Abstract
Wind pollination has evolved from insect pollination in numerous angiosperm lineages and is associated with a characteristic syndrome of morphological traits. The traits initiating transitions to wind pollination and the ecological drivers involved are poorly understood. Here, we examine this problem in Thalictrum pubescens, an ambophilous (insect and wind pollination) species that probably represents a transitional state in the evolution of wind pollination. We investigated wind-induced pollen release by forced harmonic motion by measuring stamen natural frequency (fn), a key vibration parameter, and its variability among nine populations. We assessed the repeatability of fn over consecutive growing seasons, the effect of this parameter on pollen release in a wind tunnel, and male reproductive success in the field using experimental manipulation of the presence or absence of pollinators. We found significant differences among populations and high repeatability within genotypes in fn. The wind tunnel assay revealed a strong negative correlation between fn and pollen release. Siring success was greatest for plants with lower fn when pollinators were absent, but this advantage diminished when pollinators were present. Our biomechanical analysis of the wind–flower interface has identified fn as a key trait for understanding early stages in the transition from insect to wind pollination.
Keywords: ambophily, plant biomechanics, pollen dispersal, pollen limitation, reproductive transitions, wind pollination
1. Introduction
Evolutionary transitions from insect to wind pollination have occurred repeatedly in the flowering plants and are associated with significant modifications to floral traits, resource allocation and mating patterns [1–3]. Wind pollination probably evolves when changes to the abiotic or biotic environment result in a decline in pollinator abundance or activity [4,5]. The transition is thought to be restricted to lineages with relatively simple floral structures that are easily modified to overcome biomechanical constraints on wind pollination [6,7]. Unfortunately, these hypotheses have rarely been evaluated using experiments or theoretical models and little is known about the microevolutionary processes responsible for the origins of wind pollination, despite the importance of this transition in angiosperms.
One approach to investigating transitions between pollination systems is to focus on pollination ecotypes, variants of species locally adapted to different pollen vectors through changes in floral traits [8,9]. This variation provides opportunities for investigating the ecological mechanisms underpinning shifts in pollination mode and the precise sequence of adaptive changes in floral structures [10]. Floral divergence leading to pollination ecotypes is usually attributed to regional differences in the composition and foraging habits of pollinators [11–13]. Geographical variation in pollinators may alternatively lead to a loss in pollinator services, causing selection for selfing or wind pollination [2,3]. Surprisingly, few if any species that exhibit both wind and insect pollination are known to exhibit population differentiation in either of their pollination modes, which has impeded experimental investigations into floral trait evolution under pollinator limitation. The apparent absence of intraspecific variation may simply be owing to wind pollination not receiving the same level of research interest as animal-pollinated systems. But it may also have been neglected because of limited appreciation of the biomechanical features of wind pollination mechanisms.
Wind pollination consists of three distinct physical processes: pollen release, dispersal and capture [14]. Each process is governed by different fluid dynamic principles and probably requires separate biomechanical adaptations to function effectively [1,15]. Convergence in floral traits is typical of wind-pollinated taxa (e.g. non-sticky small-sized pollen, reduced corolla size, expanded stigmatic surfaces, flexible stamens, high pollen : ovule ratio, unisexual flowers), presumably to overcome specific aerodynamic constraints [14,16]. However, the functional significance for many traits associated with wind pollination is not well supported by mechanistic studies of trait function [15], nor is it evident from the patterns of convergent evolution which traits are directly relevant for initiating the transition from insect to wind pollination [17]. Experimental studies of the biomechanics of wind–flower interactions are needed to determine the trait modifications necessary for promoting wind pollination and this is the approach we use in this investigation.
Understanding the mechanisms of pollen release is particularly important because it is the first critical step in successful wind pollination, and it is probably costly to plants that rely solely on insect pollinators, as a result of male gamete wastage. Pollen release requires the mobilization of stationary pollen by external forces to overcome its adhesion to anthers [1]. This is challenging for plants because pollen is immersed in a boundary layer of nearly still air surrounding stamens in which aerodynamic forces are generally weak [18]. Architectural features of inflorescences may be important as many wind-pollinated species position flowers away from their own foliar boundary layers, ostensibly exposing pollen to stronger wind [19]. Wind-pollinated plants may subvert constraints imposed by the boundary layer by harnessing wind energy, driving stamen motion capable of dislodging pollen. This mechanism involves turbulence-induced resonance vibration of stamens resulting in the inertial ejection of pollen from anthers [18,20]. Stamen resonance occurs when turbulent eddies impact stamens periodically at the natural frequency of vibration of stamens (fn; see [21]) resulting in large amplitude oscillations owing to the storage of vibrational energy. The kinetic energy of turbulence declines over the frequency spectrum of eddies, leading to an expected inverse relation between the probability of pollen release and fn. The natural frequency is a composite trait determined by several fundamental properties of stamens including their length and mass (see Material and Methods). We might predict that fn mediates pollen release and that selection favours lower fn, particularly under pollinator limitation.
A few species do not exhibit the classical wind pollination syndrome and still rely on a mix of insect and wind pollination (ambophily) [2]. An important unresolved issue for ambophilous species is whether it is an intermediate state in the transition from insect to wind pollination, or if it represents a mixed strategy and is an evolutionarily stable state [2,3]. Where there is local variation in the reliability of pollinator service, we might expect evolutionary responses to pollinator abundance resulting in pollination-mediated selection on floral traits, such as stamen fn, and variation among populations in pollination systems. Few studies have examined variation in insect and wind pollination among populations (e.g. [5,22,23]), and none have investigated in detail the function of vibrating stamens in different pollination environments.
Here, we investigate variation in the mechanism of pollen release and its consequences for fitness under pollinator limitation in Thalictrum pubescens, an ambophilous perennial herb. The contrasting requirements of insect versus wind pollination suggest that selection for traits promoting wind-induced pollen release will depend on the availability of pollinators. We address three questions in our study: (i) are there genetic differences among populations in the natural frequency of stamens? To address this question, we measured fn and its variability among nine populations using a simple mechanical test. We also investigated the repeatability of fn over three consecutive growing seasons in a sample of genotypes from different populations to assess whether this trait has a heritable component; (ii) does variation in the natural frequency of stamens influence the release of pollen in wind? We assayed pollen release in a wind tunnel to determine the relationship between fn and pollen release. We predicted an inverse relation between these parameters; and (iii) what is the effect of natural frequency on seed set in the presence or absence of pollinators? We manipulated the presence or absence of pollinators in the field to determine the effects of fn on seed production.
2. Materials and methods
(a). Study species
Thalictrum pubescens (Ranunculaceae) is a dioecious perennial herb native to deciduous forests and wetlands of eastern North America [24,25] growing in open and shaded habitats including woods, thickets, marshes, meadows, ditches and stream banks. Plants reproduce vegetatively by ramets and sexually by outcrossed seed. Ramets flower from late June until early August and populations are weakly protandrous. Floral displays are large with paniculate inflorescences that are 1–3 m in height consisting of numerous small white flowers. Flowers of both sexes are fragrant, produce no nectar or petals and have small white sepals that do not enclose the reproductive organs. The sexual system of T. pubescens is cryptic dioecy (see [26]) because female flowers produce, in addition to several uniovulate carpels, functioning stamens with sterile, inaperturate pollen used for pollinator attraction [24,27,28]. In both sexes, the stamens are flexible, filiform to clavate in shape and have a basifixed anther that dehisces longitudinally (see the electronic supplementary material, figure S1). In females, the stamens are slightly shorter and four times fewer in number than in males [25]. The reproductive organs are well exposed to airflows, suggesting wind pollination, but both wind and insects appear to contribute to pollination [24,27,28]. Among the approximately 190 species of Thalictrum, there is extensive variation in the floral syndromes and pollination modes with species pollinated by wind, insects or both [24]. Phylogenetic analysis indicates that insect pollination is the ancestral state and wind pollination has evolved independently several times possibly via ambophily [29,30].
(b). Sampling and cultivation of plants
In spring 2014, we excavated 10–12 genets upon shoot emergence from each of nine populations across Ontario, Canada (electronic supplementary material, figure S2). The populations were chosen to encompass the wide variation in environmental conditions in which plants occur, including road-side marshes with no canopy, the understory of closed-canopy deciduous forests and a partially shaded gravel ditch. Population sizes varied widely from 20 to approximately 2000 individuals per population. Plants were returned to a glasshouse at the University of Toronto where they were placed in 25 cm diameter soil-filled pots in a random block design and allowed to grow for two seasons.
In spring 2016, we cloned 30 male and 30 female plants from this sample of T. pubescens plants, using 4–6 equally sized rootstock divisions per genet. We chose female plants for cloning at random from the collection, whereas males comprised those with the 15 highest and lowest stamen natural frequencies as measured in 2015 (see below). Each division was placed separately into a 25 cm diameter pot and grown outdoors under shade cloth at the Koffler Scientific Reserve, University of Toronto (Newmarket, Ontario).
(c). Measuring the natural frequency of stamens
We measured variation in the natural frequency of vibration of stamens in the sample of male plants before cloning using the modal testing procedure of Timerman et al. [20]. The method uses an electrodynamic shaker to apply a controlled amount of vibration to the fixed end of a stamen. The electrodynamic shaker is similar to a loudspeaker where a current passing through a coil of wires drives its motion. To determine the natural frequency of stamens, the frequency of vibration is increased over a fixed range and the displacement of the anther is observed. Intuitively, the motion of the anther should match the motion of the electrodynamic shaker, but a more vigorous response is predicted for stamens that are excited at their natural frequencies (i.e. because they resonate). By forcing a stamen to vibrate through a range of frequencies, the natural frequency can be determined by identifying the excitation frequency that causes the most vigorous change in the displacement of the anther.
To measure the natural frequency in our sample of plants, we transferred males in their second year of growth from the glasshouse as they came into flower into a laboratory with a fluorescent light programmed at 16 h daylight intervals and 21°C. A single flower with fully extended filaments and undehisced anthers was mounted horizontally by its receptacle onto a vertically oriented SmartShaker Pro K2004E01 electrodynamic shaker (The Modal Shop, Cincinnati, Ohio). We then removed all but one stamen from the flower using fine-tipped forceps. The stamen was vibrated in the vertical plane using a low amplitude sine wave that increased in frequency from 5 to 50 Hz. The frequency range was chosen based on our preliminary analysis indicating that the natural frequency was likely to occur in this range. A sufficiently low amplitude was chosen to limit displacement of the anther until the stamen resonated. The procedure was repeated for five flowers from 51 male plants (i.e. the number of male plants excavated) and one-way analysis of variance was used to analyse differences among populations in natural frequency.
In addition to natural frequency, we also measured stamen length, L, diameter, d and mass, m, in 2015 by randomly sampling three stamens from five flowers per plant. We used these measurements to investigate the correlates of stamen natural frequency. Theory indicates that slender cantilevered structures bearing a load at the free end have a natural frequency given by where EI and L are the flexural rigidity and length of the structure, respectively, and m is the mass of the load [21]. The flexural rigidity quantifies the resistance of a structure to bending and is the product of two properties: the modulus of elasticity, E, and the second moment of area, I. The former is an intrinsic property of materials describing stiffness, whereas the latter is an intrinsic property of structures describing the geometrical distribution of material. The second moment of area is known empirically for certain shapes. In structures with a circular cross-section, the second moment of area is , where r is the radius of the cross-section. We determined the expected natural frequency for each plant by parametrizing these models with the stamen measurements and setting r = d/2. We also used linear regression to investigate correlations between stamen natural frequency and each of the stamen traits.
To determine if there was a heritable component to stamen natural frequency, we quantified the degree to which variation among genotypes contributed to the total variation in fn (i.e. the repeatability) [31]. In the summer of 2016 and 2017, we measured stamen natural frequency for three flowers from 14 of the genotypes measured in 2015 and cloned in 2016. The genotypes selected encompassed the range of natural frequencies measured from our population sample and included plants from seven populations. We measured fn in four to six clones per genotype and used the mean in our analysis. To estimate the variance components of stamen natural frequency, we used a linear mixed model with a year as a fixed factor and genotype as a random effect. The significance of the fixed factor was determined using the Satterthwaite approximation implemented in the R library lmerTest [32].
(d). Assay of pollen release in a wind tunnel
We evaluated the effects of natural frequency on pollen release in a custom-made benchtop wind tunnel at low () and high average wind speeds . Both wind speeds are likely to induce stamen resonance during flowering [18]. The test section of the wind tunnel had a length of 120 cm and a rectangular cross-section measuring 25.4 × 17.8 cm with Reynolds numbers ReL ∼ 20 000 and ReH ∼ 70 000, indicating that the air flow was turbulent (see the electronic supplementary material, appendix 1). We assayed pollen release for flowers sampled from 10 males representing each of the populations that were used to investigate variation in the natural frequency of stamens. Each plant was evaluated, in sequence, at both wind speeds with three replicates per wind speed. Prior to the experiment, we randomized the sequence of low and high wind speed trials to limit any effects of repeatedly sampling flowers from each plant. For each trial, we randomly sampled a single flower from a focal plant if at least half of its anthers were dehiscent and contained many pollen grains. The flower was placed by its pedicel into a 0.1 ml water-filled PCR tube to prevent wilting. We sealed the tube using putty to prevent the flower from moving and water from spilling. We then mounted the tube upside down to a 12 cm long, thin rod at the centre of the test section. A greased microscope slide was placed downwind 5 cm from the flower centred upon the anthers to collect pollen. During a trial, the flower was exposed to the wind for 5 min and the flower was filmed using a distance-calibrated Casio Elixim FH25 digital camera at 120 frames per second. Following a trial, we removed the flower from the test section and counted the number of dehiscent anthers using a dissection microscope. We stained pollen on the slide with fuchsine and sealed it with a 22 × 22 mm coverslip. Later, the number of pollen grains captured was counted in the area covered by the coverslip. Pollen counts were normalized by the number of dehiscent anthers to account for variation among flowers in pollen availability. To determine the acceleration of stamens and the characteristics of their motion in the wind tunnel, we used tracker video analysis and modeling software (Open Source Physics, http://www.compadre.org/osp/) to track the motion of the single most visible and rapidly vibrating stamen per video for 1 min.
(e). Determining the fitness consequences of variation in stamen natural frequency
We evaluated the effects of fn on a fitness component (seed set) in a manipulative field experiment. Our broader objective was to determine if pollinator availability could influence selection for wind pollination. Specifically, lower fn should be favoured under pollinator limitation as it would promote greater atmospheric pollen dispersal. By contrast, higher fn should be favoured when pollinator visits are abundant because more pollen would be available for biotic pollination.
We established arrays of female and male plants in seven replicated blocks located at least 200 m apart in grassy fields located throughout the field station. Each block consisted of four 1.5 × 1.5 × 2.0 m screen houses separated by 20 m in a grid formation. The screen houses were randomized in a 2 × 2 factorial design to exclude or permit insect visits to plants with either high or low fn. They were constructed in the field using 38 × 38 mm lumber and enclosed using 0.288 mm diameter (1.13 × 1.30 mm aperture) FG72 fibreglass screen (C.R. Laurence of Ontario, Concord, Ontario). Insect visits were permitted by cutting 5 cm wide slits lengthwise into the top, middle and bottom of each screened panel. We used screen houses for the insect inclusion treatment to ensure uniformity in airflow conditions among treatments.
Shortly before anthesis in July 2015, we randomly assigned one female genotype and a random pair of male genotypes with high and low fn to each block. We then paired one female clone with two male clones from each genotype and placed the pairs separately into both types of screen houses. In each screen house, we positioned the female clone at the centre and the two male clones in opposite corners. The experiment involved a total of 28 female and 56 male plants divided among seven replicates, each comprised of four female and two pairs of four male clones. We positioned Vortex cup anemometers (Inspeed, Sudbury, Massachusetts) for measuring wind speed at a height of 1.5 m at the centre of at least one screen house per block. In all, there were eight male and four female plants per block and a total of 56 male and 28 female plants from 14 male and seven female genotypes in the experiment.
During the flowering period from late June to mid-July, we observed pollinator visits to plants in our insect inclusion treatments. On each day of the experiment, from 11.00 to 15.00, we recorded the number of insect visits during a 30 min interval to plants of both sexes in four randomly chosen blocks. At fruit maturation, we sampled 10 flowers per female plant and counted the number of seeds and total number of carpels per flower. We evaluated the interactive effects of stamen natural frequency and pollinator availability on siring success, represented by proportion seed set, using a generalized linear model with quasi-binomial errors to account for over-dispersion in our data. Because each carpel has only one ovule, we considered each seed to be a successful instance of a Bernoulli trial resulting in a binomial distribution of seeds; i.e. we modelled the number of successes (seeds) and failures (unfertilized ovules). We included an additional fixed factor in our model accounting for the total number of male flowers present in each screen house as pollen availability should influence seed set. We also investigated whether fn affected the pollinator visitation rate using a one-tailed paired t-test to test the hypothesis that males with greater fn are visited more often.
3. Results
(a). Variation in stamen natural frequency
Time-varying sinusoidal excitation of the electrodynamic shaker caused stamens to vibrate vigorously (resonate) in the vertical axis within a narrow band of frequencies averaging 15.2 ± 4.14 Hz (mean ± s.d., n = 57) at the natural frequency. On average, stamens were 6.29 ± 0.96 mm (n = 51) in length, 0.40 ± 0.07 mm (n = 51) in filament diameter and 0.19 ± 0.06 mg (n = 57) in anther mass. The cantilever beam model predicted fn of stamens reasonably well based on stamen mass, length and diameter. We found a significant linear relationship between the expected and observed natural frequencies (β = 0.88, 95% confidence interval 0.50–1.26, d.f. = 48, r2 = 0.31, p < 0.001) and the model predicted an average fn of 21.8 ± 4.97 Hz (n = 50).
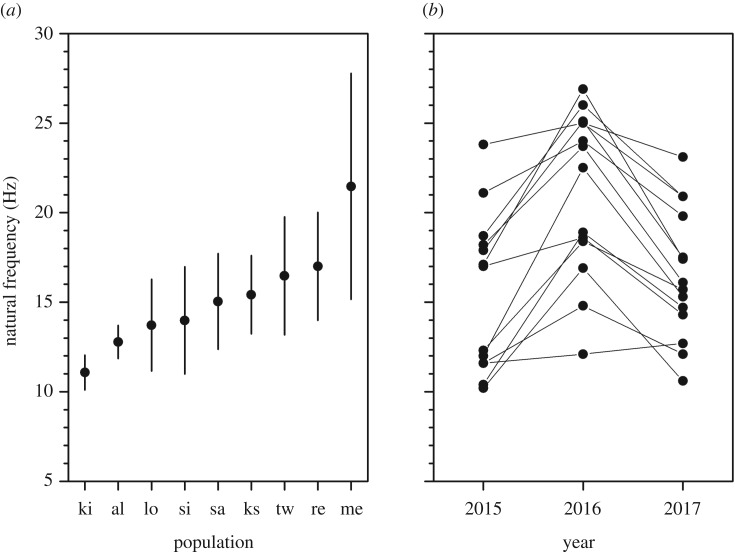
We found significant differences in stamen natural frequency (F8,49 = 4.97, p < 0.001) ranging from 11.10 to 22.77 Hz among populations of T. pubescens grown in a common glasshouse environment. This variation was associated with negative correlations with stamen length (β = −0.15, r2 = 0.70, d.f. = 7, p = 0.003) and/or anther mass (β = −0.01, r2 = 0.50, d.f. = 7, p = 0.02) among populations (electronic supplementary material, figure S1). Stamen natural frequency was also correlated within genotypes measured over three consecutive years. Specifically, we found no significant differences in stamen natural frequency among years (F1,36 = 0.085, p = 0.77) with 50.3% of the observed variation in natural frequency resulting from differences among genotypes (electronic supplementary material, table S1).
(b). Pollen release in the wind tunnel
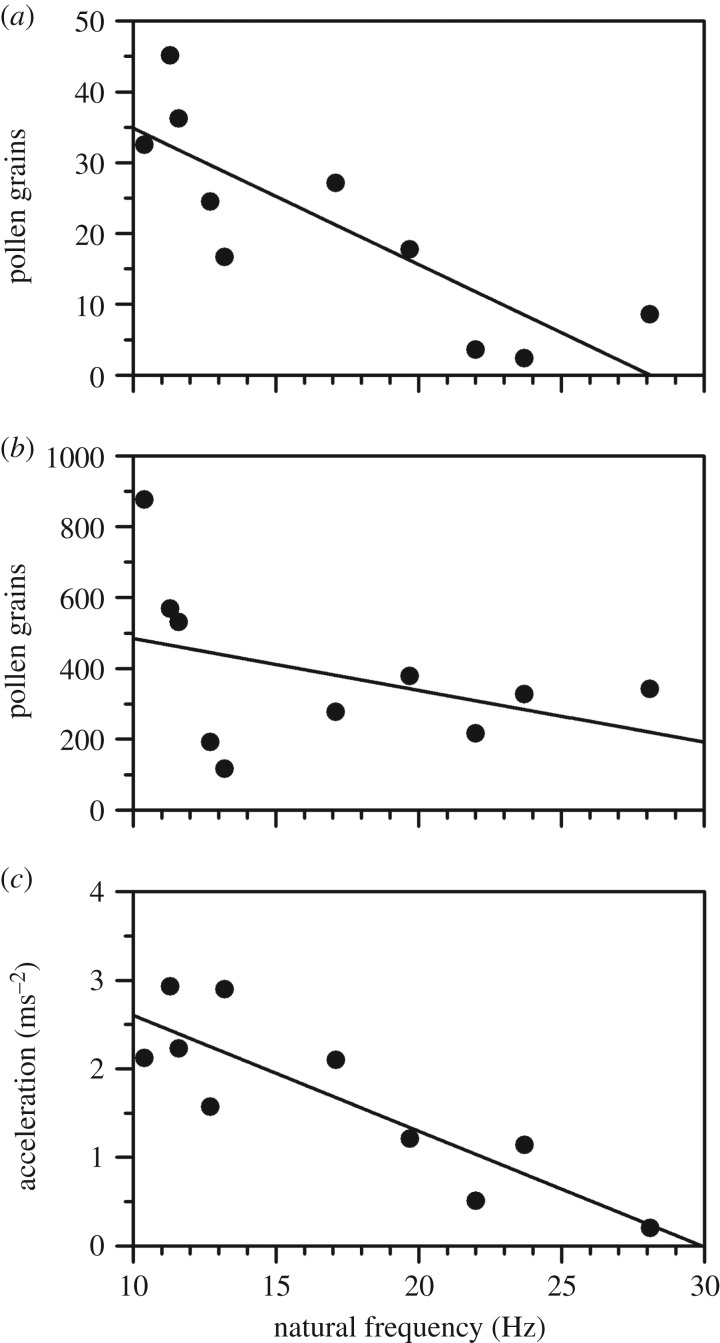
In the wind tunnel, we found a significant negative relationship between the number of pollen grains released and stamen natural frequency at an average wind speed of 1.5 m s−1 (figure 2a; r2 = 0.65, p = 0.003). This relationship did not occur at 5.0 m s−1 wind speed (r2 = 0.06, p = 0.25) but pollen release was greatest from the three plants with the lowest stamen natural frequency (figure 2b). At the lower wind speed, we also found a negative relationship between stamen acceleration and natural frequency (figure 2c; r2 = 0.71, p = 0.001). The greater acceleration for stamens with low natural frequency was owing to wind-induced stamen resonance, which did not occur in stamens with high natural frequency (electronic supplementary material, figure S3).
Figure 2.
Effect of stamen natural frequency on both pollen release at 1.5 m s−1 (a; r2 = 0.65, p < 0.01) and 5 m s−1 (b; r2 = 0.65, p < 0.003) average wind speed, and root-mean-square stamen acceleration at 1.5 m s−1 (c; r2 = 0.65, p < 0.01) for flowers of T. pubescens in a wind tunnel. Each point represents the mean of three trials per genotype.
(c). The fitness consequences of stamen natural frequency in the field
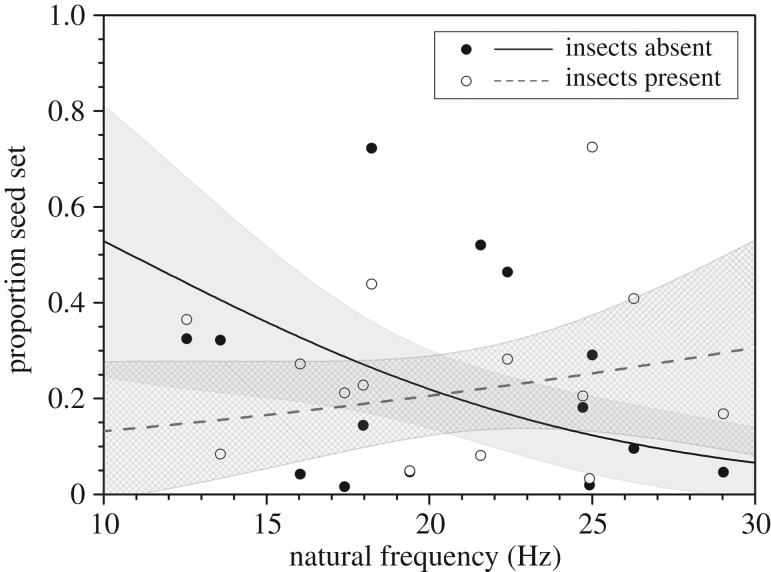
As expected, seed production depended predominantly on the number of male flowers present in screen houses (F1,26 = 19.34, p < 0.001) and not on pollinator availability (F1,24 = 0.36, p = 0.55) or stamen natural frequency (F1,25 = 1.27, p = 0.27; electronic supplementary material, table S2); but there was a significant interaction between the effects of pollinator availability and stamen natural frequency on seed set (figure 3; F1,23 = 6.08, p = 0.02). As predicted, proportion of seed set declined with natural frequency when pollinators were excluded (β = −0.14, t23 = −2.514, p = 0.019; figure 3) and tended to increase with fn when pollinators were allowed to visit flowers, although the slope was not significant (β = 0.051, t23 = 0.95, p = 0.35; figure 3). Wind speed was generally low in screen houses (mean ± s.d. = 0.83 ± 0.50 m s−1) during pollination but did not differ significantly among pollination treatments (t26 = 0.55, p = 0.59).
Figure 3.
Observed and fitted effect of stamen natural frequency in T. pubescens on seed set under insect exclusion (filled circle, solid line) and inclusion (open circle, dashed line) treatments under field conditions. Shaded regions represent the 95% confidence regions of the fitted models.
We observed pollinators visiting flowers in screen houses in which they were allowed entry. We mainly observed visitors foraging intensively on male plants for pollen but visits to females and transitions from male to female flowers were also recorded. Our observations spanned 29 h over 18 days from 17 July to 8 August 2016 and involved a total of 12 screen houses from six blocks. There was a total of 70 observation intervals and each screen house was observed an average of 5.83 ± 2.21 (mean ± s.d.) times during the study. We observed a total of 26 pollinators, resulting in an average visitation rate of 0.88 ± 0.52 (range = 0–2) visits h−1 to screen houses. Pollinators were primarily small solitary pollen-collecting bees (85%) but also hoverflies (12%) and bumblebees (4%). Within blocks, the visitation rate was significantly greater for screen houses with greater fn males (t5 = 2.27, p = 0.036). Screen houses with lower fn males received an average of 0.76 ± 0.16 visits h−1 whereas those with higher fn received 1.37 ± 0.52 visits h−1.
4. Discussion
Our investigation of wind-induced stamen vibration in T. pubescens revealed four key findings: (i) the release of pollen is inversely related to fn ; (ii) populations are differentiated with respect to fn; (iii) there is evidence of heritable between-population genetic variation in fn; and (iv) the fitness effects of fn depend on the availability of pollinators. Our study provides, to our knowledge, the first experimental evidence suggesting that pollinator availability may lead to divergent selection on traits governing wind pollination. Below, we consider the function of fn in pollination broadly, and the relevance of our findings to early stages in the evolution of wind pollination.
(a). Variation and repeatability of stamen natural frequency
Plants sampled from populations of T. pubescens grown in the glasshouse exhibited significant differences in fn (figure 1a). A plausible explanation for this finding is that the populations are differentiated with respect to fn owing to the selection for greater wind or insect pollination, depending on local conditions. We did not observe any notable features of the habitats of populations sampled, such as soil moisture or shading, that could account for this variation but acknowledge that our sample size of populations is relatively small. Future investigation of the pollination systems of populations combined with a larger field survey of fn is obviously needed to evaluate whether populations are locally adapted to the pollination environment in which they occur.
Figure 1.
Variation in stamen natural frequency of T. pubescens: (a) among populations, and (b) among genotypes over years. The population comparison occurred over one growing season whereas the genotypic comparison was over three consecutive growing seasons. Black circles represent population means and error bars represent 95% confidence intervals. Populations are in ascending order by mean.
Our measurements of fn were relatively stable within genotypes over 3 years despite clonal propagation and the growth of plants in both the glasshouse (2014–2016) and field (2016–2017). However, there was some plasticity in fn across years, particularly in 2016 when fn was greater for most genotypes (figure 1b). The spike in fn was probably because of rootstock divisions made before flowering. This procedure resulted in all clones having reduced height and flower number relative to the parental genets measured in 2015 (Δ height = 92.2 ± 40.3 cm; Δ flowers = 3158.5 ± 1706.5). Virtually all clones grew prodigiously again in 2017 and were of a similar height to plants in 2015. Over 5 years of observation, we have noted that reduced growth in T. pubescens suppresses flower size causing a reduction in stamen length (and see [33,34]). The inverse relationship between fn and stamen length suggests that poor condition may cause an increase in fn and explain the change in pattern among years we report.
(b). Wind tunnel assay of pollen release
Our study demonstrated that wind-induced stamen vibrations cause pollen release in T. pubescens. Pollen release was greatest from resonating stamens owing to the rapid acceleration of anthers, as predicted by theory [18]. Stamens with higher fn were less likely to resonate at lower wind speeds and thus released significantly fewer pollen grains. There was sufficient energy available at higher wind speeds to cause stamen resonance for all fn, but the amount of pollen released was still greatest for stamens with lower fn. We were unable to measure stamen acceleration at higher wind speeds, but our results are consistent with the prediction that more wind energy is available to excite stamens with lower fn. Resonance vibration may be detrimental to plants by causing stem buckling and lodging [35,36]. In T. pubescens, resonance vibration promoted pollen dispersal without these negative effects. Several analogous cases occur in bryophytes [37], horsetails [38] and fungi [39], and function in spore dispersal. Resonance vibration of stems may also promote pollen capture [40], and is an important mechanism in grasses and Ephedra [21,41].
Differences in pollen adhesion could also account for variation in pollen release among flowers. Adhesion forces resist wind-induced pollen release and determine the minimum acceleration of anthers required for pollen release [18,42]. Pollen adhesion arises from sticky tapetum-derived substances coating pollen grains (e.g. pollenkitt), causing them to clump [43], but may also involve electrostatic and frictional forces [1,44]. Variation in pollen adhesion has been reported in ambophilous species and is negatively associated with population differences in pollinator visits [45,46]. The relative contributions of adhesion and inertial forces (e.g. stamen acceleration) in shaping the inverse relationship between pollen release and fn (figure 2a) are unclear from our experiment, but variation in inertial forces was undoubtedly important given the concordant decline in stamen acceleration over the range of fn tested (figure 2c).
(c). Fitness consequences of stamen natural frequency in the field
In our field experiment, seed set in females depended on the pollination treatment and genotype of pollen donors. When pollinators were excluded, females produced a higher proportion of seed when paired with lower fn males (figure 3, solid line). Given the observed effect of fn on pollen release in the wind tunnel, we interpret this to mean that there was an advantage in terms of siring success for male genotypes with lower fn as they released more pollen by wind. Further investigation is needed to determine if stamens with lower fn are indeed depleted of pollen at a faster rate.
The advantage of having a lower fn was not evident when pollinators were able to visit plants, resulting in a marginal increase in the proportion of seeds with increasing fn (figure 3, dashed line). Unexpectedly, we found that allowing pollinators to visit plants in screen houses led to a reduction in seed set with lower fn males. Visitors may not have been effective pollinators of females and instead may have exported pollen out of screen houses before dispersal by wind. Indeed, 54% of the observed visitors left screen houses after foraging on male flowers. Disturbance of anthers by insect visitors may have also contributed to pollen losses owing to gravitational settling [47], further reducing pollen available for wind dispersal to females. Given the reduced level of wind pollination relative to the exclusion treatment (i.e. figure 3, fn approx. 10–20 Hz), the increased proportion of seeds with fn was probably not related to stamen biomechanics. On average, the visitation rate was nearly double that recorded in screen houses with higher fn males, suggesting that these phenotypes may be more attractive to pollinators for reasons at present unknown. Variation in flower number did not account for this difference in visitation rate to males with contrasting values of fn (r2 = 0.09, p = 0.18); however, it could have resulted from variation in floral scent. In Thalictrum, floral scent is correlated with pollination mode, with insect-pollinated species exhibiting a greater diversity and quantity of volatile organic compounds that are functionally associated with pollinator attraction in comparison with wind-pollinated species [48]. Intriguingly, individuals of T. pubescens exhibit variation in the fragrance intensity of flowers (D. Timerman 2015, personal observation), which, if related to fn, could possibly explain our results.
(d). Stamen resonance and the evolution of pollination systems
Many structural modifications to flowers and inflorescences associated with the wind pollination syndrome have evolved to achieve greater efficiency in pollen dispersal and capture [1]. These adaptations are unlikely to have arisen simultaneously but rather accumulated gradually during the transition from animal to wind pollination, with ambophily as a probable intermediate stage [2,3,6,17]. It is not known with any certainty which modifications commonly initiate the transition to wind pollination. However, because the fundamental difference between the two pollination systems involves the mechanisms of pollen dispersal, modifications to stamens, the organs of pollen release, probably played a key role in the early stages of the transition; hence, our focus on stamens as the initiators of shifts to wind pollination seems justified.
A major unresolved question concerning the evolution of wind pollination is the apparent absence of clear-cut pollination ecotypes involving animal versus wind-pollinated populations within species. Ambophilous species are generally considered to possess a mixed pollination strategy with populations employing both animal and wind pollination; but there are no quantitative estimates of the relative importance of wind versus animal pollination to siring success and seed set in any natural population of an ambophilous species, presumably because of the technical challenges in obtaining such estimates. Outside of our study, we are not aware of efforts to determine the extent to which ambophilous populations have become genetically differentiated for reproductive traits associated with wind versus animal pollination. In T. pubescens, variation among populations in fn is near continuous and although populations may differ in the relative importance of wind versus insect pollination, there is no evidence of distinct pollination ecotypes. Future work targeting functional trait variation in ambophilous species may offer the best opportunities for understanding the early stages in one of the most iconic reproductive transitions in flowering plants.
Supplementary Material
Acknowledgements
We thank Georgia Henry and George Sandler for assisting in the laboratory and field experiments, and Stephan Schneider and the Koffler Scientific Reserve for logistical assistance.
Data accessibility
Laboratory and field data: Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.2f7g889 [49].
Authors' contributions
D.T. and S.C.H.B. conceived the ideas and wrote the manuscript. D.T. designed the experiments and collected and analysed the data. Both authors contributed to writing the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada through a discovery grant to S.C.H.B. and a Canada Graduate Scholarship to D.T.
References
- 1.Niklas KJ. 1985. The aerodynamics of wind pollination. Bot. Rev. 51, 328–386. ( 10.1007/BF02861079) [DOI] [Google Scholar]
- 2.Culley TM, Weller SG, Sakai AK. 2002. The evolution of wind pollination in angiosperms. Trends Ecol. Evol. 17, 361–369. ( 10.1016/S0169-5347(02)02540-5) [DOI] [Google Scholar]
- 3.Friedman J, Barrett SCH. 2009. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515–1527. ( 10.1093/aob/mcp035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox PA. 1991. Abiotic pollination: an evolutionary escape for animal-pollinated angiosperms [and discussion]. Phil. Trans. R. Soc. Lond. B 333, 217–224. ( 10.1098/rstb.1991.0070) [DOI] [Google Scholar]
- 5.Goodwillie C. 1999. Wind pollination and reproductive assurance in Linanthus parviflorus (Polemoniaceae), a self-incompatible annual. Am. J. Bot. 86, 948–954. ( 10.2307/2656611) [DOI] [PubMed] [Google Scholar]
- 6.Linder HP. 1998. Morphology and the evolution of wind pollination. In Reproductive biology in systematics, conservation and economic botany (eds Owens SJ, Rudall PJ), pp. 123–135. Richmond, UK: Royal Botanic Gardens, Kew. [Google Scholar]
- 7.Friedman J, Barrett SCH. 2008. A phylogenetic analysis of the evolution of wind pollination in the angiosperms. Int. J. Plant Sci. 169, 49–58. ( 10.1086/522510) [DOI] [Google Scholar]
- 8.Grant V, Grant KA. 1965. Flower pollination in the phlox family. New York, NY: Columbia University Press. [Google Scholar]
- 9.Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annu. Rev. Ecol. Syst. 1, 307–326. ( 10.1146/annurev.es.01.110170.001515) [DOI] [Google Scholar]
- 10.Harder LD, Johnson SD. 2009. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol. 183, 530–545. ( 10.1111/j.1469-8137.2009.02914.x) [DOI] [PubMed] [Google Scholar]
- 11.Johnson SD. 2006. Pollinator-driven speciation in plants. In Ecology and evolution of flowers (eds Harder LD, Barrett SCH), pp. 295–310. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Anderson B, Alexandersson R, Johnson SD. 2010. Evolution and coexistence of pollination ecotypes in an African Gladiolus (Iridaceae). Evolution 64, 960–972. ( 10.1111/j.1558-5646.2009.00880.x) [DOI] [PubMed] [Google Scholar]
- 13.van der Niet T, Peakall R, Johnson SD. 2014. Pollinator-driven ecological speciation in plants: new evidence and future perspectives. Ann. Bot 113, 199–212. ( 10.1093/aob/mct290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehead DR. 1969. Wind pollination in the angiosperms: evolutionary and environmental considerations. Evolution 23, 28–35. ( 10.1111/j.1558-5646.1969.tb03490.x) [DOI] [PubMed] [Google Scholar]
- 15.Ackerman JD. 2000. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Plant Sys. Evol. 222, 167–185. ( 10.1007/BF00984101) [DOI] [Google Scholar]
- 16.Faegri K, van der Pijl L. 1966. The principles of pollination ecology. New York, NY: Pergamon Press. [Google Scholar]
- 17.Welsford MR, Hobbhahn N, Midgley JJ, Johnson SD. 2016. Floral trait evolution associated with shifts between insect and wind pollination in the dioecious genus Leucadendron (Proteaceae). Evolution 70, 126–139. ( 10.1111/evo.12821) [DOI] [PubMed] [Google Scholar]
- 18.Urzay J, Llewellyn Smith SG, Thompson E, Glover BJ. 2009. Wind gusts and plant aeroelasticity effects on the aerodynamics of pollen shedding: a hypothetical turbulence-initiated wind-pollination mechanism. J. Theor. Biol. 259, 785–792. ( 10.1016/j.jtbi.2009.04.027) [DOI] [PubMed] [Google Scholar]
- 19.Harder LD, Prusinkiewicz P. 2013. The interplay between inflorescence development and function as the crucible of architectural diversity. Ann. Bot. 112, 1477–1493. (doi:10:1093/aob/mcs252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timerman D, Greene DF, Urzay J, Ackerman JD. 2014. Turbulence-induced resonance vibrations cause pollen release in wind-pollinated Plantago lanceolata L. (Plantaginaceae). J. R. Soc. Interface 11, 20140866 ( 10.1098/rsif.2014.0866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niklas KJ. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago, IL: University of Chicago Press. [Google Scholar]
- 22.Berry PE, Calvo RN. 1989. Wind pollination, self-incompatibility, and altitudinal shifts in pollination systems in the high Andean genus Espeletia (Asteraceae). Am. J. Bot. 76, 1602–1614. ( 10.2307/2444398) [DOI] [Google Scholar]
- 23.Gomez JM, Zamora R. 1996. Wind pollination in high-mountain populations of Hormathophylla spinosa (Cruciferae). Am. J. Bot. 83, 580–585. ( 10.2307/2445916) [DOI] [Google Scholar]
- 24.Kaplan SM, Mulcahy DL. 1971. Mode of pollination and floral sexuality in Thalictrum. Evolution 25, 659–668. ( 10.2307/2406946) [DOI] [PubMed] [Google Scholar]
- 25.Davis SL. 2002. Allocation to floral structures in Thalictrum pubescens (Ranunculaceae), a cryptically dioecious species. Ann. Bot. 90, 119–126. ( 10.1093/aob/mcf158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer SS, Charlesworth D. 1991. Cryptic dioecy in flowering plants. Trends Ecol. Evol. 6, 320–325. ( 10.1016/0169-5347(91)90039-Z) [DOI] [PubMed] [Google Scholar]
- 27.Melampy MN, Hayworth AM. 1981. Sex-linked niche differentiation in two species of Thalictrum. Am. Midl. Nat. 106, 325–334. ( 10.2307/2425169) [DOI] [Google Scholar]
- 28.Davis SL. 1997. Stamens are not essential as an attractant for pollinators in females of cryptically dioecious Thalictrum pubescens Pursch. (Ranunculaceae). Sex Plant. Reprod. 10, 293–299. ( 10.1007/s004970050101) [DOI] [Google Scholar]
- 29.Soza VL, Brunet J, Liston A, Salles Smith P, Di Stilio VS. 2012. Phylogenetic insights into the correlates of dioecy in meadow-rues (Thalictrum, Ranunculaceae). Mol. Phylogenetics Evol. 63, 180–192. ( 10.1016/j.ympev.2012.01.009) [DOI] [PubMed] [Google Scholar]
- 30.Soza VL, Haworth KL, Di Stilio VS. 2013. Timing and consequences of recurrent polyploidy in meadow-rues (Thalictrum, Ranunculaceae). Mol. Biol. Evol. 30, 1940–1954. ( 10.1093/molbev/mst101) [DOI] [PubMed] [Google Scholar]
- 31.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics, 4th edn New York, NY: Longman. [Google Scholar]
- 32.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 33.Brock MT, Weinig C. 2007. Plasticity and environment-specific covariances: an investigation of floral–vegetative and within flower correlations. Evolution 61, 2913–2924. ( 10.1111/j.1558-5646.2007.00240.x) [DOI] [PubMed] [Google Scholar]
- 34.Camargo ID, Nattero J, Careaga SA, Núñez-Farfán J. 2017. Flower-level developmental plasticity to nutrient availability in Datura stramonium: implications for the mating system. Ann. Bot. 120, 603–615. ( 10.1093/aob/mcx093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flesch TK, Grant RH. 1992. Corn motion in the wind during senescence: I. Motion characteristics. Agron. J. 84, 742–747. ( 10.2134/agronj1992.00021962008400040037x) [DOI] [Google Scholar]
- 36.Sterling M, Baker C, Berry P, Wade A. 2003. An experimental investigation of the lodging of wheat. Agric. For. Meteorol. 119, 149–165. ( 10.1016/S0168-1923(03)00140-0) [DOI] [Google Scholar]
- 37.Johansson V, Lönnell N, Sundberg S, Hylander K. 2014. Release thresholds for moss spores: the importance of turbulence and sporophyte length. J. Ecol. 102, 721–729 ( 10.1111/1365-2745.12245) [DOI] [Google Scholar]
- 38.Zajączkowska U, Kucharski S, Nowak Z, Grabowska K. 2017. Morphometric and mechanical characteristics of Equisetum hyemale stem enhance its vibration. Planta 245, 835–848. ( 10.1007/s00425-017-2648-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grace J. 1977. Plant response to wind. London, UK: Academic Press. [Google Scholar]
- 40.Krick J, Ackerman JD. 2015. Adding ecology to particle capture models: numerical simulations of capture on a moving cylinder in crossflow. J. Theor. Biol. 368, 13–26. ( 10.1016/j.jtbi.2014.12.003) [DOI] [PubMed] [Google Scholar]
- 41.McCombe D, Ackerman JD. 2018. Collector motion affects particle capture in physical models and in wind pollination. Am. Nat. 192, 81–93. ( 10.1086/697551) [DOI] [PubMed] [Google Scholar]
- 42.Timerman D, Greene DF, Ackerman JD, Kevan PG, Nardone E. 2014. Pollen aggregation in relation to pollination vector. Int. J. Plant Sci. 175, 681–687. ( 10.1086/676301) [DOI] [Google Scholar]
- 43.Pacini E, Hesse M. 2005. Pollenkitt: its composition, forms and function. Flora 200, 399–415. ( 10.1016/j.flora.2005.02.006) [DOI] [Google Scholar]
- 44.Jackson ST, Lyford ME. 1999. Pollen dispersal models in Quaternary plant ecology: assumptions, parameters, and prescriptions. Bot. Rev. 65, 39–75. ( 10.1007/BF02856557) [DOI] [Google Scholar]
- 45.Stelleman P. 1984. The significance of biotic pollination in a nominally anemophilous plant: Plantago lanceolata. Proc. K. Ned. Akad. Wet. 87, 95–119. [Google Scholar]
- 46.Vroege PW, Stelleman P. 1990. Insect and wind pollination in Salix repens L. and Salix caprea L. Israel J. Bot. 39, 125–132. ( 10.1080/0021213X.1990.10677137) [DOI] [Google Scholar]
- 47.Harder LD, Thomson JD. 1989. Evolutionary options for maximizing pollen dispersal in animal-pollinated plants. Am. Nat. 133, 323–344. ( 10.1086/284922) [DOI] [Google Scholar]
- 48.Wang TN, Clifford MR, Martínez-Gómez J, Johnson JC, Riffell JA, Di Stilio VS. In press Scent matters: differential contribution of scent to insect response in flowers with insect vs. wind pollination traits. Ann. Bot. ( 10.1093/aob/mcy131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timerman D, Barrett SCH. 2018. Data from: Divergent selection on the biomechanical properties of stamens under wind and insect pollination Dryad Digital Repository. ( 10.5061/dryad.2f7g889) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Timerman D, Barrett SCH. 2018. Data from: Divergent selection on the biomechanical properties of stamens under wind and insect pollination Dryad Digital Repository. ( 10.5061/dryad.2f7g889) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Laboratory and field data: Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.2f7g889 [49].