Abstract
Ediacaran fossils document the early evolution of complex megascopic life, contemporaneous with geochemical evidence for widespread marine anoxia. These data suggest early animals experienced frequent hypoxia. Research has thus focused on the concentration of molecular oxygen (O2) required by early animals, while also considering the impacts of climate. One model, the Cold Cradle hypothesis, proposed the Ediacaran biota originated in cold, shallow-water environments owing to increased O2 solubility. First, we demonstrate using principles of gas exchange that temperature does have a critical role in governing the bioavailability of O2—but in cooler water the supply of O2 is actually lower. Second, the fossil record suggests the Ediacara biota initially occur approximately 571 Ma in deep-water facies, before appearing in shelf environments approximately 555 Ma. We propose an ecophysiological underpinning for this pattern. By combining oceanographic data with new respirometry experiments we show that in the shallow mixed layer where seasonal temperatures fluctuate widely, thermal and partial pressure (pO2) effects are highly synergistic. The result is that temperature change away from species-specific optima impairs tolerance to low pO2. We hypothesize that deep and particularly stenothermal (narrow temperature range) environments in the Ediacaran ocean were a physiological refuge from the synergistic effects of temperature and low pO2.
Keywords: Ediacaran, oxygen, temperature, respiration
1. Introduction
The role of marine oxygenation as it pertains to early animal evolution is a fundamental question in deep Earth history. Interdisciplinary research spanning palaeontology, geochemistry, and molecular biology increasingly tie changes in Earth's surface environment to the emergence and subsequent radiation of animals across the Neoproterozoic-early Palaeozoic transition approximately 800–500 million years ago (Ma). This interval is marked by evidence for extreme climate fluctuations and biogeochemical perturbations, including two long-lasting glaciation events (i.e. snowball earth glaciations) during the Cryogenian Period ca 720–635 Ma [1], and extremely positive carbonate carbon isotope records punctuated by negative excursions [2]. The first large, morphologically complex fossils do not appear in the fossil record until the Ediacaran Period ca 635–541 Ma [3]. Multi-proxy geochemical evidence suggests early animal evolution occurred against a backdrop of widespread marine subsurface anoxia [4–6]. While the absolute amount of oxygen (O2) as a percentage of present atmospheric levels (PAL) through the Neoproterozoic are contentious, researchers have traditionally considered levels of 1–10% PAL to be most likely [7,8]. During the Ediacaran and early Cambrian, redox sensitive trace metal enrichments suggest transient oxygenation events during that time [9,10]. However, regional and stratigraphic inconsistencies in the pattern of these enrichments and several other lines of evidence indicate any oxygenation must have been relatively muted or short-lived [5,6,11–14]. There is therefore emerging consensus that early animals encountered persistent and severely low O2 partial pressures (pO2), which would have had profound effects on the spatial distribution of metabolically-viable habitats [15].
For decades, these low levels of marine O2 have been opined [16–19], albeit controversially [20], as the environmental barrier to early animal evolution. However, given the striking climatic fluctuations of the Neoproterozoic, O2 is not the only environmental influence that has been considered. The stratigraphic occurrence of highly diverse, shallow-water shelfal ‘White Sea’ fossil assemblages ca 560–550 Ma [3] in exclusively siliciclastic sediments has been viewed as an indication of habitation in cold-water environments (at latitudes above low-latitude carbonate belts) [21]. To the extent that the Ediacara biota represent animals, this ‘Cold Cradle’ model of evolution posited that the greater gas solubility of O2, as well as the sluggish remineralization of nutrients by prokaryotes in cold, shallow, high-latitude waters allowed metazoan ecosystems to flourish in the later Ediacaran.
Detailed palaeontological and geochronological studies now indicate that the oldest non-algal megascopic fossil assemblage is not the White Sea assemblage, but rather is the so-called ‘Avalon assemblage’ [22–25]. This community is primarily dominated by morphologically complex soft-bodied benthic frondose fossils belonging to two recognized groups, the Arboreomorpha and Rangeomorpha. While there are phylogenetic issues with assigning Ediacaran fronds to the Metazoa ([26], but see [27]), Avalonian assemblages also contain other fossils more likely to be animals, including sponges [28] and body- and trace-fossil evidence for eumetazoan cnidarians [23]. These fossils appear in the middle Ediacaran ca 571 Ma in deep-water, aphotic slope and basinal facies [22–24,29], where they are found in situ, buried by ash beds or rapidly deposited sediments. The inferred deep-water depositional environment is supported by expansive, kilometre-scale stratigraphic sections of uninterrupted turbidites displaying thick TC-E and TD-E Bouma subdivisions, contour-parallel bottom currents, and no evidence for wave-generated sedimentary structures [30]. These stratigraphically oldest deep-water, morphologically complex fossils are found in multiple sedimentary basins worldwide including England, Newfoundland, and the Mackenzie and Wernecke Mountains of northwestern Canada. By contrast, Ediacara biota are absent from shallow-water environments on the shelf until approximately 560–555 Ma, often after the globally recognized Shuram carbon isotope excursion [31–33].
The Ediacaran fossil record therefore displays a puzzling pattern. For approximately 15 Myr large, morphologically complex eukaryotes and animals only inhabited deep-water settings. This observation is at odds with palaeontological meta-analyses which show that onshore to offshore macroevolutionary patterns predominate across multiple intervals in the later Phanerozoic (541 Ma-present) fossil record [34]. Might deep-water settings have provided a kind of physiological refugia for early metazoans in a generally low pO2 global ocean? Given that both deep- and high-latitude water masses are colder than shallow-water counterparts at low-latitudes, what ecophysiological or oceanographic differences exist between the two environments, and how do they fit within the context of the Cold Cradle hypothesis? Lastly, as Ediacaran communities did eventually radiate onto the shelf to inhabit shallow-water environments, what stressors might these ecosystems have faced? To shed light on these questions, we apply an oxygen supply index (OSI, table 1) to determine how temperature can govern O2 supply to animals at oceanographic scales. We then present new experimental respirometry data that illustrate how temperature dynamically affects the absolute pO2 tolerance of marine ectotherms. Lastly, we integrate these two approaches to re-examine bathymetric patterns within the Ediacaran fossil record in an ecophysiological context.
Table 1.
Summary definitions of key terms.
| term | abbreviation | definition |
|---|---|---|
| oxygen supply index | OSI | a term which measures the rate at which oxygen can transfer from the water column into an animal by integrating the partial pressure and diffusivity of oxygen within the water as well as its solubility |
| thermal performance curve | TPC | the thermal performance curve represents an animal's fitness due in part to thermal tolerance characteristics and temperature dependant effects on physiological and biochemical functions (e.g. fecundacy, growth, metabolic rate). Often it reflects the natural environmental temperature range of the species, that is its thermal window |
| thermal optimum | Topt | maximum performance occurs here at the peak of the TPC, often at intermediate temperature and represents maximum aerobic scope, that is the greatest difference between MMR and SMR |
| standard metabolic rate | SMR | the minimum metabolic rate that supports basic maintence requiremens of an organism while at rest and fasting. In ectotherms this rate is temperature sensitive |
| maximum metabolic rate | MMR | the maximum metabolic rate achieved by an organism during unsustainable physical activity that is limited by aerobic capacity |
| oxygen- and capacity-limited thermal tolerance | OCLTT | a concept which decribes thermally-induced hypoxemia (low levels of oxygen in blood or tissues) at both ends of an animal's thermal window due to thermal effects imparted on oxygen bioavailability, metabolic demand, and ventilatory capacity to supply enough oxygen to meet these metabolic requirements |
| critical O2 level | [O2]crit | this is the critical oxygen level below which standard metabolic rate can no longer be maintained aerobically. At this point, oxygen demand is greater than the animal's capacity to supply oxygen and anaerobic metabolic pathways begin operating |
2. Background and previous work
(a). Oxygen bioavailability in aquatic settings
The challenges of aquatic respiratory gas exchange are well known. In water at standard temperature and pressure there is approximately 30 times less O2 than in the atmosphere by concentration, and O2 diffuses approximately 2.4 × 105 times slower through water than air [35]. Furthermore, both temperature and salinity independently impact the solubility of O2 in water owing to their effects on the Henry gas coefficient. This results in seawater containing on average 25% less O2 than freshwater at a given temperature [36]. Owing to these physical constraints, limitations in environmental O2 supply have profound physiological impacts on aquatic animals in the modern ocean.
Respiratory O2 exchange cannot simply be discussed interchangeably with units of pO2 or solubility. Instead, the product of pO2, solubility, and diffusivity potential together represent the flux of O2 that can be transferred from the environment into a respiring organism. This relationship inherently describes Fick's first law of diffusion, which expresses partial pressure as proportional to its concentration gradient [37]. Putting aside organism-specific differences in surface area to volume ratios, respiratory structures (e.g. gills, pigments), or differences in pumping [38], aquatic animals can only extract O2 from the water column at an absolute rate proportional to environmental availability. This relationship has been expressed in freshwater environments as the OSI [39] (expressed here in µmol kg−1 matm m2 s−1 × 10−5):
| 2.1 |
where αO2 is the solubility of O2 in water (mol m−3 Pa−1), DO2 is the diffusivity of O2 in water (m2 s−1 × 10−9), and pO2 is the partial pressure of O2 in water (matm).
(b). Respiration physiology and temperature
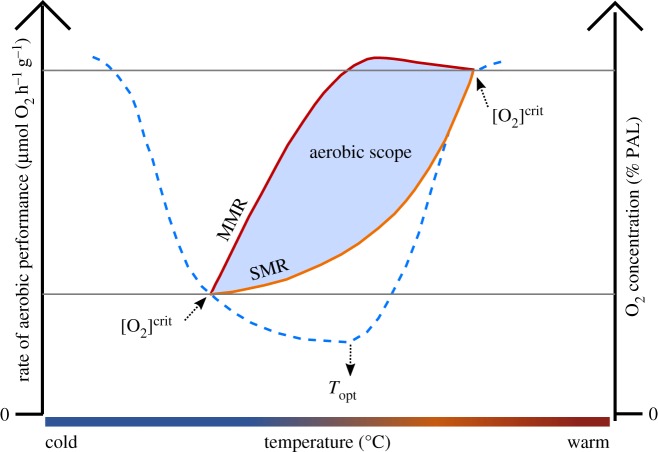
The ability to avoid hypoxia in low O2 conditions represents a physiological balance between O2 supply and demand. Temperature affects both sides of this equation [40], and for aquatic ectothermic animals, the inability to control body temperature results in metabolic rates which change significantly with ambient temperature. All ectotherms exhibit a Q10 rate coefficient in which a 10°C increase in body temperature raises metabolic rate by a factor of approximately 2–3, or about 8% per degree Celsius [41]. Despite numerous processes at the whole-organism, tissue, and enzymatic levels to partially offset this effect, these strategies are invariably not fully effective at countering the effects of temperature on metabolic rate [42]. Marine ectotherms consequently display a thermal performance curve (table 1) which often represents an organism's relative fitness across its natural environmental temperature range [43]. The thermal performance curve reflects the effects of low temperature on O2 supply and ventilation costs, and high temperatures on enzyme instability and metabolic demand. In respiratory physiology terms, the thermal performance curve inherently represents aerobic scope, because ATP yield from aerobic respiration is dramatically higher (approx. 15×) than any anaerobic glycolytic pathway. Scope is the instantaneous proportion of metabolic power available to an organism after its basal maintenance costs are met, and can be used to invest in growth, reproduction, predation and defence, and other fitness-related functions [44].
The co-limiting effects of temperature and low pO2 on aerobic scope can result in the metabolic demands of an animal exceeding its capacity for O2 supply [45]. This phenomenon is referred to as oxygen- and capacity-limited thermal tolerance (OCLTT, table 1) [46]. At its most basic level, OCLTT represents a reduction of relative aerobic scope at temperatures both above and below Topt (table 1). Above Topt, standard metabolic rate (the lowest rate of O2 consumption required for maintenance, table 1) makes up a greater proportion of aerobic scope owing to Q10 effects (figure 1) [45]. At high enough temperatures, maximum metabolic rate (table 1) can also begin to decrease owing to heat-induced damage to biochemical systems and organ (e.g. heart) failure [47,48]. Combined, warming above Topt causes a decrease in aerobic performance and lowers pO2 tolerance, causing survivorship to greatly decline [49]. At temperatures below Topt, maximum metabolic rate becomes increasingly inhibited owing to decreasing O2 bioavailability, and reduced aerobic capacity owing to kinetic reduction in circulatory and ventilatory performance. This results in a net reduction of aerobic scope despite lower metabolic rates, thus limiting low pO2 tolerance at the cold end of the spectrum as well (figure 1) [47,48,50]. Lastly, at both ends of the thermal performance curve, low ambient pO2 not only drops maximum metabolic rate (causing a reduction in the range of thermal tolerance by way of reducing overall scope), but also increases the proportion of standard metabolic rate used for ventilation, as animals must exchange more water over their respiratory surfaces in order to extract the same amount of O2 [51,52].
Figure 1.
Conceptual model of how temperature and ambient O2 interact to constrain aerobic performance in ectothermic animals. Aerobic scope is defined as the difference of rates of aerobic performance (left axis), specifically between maximum metabolic rate, MMR, and standard metabolic rate, SMR. Tolerance to low O2 levels decreases at temperatures both below and above Topt, as O2 supply capacity falls relative to O2 demand, resulting in [O2]crit occurring at progressively higher ambient O2 tensions (blue dashed line, right axis). Because Topt represents maximum rate of aerobic performance (the greatest distance between MMR and SMR on the left axis), it also corresponds to the [O2]crit minimum (on the right axis). As low ambient O2 reduces aerobic scope by lowering MMR, it consequently narrows the thermal window of environmental O2 tolerance because any temperature-related increase in SMR or reduction in MMR will consequently take up a larger proportion of aerobic scope. Figure adapted from [46].
The OCLTT relationship can be determined for a given animal by measuring what is known as the critical O2 concentration ([O2]crit, table 1), or alternatively expressed in partial pressure (), across its natural temperature range. [O2]crit represents the O2 level below which an organism is no longer able to maintain its standard metabolic rate. At the [O2]crit, aerobic scope is therefore zero, and any further decrease in O2 saturation, or change in temperature further away from Topt, will result in ATP demand exceeding O2 supply. At a metabolic level, this deficit in aerobically generated ATP triggers the onset of fermentation. This shift in metabolic pathway dramatically changes respiration rate, allowing [O2]crit to be calculated using breakpoint analysis on the O2 draw-down curve generated during closed-system experimental respirometry (electronic supplementary material, figure S9).
3. Results
(a). Oceanographic controls on oxygen supply
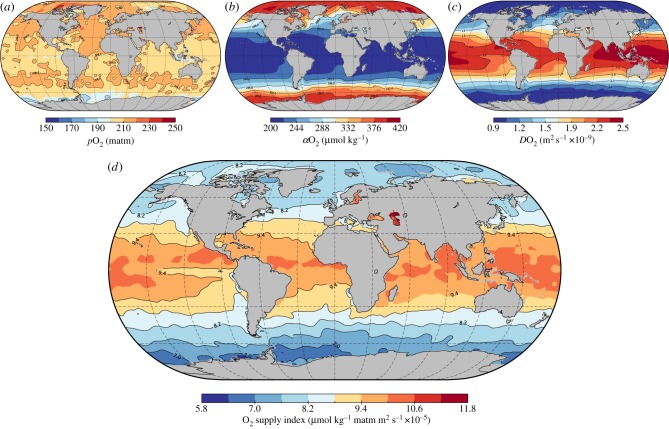
OSI has been calculated in freshwater environments, but not in the global ocean. To illustrate the behaviour of OSI in the global ocean for the first time to our knowledge, dissolved O2, salinity, density, temperature, and pressure data from the National Oceanic and Atmospheric Administration World Ocean Atlas (NOAA WOA13 V2) were used to calculate in situ pO2, diffusivity and solubility (electronic supplementary material). Temperature and salinity independently affect solubility. It is therefore possible for solubility to change dissolved O2 concentrations in the ocean without any adjustment in pO2, such as across salinity or temperature gradients. Ultimately, the total amount of O2 that is present at a given location is dependent on not just solubility, but also on atmospheric pO2 and the saturation state of the water column (electronic supplementary material, figures S1–S3). At the sea surface, there is little variation in the pO2 of seawater with latitude owing to the limited change in saturation water vapour pressure across the normal range of marine water temperatures combined with the near constant atmospheric pO2 at sea level (figure 2; electronic supplementary material, figure S7; [53]). At depth though, increases in pO2 of approximately 14% per 1000 m depth occur owing to large increases in hydrostatic pressure (electronic supplementary material, figures S4–S6 and S8; [54]). However, because pressure increases the fugacity of O2 exponentially, it also has a reciprocal effect on the solubility coefficient in water owing to increased outgassing tendency (electronic supplementary material, figure S2a). This leads to little change in OSI past the thermocline unless the water mass is undersaturated with respect to O2 (electronic supplementary material, figures S1d and S4d). For instance, at depths between 200–1000 m in the modern ocean, undersaturation (lower than equilibrium dissolved O2 concentrations) driven by remineralization of organic matter plays a significant role in developing oxygen minimum zones (OMZs) on upwelling margins (electronic supplementary material, figure S6d; [55]).
Figure 2.
Factors governing oxygen supply to animals. (a) Average annual partial pressure of O2 (pO2) in the global ocean at surface. (b) Average annual solubility of O2 (αO2) in the global ocean at surface. Values increase with latitude owing to the thermal effects on Henry's solubility coefficient. (c) Average annual diffusivity of O2 (DO2) in the global ocean at surface. (d) Average annual bioavailability of O2 in the global ocean at surface, expressed using the oxygen supply index (OSI) [39]. Despite the increased solubility of O2 in cold water, the kinematic viscosity also increases substantially, reducing the diffusivity of O2 at a rate greater than the offsetting effect on solubility. As a result, the supply of O2 to respiratory surfaces actually decreases approximately linearly as water becomes colder.
Finally, the third component of OSI, diffusivity, is largely controlled by Brownian motion, as molecular O2 exists as a dissolved gas within seawater. As such, the capacity of O2 to diffuse through the liquid medium is heavily dependent on the ratio of temperature to density of seawater, which together govern its kinematic viscosity (also known as momentum diffusivity) [56]. Given the wide thermal ranges and saline nature of seawater, it is critical to take diffusivity into account in studies of respiration physiology. However, this is not commonly done. Within the ocean, increases in viscosity owing to colder water temperatures at higher latitudes or greater depths overcome increases in solubility on OSI (electronic supplementary material, figures S4–S7). The key point here regarding O2 supply for respiration as determined from OSI is that cold marine waters at depth or high-latitudes in equilibrium saturation conditions have only 60–70% of the O2 supply available in shallow low-latitude regions (figure 2; electronic supplementary material, figures S4d and S7d). The physiological implications of this novel result are immediately apparent and probably far reaching, as cold waters in the global ocean are therefore much more difficult to respire in, despite having greater O2 solubility [39,57].
(b). Aerobic respiration and temperature
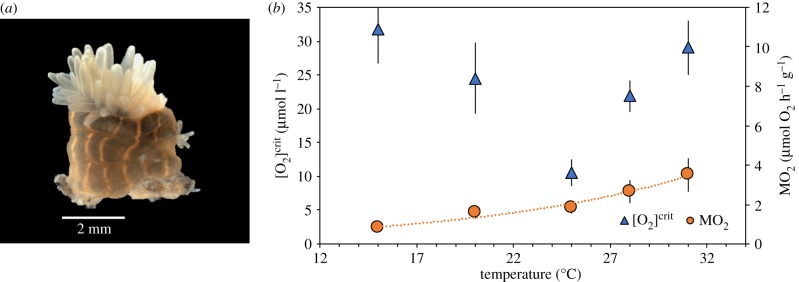
To illustrate how [O2]crit varies with temperature, we conducted 86 [O2]crit measurements on the intertidal anthozoan cnidarian Diadumene lineata (figure 3). This species of sea anemone was selected as intertidal organisms regularly encounter significant diurnal and seasonal temperature and pO2 fluctuations [58]. Furthermore, anemones possess a diploblastic body plan and therefore rely entirely on cutaneous diffusion of O2 into two epithelial tissue layers—the external ectoderm, and internal endoderm—for aerobic respiration. These layers are supported by a hydrostatic skeleton constructed of a gelatinous, metabolically-inert tissue called mesoglea. This makes the anemone body plan at least physiologically analogous, if not necessarily homologous, to many Avalonian Ediacaran organisms without circulatory or respiratory systems. The comparison may be even closer than analogue, as many workers interpret some morphologically complex Ediacaran fossils to in fact be total-group actinarian cnidarians [59–61].
Figure 3.
Variation of pO2 tolerance with temperature. (a) Intertidal anthozoan cnidarian Diadumene lineata (YPM IZ 077401) from the New England region of the western Atlantic. Image courtesy of E.A. Lazo-Wasem. (b) [O2]crit and MO2 data for 86 individuals of D. lineata binned into five experimental temperatures (±0.3°C). Mean (±s.d.) standard O2 consumption rate (MO2) increases consistently with temperature owing to the Arrhenius relationship (Q10 = 2.50, n = 70, R2 = 0.96). Absolute environmental O2 tolerance ([O2]crit) displays a bidirectional relationship (n = 86, R2 = 0.75). Triangles represent average mean values and whiskers represent standard error. These data provide, to our knowledge, the first experimental support for physiological principles hypothesized to be universal for marine ectotherms [45,46], indicating these principles are applicable to questions regarding the deep-water origin of Ediacaran organisms. (Online version in colour.)
Results of respirometry experiments demonstrate this taxon displays mass-normalized standard metabolic rates (MO2) that increase predictably with temperature (Q10 = 2.50, figure 3). For [O2]crit, mean values take the form of a concave-up parabola, demonstrating the predicted bidirectional effects that temperature has on environmental O2 tolerance. At a Topt of approximately 24°C, aerobic scope is maximal and D. lineata is able to respire aerobically well into low pO2 levels ([O2]crit of 10.4 µmol l−1 or 4.7% PAL). However, upon warming to 28°C, pO2 tolerance decreases significantly ([O2]crit of 21.9 µmol l−1 or 10.4% PAL). Cooling to 20°C produces a similar decrease in tolerance ([O2]crit of 24.5 µmol l−1 or 10.2% PAL). Such bi-directionality has been studied at the molecular level and predicted to occur at the organismal level [46–48,50,62], yet despite its purported generality as a physiological principle that affects all aquatic ectotherms [46], this relationship has not previously been demonstrated experimentally using respirometry. While the exact shape of the concave-up parabola and position of the thermal optimum almost certainly differs between organismal lineages in different environments and over evolutionary timescales, the bi-directional effects seen in these D. lineata respiratory data are likely to be universal. These novel experimental results demonstrate that hypoxia tolerance is not a single threshold value in dynamic, shallow-water marine environments, but rather is determined jointly from the effects of temperature on OSI and on an animal's supply capacity over metabolic demand.
4. Discussion
(a). Seasonality and thermal tolerance in low pO2 oceans
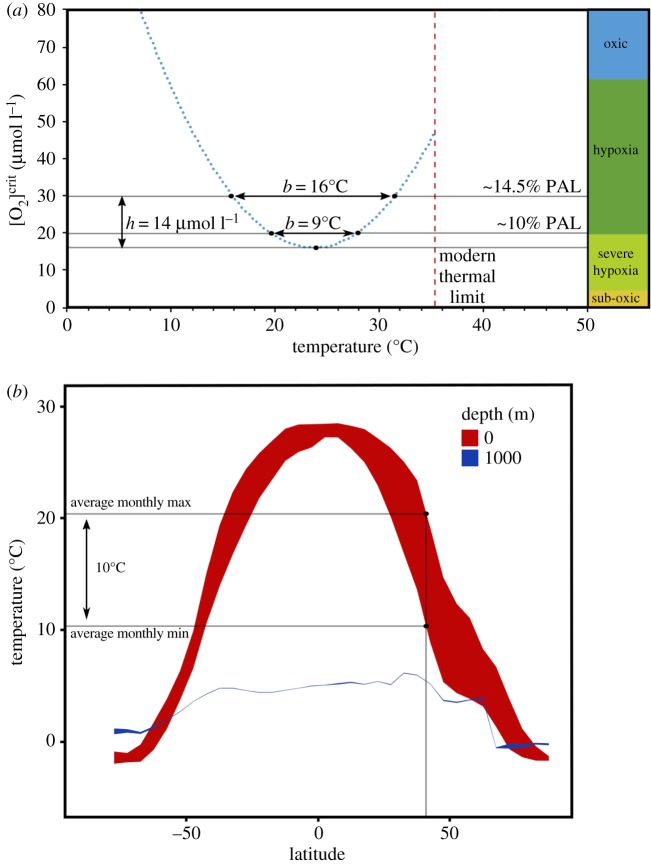
These physiological principles have significant use for understanding how low ambient O2 levels and climate might have synergistically affected early animal life. While focus on the absolute lower O2 limits for metazoan aerobic respiration [63,64] or critical thresholds on carnivory [65] provide important constraints on early animal ecosystems, the exogenous thermal environment in combination with pO2 probably governed the metabolic viability of habitats for early animal ecosystems. Critically, because ambient pO2 governs the size of aerobic scope, low pO2 narrows the thermal range over which aerobic respiration can occur. Mathematically, this can be thought of in terms of the area above a polynomial regression run through a plot of [O2]crit with respect to temperature for a given species, expressed as:
| 4.1 |
where A is the area enclosed between the parabola and a chord intersecting the y-axis at an environmental O2 level. The length of this chord between the intercepts with the parabola is b, which represents the thermal range of aerobic respiration at a given O2 concentration. h is the height from the parabola vertex ([O2]crit at Topt) to the chord, and represents aerobic scope (figure 4a). Because A in equation (4.1) is related to the product of both O2 and temperature, any decrease in aerobic scope (h) caused by lower ambient O2 concentrations will also narrow the thermal range of aerobic respiration (b). For D. lineata, ambient pO2 at 14.5% PAL corresponds to a 16°C allowable range for aerobic respiration which meets minimum requirements for maintenance, but ambient pO2 at 10% PAL results in only a 9°C allowable range (figure 4). Thermal range continues decreasing to zero at a pO2 of approximately 5% PAL, where the animal can only respire aerobically at its Topt of approximately 24°C. This relationship has significant implications when considering the impact of thermal variability in the Ediacaran ocean. pO2 levels of 10% PAL are on the high end of estimates for this interval, although in reality very little is concretely known about exact atmospheric O2 levels during this time [5,7,8]. If pO2 were closer to the lower-end estimate of 1% PAL, then presumably all megascopic metazoans would be driven deep into the respective vertices of their OCLTT parabola-space (figure 4a). This is if any could have survived at all; while metazoan macrofauna (0.3 to approximately 50 mm in size) can often have lower O2 requirements [63], theoretical, experimental, and oceanographic evidence suggest minimum pO2 levels of 1–4% PAL are needed for non-bilaterian megafauna in the absence of thermal variability [64,68,69]. Furthermore, marine animals require sustained metabolic rates to be a factor of approximately 2 to 5 greater than resting demand in order to sustain ecological activity [40]. Thus, the thermal range of low pO2 tolerance in the Ediacaran may have been considerably narrow. We note that although this discussion is couched in terms of traditional Ediacaran O2 estimates, the general principle of lower thermal range holds for any degree of low-oxygen Earth system.
Figure 4.
Impact of seasonal temperature variation on aerobic respiration in low pO2 conditions. (a) Polynomial regression run through the [O2]crit field of D. lineata (R2 = 0.75). The region above this blue dashed line represents the temperature and O2 conditions in which this species can maintain aerobic respiration (note that the regression vertex does not perfectly correspond with the lowermost measured [O2]crit value). While the exact equation of the polynomial probably differs between species, the bidirectional nature of O2 tolerance with respect to temperature is probably universal [46]. Because the area of this aerobic field (A) is dependent on the severity of hypoxia h, and the thermal range of the environment b, lower ambient concentrations of dissolved O2 result in a narrower thermal range of aerobic respiration. Black horizontal lines represent O2 levels at 14.5% PAL, 10% PAL, and the vertex of the parabola. The red dashed line is approximately 35°C, the average upper critical (lethal) long-term thermal limit of many shallow-water ectotherms living in modern tropical environments [66]. Descriptive low-O2 state boundaries are adapted from [67]. (b) Average seasonal temperature ranges across all latitudes of the global ocean at surface and 1000 m depths. Envelope height (shown here for 40°N) represents the difference between maximum and minimum mean monthly temperatures for a given latitude. Data from NOAA WOA13 V2. (Online version in colour.)
With low ambient O2 narrowing the thermal range of taxa, seasonal temperature extremes in the shallow Ediacaran surface ocean probably had a significant impact on the aerobic respiration of metazoans. The effects of seasonality in the modern ocean can be used as an example. In the mid-latitudes, average annual sea surface temperature ranges greater than 10°C at 40° N. By contrast, seasonal temperature variation in the deep ocean is minimal (less than 1°C) across almost all latitudes at 1000 m depth (figure 4b). We propose this difference ultimately governed metabolically viable habitat for early animals in the Ediacaran.
(b). A stenothermal origin for the Ediacara biota
The Cold Cradle hypothesis [21] originally posited that the Ediacara biota evolved in shallow, cold-water environments owing to increased O2 solubility. However, we have shown that O2 bioavailability in the global ocean maintains a positive linear relationship with temperature (electronic supplementary material, figure S7d). Furthermore, although cold-water has been theorized to increase aerobic scope by reducing standard metabolic rate in well-oxygenated conditions, low pO2 environments negate any benefit that this provides [39]. At a whole-organism scale in the Ediacaran, aerobic scope throughout the global ocean was likely to be very limited if estimates for a lower O2 Earth system are correct. Given the interaction of temperature on O2 supply and aerobic demand, shallow-water environments would not have had the ideal ecophysiological conditions needed to establish diverse ecosystems given seasonal temperature fluctuations.
We hypothesize it was the lack of thermal variability, and not necessarily cold conditions, that can best explain the deep-water origin of Ediacara biota. These environments would have been far below the thermocline (roughly 200 m in the modern ocean), where the global ocean is cold and nearly isothermal (figure 4b). In such conditions, the synergistic effects of temperature and low environmental pO2 on aerobic scope are muted and organisms can instead optimize their biochemical functions through compensatory adaptation to a significant degree [41,70]. Despite the lower bioavailability of O2 in cold water, this compensatory capability allows modern animals to inhabit sub-zero waters in Antarctic habitats of the Southern Ocean [42], and severely undersaturated hypoxic deep-water OMZs on continental slopes [55]. In an ecophysiological context, this means that while ambient pO2 (h in figure 4a) governs the thermal range of aerobic tolerance (width of b within the parabola, figure 4a), animals are able to shift their thermal optimum, Topt (vertex of figure 4a), to cooler temperatures via long-term adaptation [41]. The global distribution of Ediacaran genera [71], combined with the fact that multiple taxa were capable of inhabiting both deep- and shallow-water environments in the later Ediacaran, suggests that thermal acclimation capacity was unlikely to be an issue [33,72,73].
It would seem that stenothermal habitats (areas which experience only a narrow range of temperatures) at depth would be a key environmental attribute needed for animals evolving in a low pO2 world. While we hypothesize temperature variability may be the most important factor, the fact that such deep-water environments are colder than the surface ocean is not inconsequential: this would be particularly beneficial for viable aerobic habitat if the global mid-Ediacaran upper ocean was significantly warmer than the modern. Today, animals in the tropics are already near their upper thermal limits that at ecological timescales are commonly approximately 35°C [43,45,66]. In other words, though warmer waters increases O2 bioavailability approximately linearly, the increase in metabolic rates and thus O2 demand with temperature is exponential, and in such conditions this quickly leads to temperature-induced hypoxia [40,45]. In this light, if there was an environmental control on the stratigraphic appearance of Ediacaran organisms, the up-slope movement onto the shelf and eventually into littoral habitats observed between the Avalon–White Sea and White Sea–Nama assemblages [31–33] could be read as either increased ambient pO2 or global cooling. A further expectation of this model would be that if middle Ediacaran (ca 570 Ma) shallow-water fossil occurrences are discovered, we expect them to be much lower in abundance and diversity (i.e. stressed communities) than temporally equivalent deep-water communities.
5. Conclusion
The Ediacara biota appear to have originated in deep-water slope and basinal settings in a global ocean that was still dominated by widespread low pO2 conditions [5,12,33]. In marine settings, total dissolved O2 concentration is a linchpin for metazoan life, but the critical threshold values at which point it imposes limits on physiological function can vary dramatically depending on multiple environmental factors such as primary productivity and organic carbon respiration, pH, salinity, hydrostatic pressure, and especially temperature. As we demonstrate here for the first time to our knowledge, in marine environments, the bioavailability of O2 (OSI) cannot simply be inferred from absolute environmental O2 concentrations alone, but rather it is the product of solubility, partial pressure, and diffusivity together. This has important implications for previous hypotheses that have considered the effect of water temperature on O2 solubility in Ediacaran oceans [21], as diffusivity is actually a stronger lever on O2 bioavailability, and works in the opposite direction. As a result, it is energetically more costly for animals to respire in cold, viscous water, despite its greater O2 solubility [39]. Furthermore, with new experimental physiology data, we show that temperature and O2 are synergistic and together govern the aerobic scope of marine ectothermic animals. The effects of temperature on O2 supply and demand cause O2 tolerance to vary widely as temperature fluctuates. As a result, Ediacaran environments which experienced a wide range of temperatures, such as shallow-water littoral zones and microbial bioherms, would have physiologically challenged early animal communities already living in low ambient O2 conditions. We hypothesize that the apparent deep-marine origin of the Ediacara biota in cold, stenothermal environments may be the evolutionary solution to O2 and temperature co-limitation.
Supplementary Material
Acknowledgements
We thank G. Somero, C. Frieder, C. Deutsch, J. Strauss, W. Verberk, and G. Narbonne for helpful discussion, and C. Beck, H. Deres, and R. Carpenter for assistance in the laboratory and specimen collection. We thank Associate Editor Erin Saupe and two anonymous reviewers for thoughtful comments on an earlier version of this manuscript. We also thank B.H. Bhullar for facilities access, and E. Lazo-Wasem for contributing specimen photographs and curatorial assistance. This work was carried out under the Connecticut Department of Energy and Environment permit no. 1617007.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.bf43443 [74].
Authors' contributions
T.H.B. and E.A.S. designed the study. T.H.B. and L.E.E. contributed physiological data to the analysis. R.G.S. and T.H.B. performed the analysis of oceanographic data and T.H.B. analysed the results. T.H.B. wrote the manuscript with input from R.G.S., L.E.E., P.M.H. and E.A.S.
Competing interest
We declare no competing interests.
Funding
T.H.B. was supported by a NSERC Doctoral Fellowship, and grants from the AMNH Lerner-Gray Fund for Marine Research and the SICB Grants-in-Aid of Research program. We acknowledge the Sloan Research Fellowship (E.A.S. and P.M.H.) for additional support. We also thank the Yale Peabody Museum Summer Internship Program for R. Carpenter's assistance.
References
- 1.Rooney AD, Strauss JV, Brandon AD, Macdonald FA. 2015. A Cryogenian chronology: two long-lasting synchronous Neoproterozoic glaciations. Geology 43, 459–462. ( 10.1130/G36511.1) [DOI] [Google Scholar]
- 2.Macdonald FA, et al. 2010. Calibrating the Cryogenian. Science 327, 1241–1243. ( 10.1126/science.1183325) [DOI] [PubMed] [Google Scholar]
- 3.Droser ML, Tarhan LG, Gehling JG. 2017. The rise of animals in a changing environment: global ecological innovation in the late Ediacaran. Annu. Rev. Earth Planet. Sci. 45, 593–617. ( 10.1146/annurev-earth-063016-015645) [DOI] [Google Scholar]
- 4.Scott C, Lyons TW, Bekker A, Shen Y, Poulton SW, Chu X, Anbar AD. 2008. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452, 456–459. ( 10.1038/nature06811) [DOI] [PubMed] [Google Scholar]
- 5.Sperling EA, Wolock CJ, Morgan AS, Gill BC, Kunzmann M, Halverson GP, Macdonald FA, Knoll AH, Johnston DT. 2015. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 451–454. ( 10.1038/nature14589) [DOI] [PubMed] [Google Scholar]
- 6.Hardisty DS, et al. 2017. Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet. Sci. Lett. 463, 159–170. ( 10.1016/J.EPSL.2017.01.032) [DOI] [Google Scholar]
- 7.Holland HD. 2006. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B 361, 903–915. ( 10.1098/rstb.2006.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons TW, Reinhard CT, Planavsky NJ. 2014. The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307–315. ( 10.1038/nature13068) [DOI] [PubMed] [Google Scholar]
- 9.Chen X, et al. 2015. Rise to modern levels of ocean oxygenation coincided with the Cambrian radiation of animals. Nat. Commun. 6, 7142 ( 10.1038/ncomms8142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahoo SK, Planavsky NJ, Jiang G, Kendall B, Owens JD, Wang X, Shi X, Anbar AD, Lyons TW. 2016. Oceanic oxygenation events in the anoxic Ediacaran ocean. Geobiology 14, 457–468. ( 10.1111/gbi.12182) [DOI] [PubMed] [Google Scholar]
- 11.Miller AJ, Strauss JV, Halverson GP, Macdonald FA, Johnston DT, Sperling EA. 2017. Tracking the onset of Phanerozoic-style redox-sensitive trace metal enrichments: new results from basal Ediacaran post-glacial strata in NW Canada. Chem. Geol. 457, 24–37. ( 10.1016/j.chemgeo.2017.03.010) [DOI] [Google Scholar]
- 12.Tostevin R, et al. 2016. Low-oxygen waters limited habitable space for early animals. Nat. Commun. 7, 12818 ( 10.1038/ncomms12818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace MW, Hood A, Shuster A, Greig A, Planavsky NJ, Reed CP. 2017. Oxygenation history of the Neoproterozoic to early Phanerozoic and the rise of land plants. Earth Planet. Sci. Lett. 466, 12–19. ( 10.1016/j.epsl.2017.02.046) [DOI] [Google Scholar]
- 14.Krause AJ, Mills BJW, Zhang S, Planavsky NJ, Lenton TM, Poulton SW. 2018. Stepwise oxygenation of the Paleozoic atmosphere. Nat. Commun. 9, 4081 ( 10.1038/s41467-018-06383-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhard CT, Planavsky NJ, Olson SL, Lyons TW, Erwin DH. 2016. Earth's oxygen cycle and the evolution of animal life. Proc. Natl Acad. Sci. USA 113, 8933–8938. ( 10.1073/pnas.1521544113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nursall JR. 1959. Oxygen as a prerequisite to the origin of the Metazoa. Nature 183, 1170–1172. ( 10.1038/1831170b0) [DOI] [Google Scholar]
- 17.Cloud PE., Jr 1968. Atmospheric and hydrospheric evolution on the primitive Earth. Science 160, 729–736. ( 10.2307/1724303) [DOI] [PubMed] [Google Scholar]
- 18.Knoll AH, Carroll SB. 1999. Early animal evolution: emerging views from comparative biology and geology. Science 284, 2129–2137. ( 10.1126/science.284.5423.2129) [DOI] [PubMed] [Google Scholar]
- 19.Canfield DE, Poulton SW, Narbonne GM. 2007. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315, 92–95. ( 10.1126/science.1135013) [DOI] [PubMed] [Google Scholar]
- 20.Butterfield NJ. 2018. Oxygen, animals and aquatic bioturbation: an updated account. Geobiology 16, 3–16. ( 10.1111/gbi.12267) [DOI] [PubMed] [Google Scholar]
- 21.Fedonkin MA. 2003. The origin of the Metazoa in the light of the Proterozoic fossil record. Paleontol. Res. 7, 9–41. ( 10.2517/prpsj.7.9) [DOI] [Google Scholar]
- 22.Noble SR, Condon DJ, Carney JN, Wilby PR, Pharaoh TC, Ford TD. 2015. U-Pb geochronology and global context of the Charnian Supergroup, UK: constraints on the age of key Ediacaran fossil assemblages. Geol. Soc. Am. Bull. 127, 250–265. ( 10.1130/B31013.1) [DOI] [Google Scholar]
- 23.Liu AG, Kenchington CG, Mitchell EG. 2015. Remarkable insights into the paleoecology of the Avalonian Ediacaran macrobiota. Gondwana Res. 27, 1355–1380. ( 10.1016/j.gr.2014.11.002) [DOI] [Google Scholar]
- 24.Pu JP, Bowring SA, Ramezani J, Myrow P, Raub TD, Landing E, Mills A, Hodgin E, Macdonald FA. 2016. Dodging snowballs: geochronology of the Gaskiers glaciation and the first appearance of the Ediacaran biota. Geology 44, 955–958. ( 10.1130/G38284.1) [DOI] [Google Scholar]
- 25.Boag TH, Darroch SAF, Laflamme M. 2016. Ediacaran distributions in space and time: testing assemblage concepts of earliest macroscopic body fossils. Paleobiology 42, 574–594. ( 10.1017/pab.2016.20) [DOI] [Google Scholar]
- 26.Xiao S, Laflamme M. 2009. On the eve of animal radiation: phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol. Evol. 24, 31–40. ( 10.1016/j.tree.2008.07.015) [DOI] [PubMed] [Google Scholar]
- 27.Hoyal CJF, Han J. 2018. Cambrian petalonamid Stromatoveris phylogenetically links Ediacaran biota to later animals. Palaeontology 61, 813–823. ( 10.1111/pala.12393) [DOI] [Google Scholar]
- 28.Sperling EA, Peterson KJ, Laflamme M. 2011. Rangeomorphs, Thectardis (Porifera?) and dissolved organic carbon in the Ediacaran oceans. Geobiology 9, 24–33. ( 10.1111/j.1472-4669.2010.00259.x) [DOI] [PubMed] [Google Scholar]
- 29.Waggoner B. 1999. Biogeographic analyses of the Ediacara biota: a conflict with paleotectonic reconstructions. Paleobiology 25, 440–458. ( 10.1017/S0094837300020315) [DOI] [Google Scholar]
- 30.Wood DA, Dalrymple RW, Narbonne GM, Gehling JG, Clapham ME. 2003. Paleoenvironmental analysis of the late Neoproterozoic Mistaken Point and Trepassey formations, southeastern Newfoundland. Can. J. Earth Sci. 40, 1375–1391. ( 10.1139/e03-048) [DOI] [Google Scholar]
- 31.Macdonald FA, Strauss JV, Sperling EA, Halverson GP, Narbonne GM, Johnston DT, Kunzmann M, Schrag DP, Higgins JA. 2013. The stratigraphic relationship between the Shuram carbon isotope excursion, the oxygenation of Neoproterozoic oceans, and the first appearance of the Ediacara biota and bilaterian trace fossils in northwestern Canada. Chem. Geol. 362, 250–272. ( 10.1016/j.chemgeo.2013.05.032) [DOI] [Google Scholar]
- 32.Grazhdankin D. 2014. Patterns of evolution of the Ediacaran soft-bodied biota. J. Paleontol. 88, 269–283. ( 10.1666/13-072) [DOI] [Google Scholar]
- 33.Narbonne GM, Laflamme M, Trusler PW, Dalrymple RW, Greentree C. 2014. Deep-water Ediacaran fossils from northwestern Canada: taphonomy, ecology, and evolution. J. Paleontol. 88, 207–223. ( 10.1666/13-053) [DOI] [Google Scholar]
- 34.Jablonski D, Sepkoski JJ, Bottjer DJ, Sheehan PM. 1983. Onshore-offshore patterns in the evolution of Phanerozoic shelf communities. Science 222, 1123–1125. ( 10.1126/science.222.4628.1123) [DOI] [PubMed] [Google Scholar]
- 35.Dejours P. 1989. From comparative physiology of respiration to several problems of environmental adaptions and to evolution. J. Physiol. 410, 1–19. ( 10.1113/jphysiol.1989.sp017517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benson BB, Krause D. 1984. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol. Oceanogr. 29, 620–632. ( 10.4319/lo.1984.29.3.0620) [DOI] [Google Scholar]
- 37.Piiper J, Dejours P, Haab P, Rahn H. 1971. Concepts and basic quantities in gas exchange physiology. Respir. Physiol. 13, 292–304. ( 10.1016/0034-5687(71)90034-X) [DOI] [PubMed] [Google Scholar]
- 38.Hofmann AF, Peltzer ET, Brewer PG. 2013. Kinetic bottlenecks to respiratory exchange rates in the deep-sea. Part 1: oxygen. Biogeosciences 10, 5049–5060. ( 10.5194/bg-10-5049-2013) [DOI] [Google Scholar]
- 39.Verberk WCEP, Bilton DT, Calosi P, Spicer JI. 2011. Oxygen supply in aquatic ectotherms: partial pressure and solubility together explain biodiversity and size patterns. Ecology 92, 1565–1572. ( 10.1890/10-2369.1) [DOI] [PubMed] [Google Scholar]
- 40.Deutsch C, Ferrel A, Seibel B, Pörtner HO, Huey RB. 2015. Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1135. ( 10.1126/science.aaa1605) [DOI] [PubMed] [Google Scholar]
- 41.Somero GN, Lockwood BL, Tomanek L. 2017. Biochemical adaptations: response to environmental challenges from life's origins to the Anthropocene, 1st edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 42.Peck LS, Webb KE, Bailey DM. 2004. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct. Ecol. 18, 625–630. ( 10.1111/j.0269-8463.2004.00903.x) [DOI] [Google Scholar]
- 43.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2, 686–690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 44.Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. 2012. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 79, 1–15. ( 10.1016/J.MARENVRES.2012.04.003) [DOI] [PubMed] [Google Scholar]
- 45.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97. ( 10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 46.Pörtner H-O. 2010. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893. ( 10.1242/jeb.037523) [DOI] [PubMed] [Google Scholar]
- 47.Melzner F, Bock C, Pörtner H-O. 2006. Critical temperatures in the cephalopod Sepia officinalis investigated using in vivo 31P NMR spectroscopy. J. Exp. Biol. 209, 891–906. ( 10.1242/jeb.02054) [DOI] [PubMed] [Google Scholar]
- 48.Melzner F, Bock C, Pörtner HO. 2006. Temperature-dependent oxygen extraction from the ventilatory current and the costs of ventilation in the cephalopod Sepia officinalis. J. Comp. Physiol. B 176, 607–621. ( 10.1007/s00360-006-0084-9) [DOI] [PubMed] [Google Scholar]
- 49.Vaquer-Sunyer R, Duarte CM. 2011. Temperature effects on oxygen thresholds for hypoxia in marine benthic organisms. Glob. Chang. Biol. 17, 1788–1797. ( 10.1111/j.1365-2486.2010.02343.x) [DOI] [Google Scholar]
- 50.Zielinski S, Pörtner HO. 1996. Energy metabolism and ATP free-energy change of the intertidal worm Sipunculus nudus below a critical temperature. J. Comp. Physiol. B 166, 492–500. ( 10.1007/s003600050037) [DOI] [Google Scholar]
- 51.Kristensen E. 1983. Ventilation and oxygen uptake by three species of Nereis (Annelida: Polychaeta). I. Effects of hypoxia. Mar. Ecol. Prog. Ser. 12, 289–297. ( 10.3354/meps012289) [DOI] [Google Scholar]
- 52.Perry SF, Jonz MG, Gilmour KM. 2009. Oxygen sensing and the hypoxic ventilatory response. Fish Physiol. 27, 193–253. ( 10.1016/S1546-5098(08)00005-8) [DOI] [Google Scholar]
- 53.Hofmann AF, Peltzer ET, Walz PM, Brewer PG. 2011. Hypoxia by degrees: establishing definitions for a changing ocean. Deep Sea Res. Part I 58, 1212–1226. ( 10.1016/j.dsr.2011.09.004) [DOI] [Google Scholar]
- 54.Enns T, Scholander PF, Bradstreet ED. 1965. Effect of hydrostatic pressure on gases dissolved in water. J. Phys. Chem. 69, 389–391. ( 10.1021/j100886a005) [DOI] [PubMed] [Google Scholar]
- 55.Levin L. 2003. Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr. Mar. Biol. Annu. Rev. 41, 1–45. [Google Scholar]
- 56.St-Denis CE, Fell CJD. 1971. Diffusivity of oxygen in water. Can. J. Chem. Eng. 49, 885 ( 10.1002/cjce.5450490632) [DOI] [Google Scholar]
- 57.Spicer JI, Gaston KJ. 1999. Amphipod gigantism dictated by oxygen availability? Ecol. Lett. 2, 397–403. ( 10.1046/j.1461-0248.1999.00105.x) [DOI] [Google Scholar]
- 58.Somero GN. 2002. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr. Comp. Biol. 42, 780–789. ( 10.1093/Icb/42.4.780) [DOI] [PubMed] [Google Scholar]
- 59.Liu AG, Matthews JJ, Menon LR, McIlroy D, Brasier MD. 2014. Haootia quadriformis n. gen., n. sp., interpreted as a muscular cnidarian impression from the Late Ediacaran period (approx. 560 Ma). Proc. R. Soc. B 281, 20141202 ( 10.1098/rspb.2014.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu AG, McIlroy D, Brasier MD. 2010. First evidence for locomotion in the Ediacara biota from the 565 Ma Mistaken Point Formation, Newfoundland. Geology 38, 123–126. ( 10.1130/G30368.1) [DOI] [Google Scholar]
- 61.Gehling JG. 1988. A cnidarian of actinian-grade from the Ediacaran Pound Subgroup, South Australia. Alcheringa 12, 299–314. ( 10.1080/03115518808619129) [DOI] [Google Scholar]
- 62.Sommer A, Klein B, Pörtner HO. 1997. Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina (L.). J. Comp. Physiol. B 167, 25–35. ( 10.1007/s003600050044) [DOI] [Google Scholar]
- 63.Sperling EA, Halverson GP, Knoll AH, Macdonald FA, Johnston DT. 2013. A basin redox transect at the dawn of animal life. Earth Planet. Sci. Lett. 371–372, 143–155. ( 10.1016/J.EPSL.2013.04.003) [DOI] [Google Scholar]
- 64.Mills DB, Ward LM, Jones C, Sweeten B, Forth M, Treusch AH, Canfield DE. 2014. Oxygen requirements of the earliest animals. Proc. Natl Acad. Sci. USA 111, 4168–4172. ( 10.1073/pnas.1400547111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sperling EA, Frieder CA, Raman AV, Girguis PR, Levin LA, Knoll AH. 2013. Oxygen, ecology, and the Cambrian radiation of animals. Proc. Natl Acad. Sci. USA 110, 13 446–13 451. ( 10.1073/pnas.1312778110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen KDT, Morley SA, Lai CH, Clark MS, Tan KS, Bates AE, Peck LS. 2011. Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS ONE 6, e29340 ( 10.1371/journal.pone.0029340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sperling EA, Knoll AH, Girguis PR. 2015. The ecological physiology of Earth's second oxygen revolution. Annu. Rev. Ecol. Evol. Syst. 46, 215–235. ( 10.1146/annurev-ecolsys-110512-135808) [DOI] [Google Scholar]
- 68.Runnegar B. 1991. Precambrian oxygen levels estimated from the biochemistry and physiology of early eukaryotes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 97, 97–111. ( 10.1016/0031-0182(91)90186-U) [DOI] [Google Scholar]
- 69.Levin LA, Huggett CL, Wishner KF. 1991. Control of deep-sea benthic community structure by oxygen and organic gradients in the eastern Pacific Ocean. J. Mar. Res. 49, 763–800. [Google Scholar]
- 70.White CR, Alton LA, Frappell PB. 2012. Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc. R. Soc. B 279, 1740–1747. ( 10.1098/rspb.2011.2060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowyer F, Wood RA, Poulton SW. 2017. Controls on the evolution of Ediacaran metazoan ecosystems: a redox perspective. Geobiology 15, 516–551. ( 10.1111/gbi.12232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gehling JG, Droser ML. 2013. How well do fossil assemblages of the Ediacara biota tell time? Geology 41, 447–450. ( 10.1130/G33881.1) [DOI] [Google Scholar]
- 73.Grazhdankin DV, Balthasar U, Nagovitsin KE, Kochnev BB. 2008. Carbonate-hosted avalon-type fossils in arctic Siberia. Geology 36, 803–806. ( 10.1130/G24946A.1) [DOI] [Google Scholar]
- 74.Boag TH, Stockey RG, Elder LE, Hull PM, Sperling EA. 2018. Data from: Oxygen, temperature, and the deep-marine stenothermal cradle of Ediacaran evolution Dryad Digital Repository. ( 10.5061/dryad.bf43443) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Boag TH, Stockey RG, Elder LE, Hull PM, Sperling EA. 2018. Data from: Oxygen, temperature, and the deep-marine stenothermal cradle of Ediacaran evolution Dryad Digital Repository. ( 10.5061/dryad.bf43443) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.bf43443 [74].