage in high light conditions, the abaxial epidermis could increase light harvesting in low light conditions, by reflecting parts of the light back into the mesophyll, that would have otherwise been transmitted and lost for photosynthesis. However, investigating this hypothesis experimentally has proved difficult for a number of reasons. (i) Separating the different layers: unfortunately, it is not possible to remove either of the epidermal layers without damaging the mesophyll. Ideally, we would have measured total transmission through the native frond, and then removed the abaxial epidermis and measured transmission through the adaxial epidermis plus mesophyll. This issue could be circumnavigated by just removing the respective other epidermis, taking spectra thereof and then subtracting them from the spectra of the native frond, but there are additional complications. Even though it is possible to remove either epidermis intact and carefully scrape off remaining mesophyll tissue with a razor blade, even after rinsing, there is always a small amount of chlorophyll left on the epidermis, which is impossible to remove fully without destroying the epidermis, and which is impossible to quantify. This small amount of chlorophyll will always influence measurements in a non-controllable way. (ii) Different amounts of chloroplasts: fronds will have different amounts of chlorophyll, and possibly varying ratios of the different types like chlorophyll a and b [40,41]. (iii) Different thicknesses of fronds: we tried to only pick mature fronds of similar length for analysis to keep results comparable (younger fronds are less stiff and still more flexible), but the plants grow really slowly, and the fronds possibly thicken with age. (iv) Curvature of fronds: the curvature of fronds varies considerably, and with it the surface area which is horizontal. This surface property will especially influence reflection properties, unless it is possible to decrease the spot size enough, in which case a large amount of measurements is necessary to obtain meaningful statistics. While the issue of the curvature of fronds (iv) could be circumvented by investing a large amount of working hours, tackling the issues of different amounts of chloroplasts (ii) and the different thicknesses of fronds (iii) are more challenging. Carrying out the integrating sphere measurements requires separating the three layers, and the mesophyll is always destroyed in the process. Hence, the thickness and chlorophyll content could not be determined on the same area of frond either way. Again, carrying out these analyses on a large amount of fronds to obtain meaningful statistics to then relate to any part of frond might be an option.
From our transmission data of the native fronds, we observed that there is very little transmission for most part of the visible spectrum, except for a small spectral area peaking at 550 nm, approximately from 510 to 590 nm. We found this interesting, since the area more or less coincides with the main reflection range of the abaxial epidermis. At the same time, however, there is also an absorption minimum for chlorophyll in this range [40,41], suggesting that there possibly is no optical function of the abaxial reflection.
5. Conclusion
In conclusion, we observed that the cell walls of M. thailandicum produce structural coloration on both the adaxial and abaxial epidermal surface by a helicoidal architecture of cellulose microfibrils. While there is a large variation in the optical response of the fronds, we find significant trends in the response of the adaxial and abaxial epidermis: the adaxial cell walls cause a much more well-defined reflection than the abaxial cell walls. While the biosynthesis of the plant cell wall and the biological significance of the differences between adaxial and abaxial epidermis are still far from being understood, we speculate that there might be a function in such an optical response, and suggest that there is still a lot to do to understand the strategies that plants use to manage light transport in their tissues.
Supplementary Material
Acknowledgements
We thank Bonan Zhu and Gen Kamita for development of software for hyperspectral imaging of the single cell. Y.O. thanks the NanoBio-ICMG platform (FR 2607) for granting access to the electron microscopy facility.
Data accessibility
Additional data are available at: https://doi.org/10.17863/CAM.26156.
Authors' contributions
L.M.S., H.W. and S.V. designed research; L.M.S., Y.O., V.E.J., C.R.L. and S.V. performed research; L.M.S., Y.O., V.E.J. and S.V. analysed data; L.M.S. and S.V. led the writing of the manuscript; Y.O., V.E.J., C.R.L. and H.W. contributed to discussions. L.M.S. planned experiments, performed optical microscopy and photography, analysed and processed data. Y.O. performed electron microscopy. V.E.J. performed simulations, automated data acquisition, data processing and data analysis. L.M.S. and S.V. performed integrating sphere measurements.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the European Research Council (ERC-2014-STG H2020 639088), the BBSRC David Phillips Fellowship (BB/K014617/1), the EPSRC (EP/N016920/1) and the European Commission, Marie Curie Fellowship (LODIS 701455).
References
- 1.Johansen VE, Onelli OD, Steiner LM, Vignolini S. 2017 doi: 10.1007/978-3-319-74144-4_3. Photonics in nature: from order to disorder. In Functional surfaces in biology III , vol. 10 (eds SN Gorb, EV Gorb), pp. 53–89. Cham, Switzerland: Springer International Publishing. ( ) [DOI]
- 2.Lee DW. 2007. Nature’s palette: the science of plant color. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424, 852–855. ( 10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 4.Parker AR. 2000. 515 million years of structural colour. J. Opt. A: Pure Appl. Opt. 2, R15–R28. ( 10.1088/1464-4258/2/6/201) [DOI] [Google Scholar]
- 5.Kinoshita S. 2008. Structural colors in the realm of nature. Singapore: World Scientific; ( 10.1142/6496) [DOI] [Google Scholar]
- 6.Whitney HM, Kolle M, Andrew P, Chittka L, Steiner U, Glover BJ. 2009. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323, 130–133. ( 10.1126/science.1166256) [DOI] [PubMed] [Google Scholar]
- 7.Moyroud E. et al. 2017. Disorder in convergent floral nanostructures enhances signalling to bees. Nature 550, 469–474. ( 10.1038/nature24285) [DOI] [PubMed] [Google Scholar]
- 8.Vignolini S, Rudall PJ, Rowland AV, Reed A, Moyroud E, Faden RB, Baumberg JJ, Glover BJ, Steiner U. 2012. Pointillist structural color in Pollia fruit. Proc. Natl Acad. Sci. USA 109, 15 712–15 715. ( 10.1073/pnas.1210105109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vignolini S, Gregory T, Kolle M, Lethbridge A, Moyroud E, Steiner U, Glover BJ, Vukusic P, Rudall PJ. 2016. Structural colour from helicoidal cell-wall architecture in fruits of Margaritaria nobilis. J. R. Soc. Interface 13, 20160645 ( 10.1098/rsif.2016.0645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW. 1991. Ultrastructural basis and function of iridescent blue colour of fruits in Elaeocarpus. Lett. Nat. 349, 260–262. ( 10.1038/349260a0) [DOI] [Google Scholar]
- 11.Strout G, Russell SD, Pulsifer DP, Erten S, Lakhtakia A, Lee DW. 2013. Silica nanoparticles aid in structural leaf coloration in the Malaysian tropical rainforest understorey herb Mapania caudata. Ann. Bot. 112, 1141–1148. ( 10.1093/aob/mct172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham RM, Lee DW, Norstog K. 1993. Physical and ultrastructural basis of blue leaf iridescence in two neotropical ferns. Am. J. Bot. 80, 198–203. ( 10.1002/j.1537-2197.1993.tb13789.x) [DOI] [Google Scholar]
- 13.Thomas KR, Kolle M, Whitney HM, Glover BJ, Steiner U. 2010. Function of blue iridescence in tropical understorey plants. J. R. Soc. Interface 7, 1699–1707. ( 10.1098/rsif.2010.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs M, Lopez-Garcia M, Phrathep O-P, Lawson T, Oulton R, Whitney HM. 2016. Photonic multilayer structure of Begonia chloroplasts enhances photosynthetic efficiency. Nat. Plants 2, 16162 ( 10.1038/nplants.2016.162) [DOI] [PubMed] [Google Scholar]
- 15.Vignolini S, Moyroud E, Glover BJ, Steiner U. 2013. Analysing photonic structures in plants. J. R. Soc. Interface 10, 20130394 ( 10.1098/rsif.2013.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilts BD, Whitney HM, Glover BJ, Steiner U, Vignolini S. 2014. Natural helicoidal structures: morphology, self-assembly and optical properties. Mater. Today Proc. 1, 177–185. ( 10.1016/j.matpr.2014.09.021) [DOI] [Google Scholar]
- 17.Wilts BD, Dumanli AG, Middleton R, Vukusic P, Vignolini S. 2017. Invited article: chiral optics of helicoidal cellulose nanocrystal films. APL Photon. 2, 0408011–0408017. ( 10.1063/1.4978387) [DOI] [Google Scholar]
- 18.Neville AC, Levy S. 1985. The helicoidal concept in plant cell wall ultrastructure and morphogenesis. In Biochemistry of plant cell walls (eds CT Brett, JR Hillmann). Society for Experimental Biology, no. 28. Cambridge, UK: Cambridge University Press.
- 19.Roland JC, Reis D, Vian B, Satiat-Jeunemaitre B, Mosiniak M. 1987. Morphogenesis of plant cell walls at the supramolecular level: internal geometry and versatility of helicoidal expression. Protoplasma 140, 75–91. ( 10.1007/BF01273716) [DOI] [Google Scholar]
- 20.Jewell SA, Vukusic P, Roberts NW. 2007. Circularly polarized colour reflection from helicoidal structures in the beetle Plusiotis boucardi. New J. Phys. 9, 99 ( 10.1088/1367-2630/9/4/099) [DOI] [Google Scholar]
- 21.Sharma V, Crne M, Park JO, Srinivasarao M. 2009. Structural origin of circularly polarized iridescence in jeweled beetles. Science 325, 449–451. ( 10.1126/science.1172051) [DOI] [PubMed] [Google Scholar]
- 22.Middleton R, Steiner U, Vignolini S. 2017. Bio-mimetic structural colour using biopolymers. In Bio-inspired polymers, pp. 555–585. Cambridge, UK: Royal Society of Chemistry. ( 10.1039/9781782626664-00555) [DOI]
- 23.Gould KS, Lee DW. 1996. Physical and ultrastructural basis of blue leaf iridescence in four Malaysian understory plants. Am. J. Bot. 83, 45–50. ( 10.1002/j.1537-2197.1996.tb13872.x) [DOI] [Google Scholar]
- 24.Boonkerd T, Nooteboom HP. 2001. A new species of Microsorum (Polypodiaceae) from Thailand. Blumea 46, 581–583. [Google Scholar]
- 25.Petchsri S, Boonkerd T, Baum BR. 2012. Phenetic study of the Microsorum punctatum complex (Polypodiaceae). ScienceAsia 38, 1–12. ( 10.2306/scienceasia1513-1874.2012.38.001) [DOI] [Google Scholar]
- 26.Kreier H-P, Zhang X-C, Muth H, Schneider H. 2008. The microsoroid ferns: inferring the relationships of a highly diverse lineage of Paleotropical epiphytic ferns (Polypodiaceae, Polypodiopsida). Mol. Phylogenet. Evol. 48, 1155–1167. ( 10.1016/j.ympev.2008.05.001) [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ImageJ. 2018 An open platform for scientific image analysis. See https://imagej.net/Welcome.
- 29.Berreman4×4. 2018 Python implementation of Berreman's 4×4 matrix method. See https://github.com/Berreman4x4/Berreman4x4.
- 30.Dumanli AG, van der Kooij HM, Kamita G, Reisner E, Baumberg JJ, Steiner U, Vignolini S. 2014. Digital color in cellulose nanocrystal films. ACS Appl. Mater. Interfaces 6, 12 302–12 306. ( 10.1021/am501995e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sultanova N, Kasarova S, Nikolov I. 2009. Dispersion properties of optical polymers. Acta Phys. Polon. A Gen. Phys. 116, 585–587. ( 10.12693/APhysPolA.116.585) [DOI] [Google Scholar]
- 32.Cosgrove DJ, Jarvis MC. 2012. Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci. 3, 204 ( 10.3389/fpls.2012.00204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouligand Y. 1972. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 4, 189–217. ( 10.1016/S0040-8166(72)80042-9) [DOI] [PubMed] [Google Scholar]
- 34.Buchanan BB, Gruissem W, Jones RL. 2015. Biochemistry and molecular biology of plants, 2nd edn Rockville MD: American Society of Plant Biologists. [Google Scholar]
- 35.Denef J-F, Cordier AC, Mesquita M, Haumont S. 1979. The influence of fixation procedure, embedding medium and section thickness on morphometric data in thyroid gland. Histochem. Cell Biol. 63, 163–171. ( 10.1007/BF00644538) [DOI] [PubMed] [Google Scholar]
- 36.Mollenhauer HH. 1993. Artifacts caused by dehydration and epoxy embedding in transmission electron microscopy. Microsc. Res. Tech. 26, 496–512. ( 10.1002/jemt.1070260604) [DOI] [PubMed] [Google Scholar]
- 37.Frka-Petesic B, Kamita G, Guidetti G, Vignolini S. In preparation The angular optical response of cellulose nanocrystal films explained by the structural distortions of the arrested suspension upon drying. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berreman DW. 1972. Optics in stratified and anisotropic media: 4×4-matrix formulation. J. Opt. Soc. Am. 62, 502–510. ( 10.1364/JOSA.62.000502) [DOI] [Google Scholar]
- 39.Carter IE, Weir K, McCall MW, Parker AR. 2016. Variation in the circularly polarized light reflection of Lomaptera (Scarabaeidae) beetles. J. R. Soc. Interface 13, 20160015 ( 10.1098/rsif.2016.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordón GB, Lagorio MG. 2007. Optical properties of the adaxial and abaxial faces of leaves. Chlorophyll fluorescence, absorption and scattering coefficients. Photochem. Photobiol. Sci. 6, 873–882. ( 10.1039/b617685b) [DOI] [PubMed] [Google Scholar]
- 41.Wolf FT. 1958. Chlorophylls A and B in the Pteridophytes. Bull. Torrey Bot. Club 85, 1–4. ( 10.2307/2482444) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data are available at: https://doi.org/10.17863/CAM.26156.