Abstract
Organisms cope with nutritional variation via developmental plasticity, adjusting trait size to nutrient availability for some traits while enabling others to develop in a nutritionally robust manner. Yet, the developmental mechanisms that regulate organ-specific growth across nutritional gradients remain poorly understood. We assessed the functions of members of the insulin/insulin-like signalling pathway (IIS) in the regulation of nutrition sensitivity and robustness in males of the horn-polyphenic beetle Onthophagus taurus, as well as potential regulatory interactions between IIS and two other growth-regulating pathways: Doublesex and Hedgehog signalling. Using RNAinterference (RNAi), we experimentally knocked down both insulin receptors (InR1 and InR2) and Foxo, a growth inhibitor. We then performed morphometric measurements on horns, a highly nutrition-sensitive trait, and genitalia, a largely nutrition-insensitive trait. Finally, we used quantitative real-time polymerase chain reaction to assess expression levels of doublesex and the Hedgehog signalling gene smoothened following IIS-RNAi. Our results suggest that nutrition responsiveness of both traits is regulated by different IIS components, which transduce nutritional conditions to both Doublesex and Hedgehog pathways, albeit via different IIS pathway members. Combined with previous studies, our findings suggest that separate origins of trait exaggeration among insect lineages were enabled through the independent co-option of IIS, yet via reliance on different components therein.
Keywords: allometry, growth, developmental plasticity, scaling relationships
1. Introduction
Variation in nutrition is one of the most fundamental and widespread challenges organisms face during development [1–3]. Organisms can meet this challenge by adjusting their phenotype through the process of developmental plasticity, thereby allowing a single genotype normally able to develop into a range of phenotypes to select trait values most adaptive given prevailing nutritional conditions [4]. Yet, different traits of the same individual may differ in what constitutes the most adaptive level of plasticity. Sexually selected traits such as weapons, for example, are often greatly exaggerated in larger individuals owing to their significance in aggressive interactions, yet muted in smaller individuals, and thus commonly exhibit extreme sensitivity to variation in nutrition [5,6]. On the other hand, legs or wings function in strict proportion to overall body size, deviations from which may carry severe fitness penalties, and thus commonly exhibit moderate nutrition sensitivity [7]. Lastly, some traits carry out functions that require a more constant absolute size regardless of overall body size or that of other structures; for example, male genitalia in insects selected to fit a wide range of female genitalia, or the central nervous system in most animals [8–10]. Such traits may exhibit minimal nutritional responsiveness during ontogeny and are said to be robust to nutritional perturbations. As a consequence, the degree of nutrition responsiveness—from highly plastic to robust—can vary strikingly among different traits even within the same individual organism and in response to the same nutritional gradient.
When the growth rate of different traits varies in response to the same nutritional gradient, individuals composed of differentially responsive traits will grow not just to different overall body sizes, but will also develop different overall shapes. This makes the study of nutrition-dependent growth central to our understanding of the developmental regulation and evolutionary diversification of shape. Plastic responses to nutrition and their effects on scaling and shape can be investigated through the study of static allometries. Static allometries enable quantitative assessments of organ sizes relative to body sizes across individuals of the same population [11], and numerous studies now illustrate how the differential scaling of traits within species and evolutionary changes in scaling relationships among species have played critical roles in the genesis of morphological diversity (e.g. insect wings [12] and beetle horns [13]). What is less well understood, however, are the developmental mechanisms that regulate precise, organ-specific growth responses across nutritional gradients: how do organisms regulate extreme growth responses of some traits while shielding others from environmental fluctuations?
Some mechanisms regulating relative growth are starting to be elucidated in increased detail, at least in model organisms such as Drosophila. Chief among them is the insulin/insulin-like signalling pathway (IIS), a highly conserved pathway now recognized as mediating nutrition-responsive growth across phyla, from humans to insects [14,15]. In insects, high nutrition conditions induce the release of insulin-like peptides (ILPs) primarily from the insulin-producing cells in the brain into the haemolymph. The ILPs circulate through the haemolymph and reach target tissues where they bind to the insulin receptor (InR) and activate a phosphokinase signal transduction cascade that induces cell growth and proliferation [14]. Importantly, this pathway has been implicated in mediating differential nutrition sensitivity across organs within an individual [16]. In Drosophila, wings, legs and palps scale in proportion to body size [17], while other body parts such as the central nervous system [9] and genitalia [16] are far less sensitive to variation in nutrition, and differential sensitivities to nutrition have been linked to differential IIS activity. For example, genitalia achieve reduced nutritional sensitivity via the Forkhead box, subgroup O (Foxo), a growth inhibitor downstream of the InR [16]. This transcription factor is normally activated during low nutrition conditions and inactive during high nutrition conditions. However, by maintaining low Foxo expression levels even under low nutrition conditions, genitalia maintain a relatively constant absolute genitalia size across all nutritional environments [16]. While these findings have greatly advanced our understanding on how organisms translate a nutritional gradient into diverse and tissue-specific responses, these insights have been largely restricted to model organisms and relatively conventional types of nutritional responsiveness. By contrast, the developmental regulation of more complex nutritional responsiveness such as extreme trait exaggeration (as in weapons or ornaments) or polyphenic trait expression (i.e. presence/absence of trait expression in nutritionally cued morphs) remains poorly understood, even though both are widespread in nature. Thus, understanding the developmental underpinnings of more complex types of nutrition-responsive growth will require the use of more appropriate experimental model systems.
A recent study in the rhinoceros beetle (Trypoxylus dichotomus) has begun to elucidate the regulation of nutrition-responsive growth of exaggerated sexually selected traits and showed that InR may be involved in mediating differential nutritional sensitivities across different organs [5]. Specifically, InR knockdown during late larval development greatly affected the nutritional response in normally exaggerated horns, while moderately nutrition-sensitive wings were only mildly affected, and nutritionally non-responsive genitalia not at all. These results suggested that differential InR expression may be an important and possibly universal mechanism enabling differential growth of different organs in response to the same nutritional gradient. Furthermore, what role the IIS plays in the regulation of more complex scaling relationships such as polyphenisms is starting to be elucidated in hemipterans. Recent studies have shown that two InR paralogues are involved in the regulation of winged versus wingless morphs in the planthopper Nilaparvata lugens [18], whereas quantitative changes in Foxo function may be underlying the recent evolution of a novel reaction norm in the soapberry bug Jadera haematoloma [19]. These findings raise the possibility that this pathway may be a hotspot for the evolution of discontinuous and differentially responsive, nutrient-responsive organ growth [19]. Here, we use the polyphenic horned beetle Onthophagus taurus to investigate the role of IIS signalling in the development and evolution of extreme levels of nutritional responsiveness (horns) and robustness (genitalia).
Onthophagus taurus is a dung beetle common to the Mediterranean and secondarily introduced to North America and Australia. Males of this species develop, as is common for many members of this genus, into two alternative morphs depending on larval nutrition: high larval nutrition results in the development of large, horned, major males which rely on aggressive fighting behaviour to secure females, whereas low nutritional conditions result in the development of small, hornless, minor males which rely on non-aggressive sneaking behaviours and sperm competition to secure mating opportunities. The resulting horn length–body size allometry is strongly sigmoidal, and hornless and horned morphs are separated by a sharp body size threshold. Intermediate morphologies do exist in natural populations, but are comparatively exceedingly rare [20].
Recent studies in this system have shown that at least two pathways are critical regulators of body size and nutrition-dependent formation of horns. Doublesex (Dsx), the cardinal member of the somatic sex-determination pathway, promotes horn growth in large, high nutrition males [21], and dsxRNAi eliminates nutrition-responsive horn growth in large males. By contrast, the Hedgehog (Hh) signalling pathway, most widely studied for its role in patterning anterior/posterior polarity, actively inhibits horn growth in small, low nutrition males, and knockdown of smoothened (smo), a key activator of Hh signalling, induces horns in male larvae otherwise fated to develop into hornless, minor males [22]. Combined, these studies demonstrated that both Dsx and Hh signalling are critical for the body size-specific induction or repression of horns, respectively, and the proper formation of the threshold body size separating alternate male morphs. However, how the action of either pathway is coordinated in light of a given individual's nutritional status remains unclear. Specifically, whether Dsx or Hh signalling (or both) are linked to components of IIS signalling and thus nutrition has yet to be examined.
In this study, we focused on the two insulin receptor paralogues common to most insects, InR1 and InR2, as well as Foxo, to assess the possible functions of diverse IIS pathway members in the regulation of nutrition sensitivity and robustness. We contrasted the development of horns, a highly nutrition-sensitive trait, with that of male genitalia (specifically, the aedeagus), a nutrition-insensitive trait. Furthermore, we tested whether IIS signalling could be acting upstream of either Dsx or Hh signalling, or both, thereby providing one or both pathways with information regarding prevailing nutritional conditions during beetle development.
2. Material and methods
(a). Beetle husbandry
Onthophagus taurus beetles were collected near Bloomington, IN and Chapel Hill, NC, and reared and maintained in laboratory colonies as described previously [23].
(b). Foxo, InR1 and InR2 cloning and RNAi knockdown
Primers were designed against O. taurus Foxo, InR1 and InR2, and corresponding fragments were cloned and sequenced to verify identity. Double Stranded RNA (dsRNA) for RNAi injections was generated as previously described [21]. Control dsRNA was generated following the same procedure using a vector sequence. The following dsRNA concentrations (dissolved in injection buffer) were used: control injections and FoxoRNAi (1 µg of dsRNA); InR1RNAi (0.5, 1, 3, 6 or 9 µg); InR2RNAi (0.25, 1 µg) and InR1 + 2RNAi (0.5 µg). Concentrations were varied in some cases in an attempt to improve penetrance and reduce mortality. All injections were executed during the last (=third) larval instar.
(c). Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to assess putative interactions between our genes of interest and smo and dsx. Glyceraldehyde 3-phosphate dehydrogenase and actin were used as reference genes in line with previous studies [24]. Primer sequences and fragment lengths are listed in the electronic supplementary material, table S2. Primer efficiency was tested for all genes using standard curves (see the electronic supplementary material). Whole-body samples were obtained from two developmental time points (24 h after dsRNAi injections and 24 h after pupation) to assess if effects on transcript abundance and pathway interactions are maintained across developmental stages. Three technical replicates were used for each sample. Larval samples were run separately for each individual to further control for any developmental timing effects, while pupal samples were pooled after RNA extractions. Larval samples included control (n = 5), FoxoRNAi (n = 6), InR1RNAi (n = 3), InR2RNAi (nsmo = 4, ndsx = 5) and InR1 + 2RNAi (n = 3). Pupal samples included control (n = 7), FoxoRNAi (n = 11), InR1RNAi (n = 6) or InR2RNAi (n = 4). RNA was extracted using the Direct-zol RNA MiniPrep kit (Zymo Research; see the electronic supplementary material for details).
(d). Allometric measurements and analyses
dsRNA-injected adults were measured using a two-dimensional morphometric set-up. Thorax width was used as a measure of body size and horn size (head and thoracic) was measured as previously described [25]. For genitalia length, paramere and phallobase were measured as shown in the electronic supplementary material, figure S1. Following previous studies [26,27], we analysed the sigmoidal horn length–body size allometry by separately fitting a sigmoidal four-parameter equation to measurements obtained from control-injected and RNAi individuals, and using Welch's t-test to compare parameter means between control-injected and RNAi treatment groups. To further test the specific hypothesis that FoxoRNAi was linearizing the normally sigmoidal body size–horn length allometry, we used the Akaike information criterion (AIC) alongside the comparison of R2 values to compare sigmoidal versus linear models fitted to allometric measurements obtained from control and FoxoRNAi individuals. Lastly, for a subset of our treatments, we also used a residuals analysis as in [24], calculating the difference between observed and expected horn length for a specific body size for all individuals, followed by a Mann–Whitney U-test. For the genitalia–body size allometry, we performed a linear model followed by model selection.
3. Results
(a). FoxoRNAi linearizes the sigmoidal body size–horn size allometry
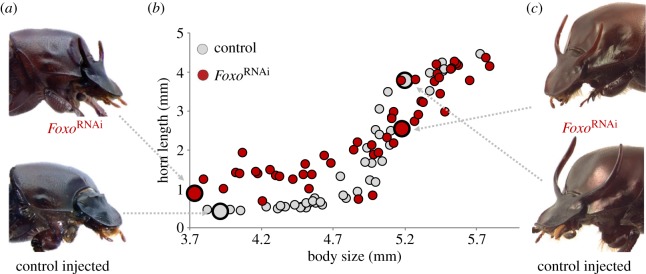
Recall that horn development in male O. taurus is extremely sensitive to nutrition, resulting in the formation of two alternative horned and hornless morph separated by a sharply defined body size threshold. To investigate the role of Foxo in this nutritionally cued male polyphenism, we used RNAinterference-mediated transcript depletion of Foxo followed by morphometric assessments of horn length in relation to body size. FoxoRNAi profoundly altered this normally strongly sigmoidal scaling relationship by inducing relatively longer horns in small, low nutrition males while simultaneously modestly reducing horn length in several large, high nutrition males, compared to control-injected individuals (figure 1a,b). Fitting allometric (sigmoidal) models to FoxoRNAi and control-injected individuals revealed a significant difference in three of the parameters analysed: a reduction in amplitude (p = 0.014), a shift in the inflection point to larger body sizes (p = 0.019) and an elevation of the y-intercept (p < 0.0001, nFoxoRNAi = 52, ncontrol = 49; figure 1). We did not recover a significant effect on the slope (p = 0.826).
Figure 1.
Effect of FoxoRNAi on the highly nutrition-sensitive, sigmoidal scaling relationship between body size and horn length. FoxoRNAi induced horn growth in low nutrition, normally hornless males (a,b), while modestly reducing horn growth in some high nutrition, normally fully horned males (b,c). Comparing control-injected (grey) and knockdown individuals (red) using independently fitted four-parameter Hill equations followed by Welch's t-tests to compare parameter estimates revealed a significant difference in amplitude, inflection point, y-intercept, but not slope. Comparing AIC and R2 values between sigmoidal and linear models for both treatment groups revealed a major difference in fit for control-injected individuals, indicating that a sigmoid model most accurately captures the scaling relationship between body size and horn length. By contrast, fitting a sigmoid model to FoxoRNAi individuals only modestly improves fit compared with a simple linear regression, suggesting that FoxoRNAi partly transforms body size–horn length allometry from strongly sigmoidal towards a more linear relationship. (Online version in colour.)
To further assess the hypothesis that FoxoRNAi linearizes the normally sigmoidal body size–horn length allometry, we fitted either a sigmoidal or a linear model to each of our treatment groups and examined both R2 and AIC values. As expected, R2 values are maximized in control-injected animals when a sigmoid model is applied (R2,sigmoid = 0.943, R2,linear = 0.671); however, this discrepancy in fit decreases in FoxoRNAi males (R2,sigmoid = 0.884, R2,linear = 0.759). Similarly, AIC values indicate a superior fit of the sigmoid model to control-injected individuals (AICsigmoid = 33.73, AIClinear = 115.25), but show that this discrepancy declines in FoxoRNAi males owing to both a reduction in fit of the sigmoidal model (AICsigmoid = 61.19) and a commensurate increase in the linear model's fit (AIClinear = 95.15). Combined, these results support the hypothesis that FoxoRNAi significantly lessens the sigmoidal nature of the body size–horn length allometry, transforming it instead towards a more linear scaling relationship.
(b). InRRNAi has no effect on the body size–horn size allometry
Mining the sequence data generated through several earlier studies [28–30] revealed the existence of two InR paralogues in the O. taurus genome (electronic supplementary material, figure S2). More detailed analysis further revealed that expression levels of InR1 and InR2 differ across developmental stages (electronic supplementary material, figure S3a), and—at the pupal stage—across tissues (electronic supplementary material, figure S3b,c). InR duplications are common among arthropods, particulary across insects (reviewed in [31]) and a gene phylogenetic reconstruction shows that Ot-InR1 is orthologous to the InR of other insects that exhibit a single InR copy (i.e. Drosophila melanogaster, the silkmoth Bombyx mori, the Colorado potato beetle Leptinotarsa decemlineata), while Ot-InR2 clusters with the InR2 of insects possessing duplicate InRs (i.e. the planthopper N. lugens, the red flour beetle Tribolium castaneum). Knockdown of Ot-InR1 or Ot-InR2 individually or in combination had no significant effect on the sigmoidal body size–horn size allometry (electronic supplementary material, figure S4), and analysis of residual horn lengths showed no significant effect following InR1 (W = 1004, p = 0.115, nInR1RNAi = 34, ncontrol = 49; electronic supplementary material, figure S4 a,b), InR2 (W = 643, p = 0.2, nInR2RNAi = 22, ncontrol = 49; electronic supplementary material, figure S4c,d) or InR1 + 2 (W = 410, p = 0.931, nInR1+2RNAi = 17, ncontrol = 49; electronic supplementary material, figure S4e,f) knockdown. These results are in marked contrast to earlier studies using an independent radiation of horned beetles in the subfamily Dynastinae (T. dichotomus [5]), which documented a major function of InR1 in horn development (no analysis of InR2 was conducted). Lastly, dsRNA injections in O. taurus, particularly at high concentrations, often resulted in a moulting phenotype similar to what has been reported for the red flour beetle T. castaneum [32] (electronic supplementary material, figure S5; InR1RNAi: 46%, InR2RNAi: 31%, InR1 + 2RNAi: 27%). Individuals with this phenotype were not able to ecdyse properly from the last larval to pupal moult and instead continued their development while remaining trapped within the larval cuticle.
(c). Foxo regulates nutrition sensitivity while InR regulates overall size of genitalia
Next, we investigated whether insulin signalling may also play a role in the regulation of nutrition insensitivity, i.e. the buffering of growth in the face of nutritional variation. To do so we focused on the development of male genitalia, in particular the aedeagus, a structure whose absolute size changes only modestly as a function of larval nutrition. Specifically, we measured aedeagus length in control-injected, FoxoRNAi, InR1RNAi, InR2RNAi and InR1 + 2RNAi individuals. Our results suggest that FoxoRNAi significantly altered the body size–aedeagus scaling relationship (t = 4.011, p = 0.0001) resulting in a further decrease of the slope of the genitalia–body size allometry (t = −4.122, p < 0.0001; figure 2a; electronic supplementary material, table S3). By contrast, InR1RNAi resulted in a reduction of aedeagus size relative to body size across all body sizes (t = −4.502, p < 0.0001; figure 2b; electronic supplementary material, table S3), while leaving the magnitude of the nutritional response across body sizes unaltered. Qualitatively, similar results were obtained through InR2RNAi (figure 2c; electronic supplementary material, table S3) and InR1 + 2RNAi (figure 2d; electronic supplementary material, table S3; InR2treatment: t = −2.574, p = 0.0123; InR2body size: t = 13. 284, p < 0.0001; InR1 + 2treatment: t = −4.995, p < 0.0001; InR1 + 2body size: t = 14.994, p < 0.0001), suggesting that InR1 and InR2 have similar and body size independent growth promoting roles during genitalia development.
Figure 2.
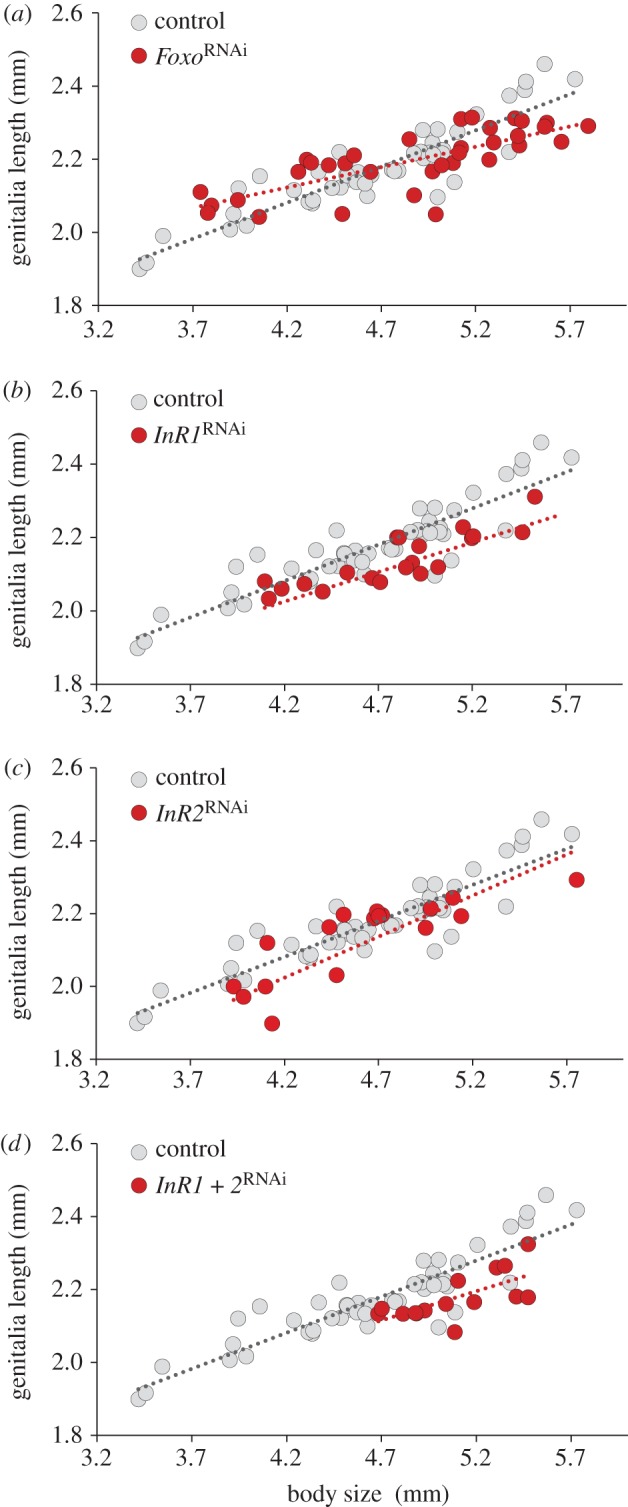
Effects of FoxoRNAi, InR1RNAi, InR2RNAi and InR1 + 2RNAi on the largely nutrition-insensitive scaling relationship between body size and male genitalia length. Shown are control-injected (grey) and knockdown (red) individuals. (a) FoxoRNAi resulted in a significant decrease in the slope of the body size-genitalia allometry, while (b) InR1RNAi, (c) InR2RNAi and (d) InR1 + 2RNAi significantly reduced genitalia size across all body sizes. The same control individuals are shown across all four panels. (Online version in colour.)
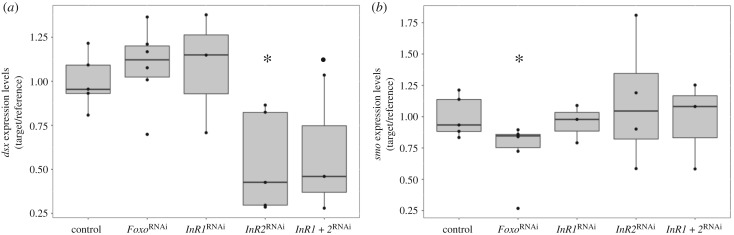
(d). dsx and smo expression levels may be regulated by the insulin signalling pathway
As introduced above, previous work identified two pathways critical for the expression of the nutritionally cued horn polyphenism in O. taurus: dsx signalling promotes horn formation under high nutrition conditions only, whereas Hh signalling inhibits horn formation under low nutrition conditions only. However, exactly how nutritional variation is transduced to then affect the action of one or both pathways is unknown. To examine whether and how insulin signalling pathway members could be interacting with one or both pathways, we used qRT-PCR to assess expression levels of dsx and the Hh signalling gene smo across various knockdown backgrounds. Larval dsx expression decreased following InR2RNAi (p = 0.029; nInR2RNAi = 5, ncontrol = 5; figure 3a), and a marginally significant decrease was also detected following InR1 + 2RNAi (p = 0.083; nInR1 + 2RNAi = 3, ncontrol = 5). However, no effect was detected for InR1RNAi (p = 0.675; nInR1RNAi = 3, ncontrol = 5) or FoxoRNAi (p = 0.499; nFoxoRNAi = 6, ncontrol = 5), suggesting that InR2, but not InR1 or Foxo, promotes dsx expression levels in larval O. taurus. By contrast, larval smo expression levels decreased after FoxoRNAi (p = 0.01; nFoxoRNAi = 6, ncontrol = 5; figure 3b), but not when InR1 (p = 0.725; nInR1RNAi = 3, ncontrol = 5), InR2 (p = 0.693; nInR2RNAi = 4, ncontrol = 5) or InR1 + 2 (p = 0.885; nInR1+2RNAi = 3, ncontrol = 5) were downregulated, suggesting that Foxo, but not InR1 or InR2, may promote smo expression levels in wild-type larvae.
Figure 3.
Larval dsx and smo expression levels in control-injected individuals and following FoxoRNAi, InR1RNAi, InR2RNAi and InR1 + 2RNAi. (a) qRT-PCR using whole-body larvae shows that dsx expression levels are unaffected by FoxoRNAi or InR1RNAi, but significantly reduced following InR2RNAi and marginally significantly reduced following InR1 + 2RNAi. This suggests that InR2, but not InR1, promotes dsx expression in wild-type individuals. (b) smo expression levels are significantly reduced following FoxoRNAi, but unaffected by InR1RNAi, InR2RNAi and InR1 + 2RNAi, suggesting that Foxo promotes smo expression in wild-type individuals. Box plots show 25% and 75% quartiles (boxes), medians (lines dividing the boxes), outermost values (whiskers), outliers (dots) and data points (dots overlapping with boxes and whiskers). *p < 0.05, •p < 0.1.
A similar approach at the pupal stage yielded, in part, strikingly different results. While FoxoRNAi did not affect dsx expression 24 h after larval injection, in the resulting pupae dsx expression exhibited an increase by 34% (electronic supplementary material, figure S6a). Similarly, pupal smo expression increased following both FoxoRNAi and InR1RNAi, whereas larvae had exhibited a decrease or no change in expression, respectively (electronic supplementary material, figure S6b). These results suggest that the nature of interactions between dsx, smo and members of IIS may vary throughout development and/or that later developmental stages may be affected through compensatory adjustments in expression dynamics as a result of RNAi perturbations. More generally, these results raise the possibility that IIS may provide nutritional information critical for the subsequent nutrition-dependent action of body-part specific growth regulators.
4. Discussion
Static allometries are the product of developmental mechanisms matching relative growth of body parts to overall body size, and evolutionary changes in these mechanisms underlie the wide diversity of scaling relationships observed in nature [12]. Understanding the mechanisms that relate the growth of parts to that of the entire organism and to the evolutionary diversification of organismal shape has been a major objective of a long-standing research programme at the interface of developmental biology and physiology. A large number of studies have now established the IIS pathway as an important regulator in the fine tuning of allometric scaling. However, comparatively fewer studies have examined the significance of the IIS pathway in organisms that show more extreme growth responses to nutritional conditions (e.g. [5]), or organisms whose growth responses are nonlinear, thereby enabling the widespread formation of alternative morphs or casts. Here, we use the polyphenic beetle O. taurus to investigate the potential functions of multiple members of the IIS pathway in the regulation of disparate growth responses by investigating two body regions that exhibit highly disparate levels of nutrition-responsive growth: (i) head horns characterized by the highly nonlinear, explosive and bimodal development, and (ii) the nutritionally canalized and largely unresponsive male copulatory organ. Our results suggest that different IIS pathway members regulate nutrition responsiveness in different body parts, and propose a candidate mechanism for the evolutionary transition from linear to sigmoidal scaling relationships. By comparing our findings to previous work, our results raise the possibility that separate origins of trait exaggeration among horned beetle lineages were enabled through the independent co-option of the same signalling pathway, yet via reliance on different components therein.
(a). Foxo's role in the development and evolution of sigmoidal horn–body size scaling
FoxoRNAi resulted in a significant increase in horn lengths of small, normally hornless males, suggesting that Foxo inhibits horn growth in low nutrition wild-type individuals. This is in marked contrast to a previous study in Onthophagus nigriventris, where FoxoRNAi had no effect on thoracic horn development [24]. However, our results are broadly consistent with separate previous studies, identifying Foxo as a growth inhibitor active when nutrition is scarce [16]. Similarly, FoxoRNAi resulted in a modest decrease in horn lengths of a subset of larger males, in particular those whose body sizes place them close to the horned side of the size threshold. This result is also consistent with prior findings in Drosophila and mammals, which identified Foxo as a growth sensitizer, upregulating InR under low nutrition conditions and priming tissues ready to proliferate in case further nutrition becomes available [33,34]. Combined, these results support the hypothesis that FoxoRNAi therefore lessens the sigmoidal nature of the normally strongly biphasic body size–horn length allometry, thereby transforming it towards a more linear scaling relationship. While this allometric transformation is incomplete, it nevertheless raises the possibility that recruitment of Foxo-mediated inhibition of horn growth at low nutrition coupled with growth sensitization at intermediate to high nutritional conditions could have played critical roles in the evolutionary transition from ancestral linear allometries to derived sigmoid scaling relationships [35]. This hypothesis is further supported by a previous study, which recovered strikingly high Foxo expression levels in horn tissue (particularly in small males), compared to genitalia or brain tissue (electronic supplementary material, figure S7; [29]). This raises the possibility that an increase in Foxo expression levels could have been a key step in the evolutionary transition from a linear to a sigmoidal allometry. Comparative functional analysis of Foxo and other putative growth regulators in species with varying degrees of male polyphenism could shed further light on the regulation and evolution of nonlinear scaling relationships.
(b). Foxo regulates both plasticity and robustness in a tissue-specific manner
Previous work in Drosophila implicates Foxo in the regulation of tissue-specific nutritional plasticity [16]. In most traits studied, to date, low nutrition results in the upregulation of Foxo and subsequent growth inhibition. However, in Drosophila genitalia, Foxo expression remains very low even when nutrition is scarce, which in turn is thought to reflect one of the mechanisms maintaining the shallow, largely nutrition-insensitive allometry of fly genitalia. By maintaining low Foxo expression levels, genitalia in low nutrition animals are able to ‘ignore’ their nutritional status and grow to similar sizes as those of medium or high nutrition individuals [16]. Similarly, experimental upregulation of Foxo in genitalia and wings increased nutrition sensitivity by decreasing trait size in small individuals [16]. In O. taurus, Foxo expression levels are much higher in head horns compared to other traits (electronic supplementary material, figure S7; [29]), and our FoxoRNAi results are consistent with findings in Drosophila: by decreasing Foxo expression levels in the highly plastic head horns, we were able to decrease nutritional plasticity. However, this was also true, albeit to a lesser degree, for O. taurus genitalia. Here, the already shallow slope of the aedeagus body size allometry was further decreased. Even though male genitalia of both wild-type Drosophila and O. taurus share similar allometric slopes when plotted on a log–log scale (Drosophila: [16]; O. taurus: S. Casasa 2018, unpublished data), FoxoRNAi has no effect in flies [16] yet results in a significant further decrease of the allometric slope in O. taurus (figure 2a). This additional slope reduction in FoxoRNAi genitalia suggests that (i) even modest Foxo expression levels may be sufficient to instruct a shallow, but nevertheless nutrient-sensitive allometry in O. taurus, and (ii) that the observed effect in O. taurus genitalia, but its absence in Drosophila, could be owing to a greater overall nutrition sensitivity in O. taurus. Together, our results suggest that low-to-moderate Foxo expression levels may be contributing to the nutritional robustness of genitalia. Further studies exploring interacting nutrient-sensing systems (i.e. TOR or Hippo signalling) could help us better understand the mechanisms of nutritional insensitivity in genitalia.
(c). InR regulates aedeagus but not horn growth
In an important earlier study, Emlen et al. [5] reported a highly significant decrease in horn length in the rhinoceros beetle T. dichotomus (Dynastinae) following InR1RNAi. The same study also reported a modest reduction of adult wing size, but no effect on male aedeagus length, following the same manipulation. Based on these results, Emlen et al. [5] proposed that the IIS pathway in general and differential InR expression in particular constitute a central regulator of relative trait size and nutritional plasticity during insect development, and proposed it as a critical facilitator of honest signalling underlying the evolutionary origin and maintenance of exaggerated secondary sexual traits across animals. By contrast, we were unable to detect any measurable effect of InR1RNAi, InR2RNAi or InR1 + 2RNAi on the horn length–body size allometry in O. taurus. At the same time, we did recover a highly significant reduction in male genitalia size. Recall that injection of high dsRNA concentrations of either construct resulted in a lethal moulting phenotype that could have masked potential horn phenotypes. However, this appears unlikely because lower concentrations were sufficient to yield a highly repeatable reduction in genitalia size alongside successful and complete eclosion.
Trypoxylus dichotomus (Dynaestine) and O. taurus (Scarabeinae) belong to different subfamilies of scarab beetles which independently evolved exaggerated horns and horn-like structures [36,37], though only the latter also evolved pronounced male polyphenisms. Our results raise the possibility that as both lineages independently evolved exaggerated horns, they each did so by independently recruiting the IIS into the regulation of relative horn size, yet by using different pathway components for different sets of traits: dynastine beetles may rely on differential expression of at least one of the insulin receptors to promote different degrees of nutrition-responsive growth across traits including horns (high levels, high sensitivity and exaggeration) and genitalia (low levels, insensitive, no exaggeration). By contrast, scarabaeine beetles may use the insulin receptor only to facilitate nutrition-insensitive growth of male genitalia and instead use Foxo-mediated differential inhibition in both horns and genitalia to enable different types of nutrition-responsive growth—polyphenic in the case of horns and largely nutritionally insensitive in the case of genitalia. A partly similar scenario appears to emerge from recent findings in hemipterans: while two InR paralogues are involved in the regulation of alternative wing polyphenic morphs in the planthopper N. lugens, evolutionary changes in Foxo function seem to underlie divergences in wing polyphenisms between populations of the soapberry bug J. haematoloma [18,19]. Our results thus generally support the broader significance of IIS in the evolutionary diversification of nutrition-responsive growth, but may call into question the claim that conserved insulin signalling is a universal mechanism of simple trait exaggeration. Instead, IIS may be a common pathway recruited into the sensitivity of nutrition responsiveness, but the precise mechanisms (i.e. IIS components) involved may differ greatly across taxa.
Our results also suggest the possibility of developmental interactions between InR and moulting hormones, as indicated by the moulting phenotype detected following high-dosage InR1/2RNAi injections noted earlier. Previous studies in insects documented interactions between IIS and two major moulting hormones, ecdysone and juvenile hormone (JH; [38,39]). Interestingly, ecdysone has also been implicated in the regulation of Drosophila imaginal disc growth [40], and JH contributes to the regulation of nutrition-responsive growth in stag beetle mandibles [41]. Comparative studies across taxa and pathways are needed to further disentangle the developmental and evolutionary routes to differential nutritional plasticity across trait types.
(d). Insulin/insulin-like signalling pathway interacts with both dsx and smo to regulate nutrition-sensitive growth
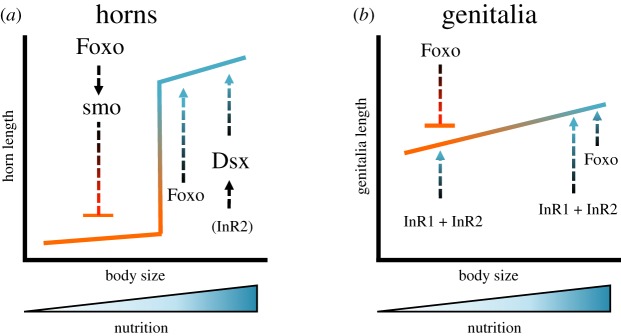
Recall that previous work identified both dsx and Hh signalling as critical regulators of nutrition-responsive growth in Onthophagus. While the male isoform of dsx promotes horn formation under high nutrition conditions only [21], Hh signalling via smo actively inhibits horn growth under low nutrition conditions only [22]. One of the major questions raised by these results concerns how either pathway may be functionally linked to nutritional conditions experienced during growth. An indirect hint was obtained from subsequent work, which pursued a genome-wide screen to identify direct and indirect dsx target genes [29]: conspicuously, absent among the otherwise enormous diversity of genes and pathways that both possessed Dsx-binding motifs in their promotor region and responded in their expression to experimental dsx downregulation were any members of the IIS. This raised the possibility that IIS may instead be operating upstream of dsx and perhaps smo as well. Lastly, results presented here show that FoxoRNAi, but not InR1/2RNAi, partly phenocopies horn phenotypes induced by both dsx and smo RNAi. To test the hypothesis that IIS pathway members may be regulating dsx and/or smo to promote or inhibit horn growth, respectively, we assessed smo and dsx expression in various RNAi backgrounds using whole-body RNA extractions. While this approach does not allow us to assess organ-specific pathway interactions, our results nevertheless provide a roadmap for further study into the regulation of dsx and smo by components of IIS signalling in the regulation of nutrition-responsive growth. We find that FoxoRNAi (but not InR1RNAi, InR2RNAi or InR1 + 2RNAi) results in a reduction of smo expression, suggesting that Foxo promotes smo expression in wild-type males. Because dsx inhibits smo at high but not low nutrition [29, E. Zattara 2018, unpublished data], this proposed interaction between Foxo and smo may therefore only be phenotypically relevant at low nutrition and explain why the experimental downregulation of either gene results in the induction of horns in small, low nutrition males only (figure 4).
Figure 4.
Proposed model for the regulation of horn and genitalia allometries by insulin signalling and interacting pathways. (a) Regulation of high levels of nutritional responsiveness during horn formation across a nutritional gradient. Low nutrition conditions result in the upregulation of Foxo, promoting smoothened (smo) expression in the process (directly or indirectly), which in turn inhibits proliferation of horn tissue, resulting in a hornless male phenotype (this study and [22]). In these individuals, FoxoRNAi results in the disinhibition and induction of horn growth. In intermediate-sized males, Foxo acts as a growth sensitizer resulting in disproportionate growth responses and the reduction in horn growth in FoxoRNAi individuals. Lastly, high nutrition conditions result in Foxo-independent expression of the male isoform of doublesex (dsxm) and the promotion of horn proliferation [21]. Results presented here provide partial support that InR2 expression promotes dsxm expression under these conditions (thus InR2 is presented in parentheses). (b) Regulation of low levels of nutritional responsiveness during genitalia growth across a nutritional gradient. Foxo inhibits genitalia growth under low nutrition condition, but promotes genitalia growth under high nutrition conditions. As a consequence, FoxoRNAi males possess relative larger genitalia under low nutrition condition, but relatively smaller under high nutrition conditions, resulting in a shallower genitalia–body size allometry. By contrast, InR1 and InR2 promote genitalia growth regardless of nutritional conditions (figure 2), and InR1/2RNAi results in reduced genitalia sizes across the entire range of body sizes. How InR1/2 and Foxo interact during genitalia formation remains to be determined. Effect size is indicated by font size, and blue arrows and orange bars indicate interactions (growth induction or inhibition, respectively) supported by RNAi or qRT-PCR data (this study and [21,22]). (Online version in colour.)
Conversely, we also find that InR2RNAi (but not FoxoRNAi, or InR1RNAi) results in a reduction of dsx but not smo, consistent with a role of one of the insulin receptor paralogues in promoting dsx expression and thereby horn growth. In contrast to our FoxoRNAi results, however, InR2RNAi does not phenocopy dsxRNAi horn phenotypes, rendering these findings in need of further study. Collectively, our results thus provide important evidence, supporting that IIS signalling may provide essential nutritional information to the Hh (via Foxo) and possible Dsx (via InR2) pathways which then transduce this information to instruct organ-specific growth responses (figure 4).
In conclusion, our results suggest that in the horn-polyphenic beetle O. taurus, both nutrition sensitivity and insensitivity may be regulated by diverse components of the IIS pathway and their interactions with dsx and Hh signalling across different body parts. The growing number of studies implicating diverse IIS components in both hemi- and holometabolous insects supports the hypothesis that this pathway may be a hotspot for the evolution of nutrient-sensitive trait growth [5,18,19], including, as suggested by the results of this study, the evolutionary transition from linear to strongly sigmoidal allometries. However, the specific components of IIS and their interactions with other pathways (e.g. TOR, Hippo, Dsx and Hh) will require further investigation to disentangle the developmental and evolutionary routes to differential nutritional plasticity across trait types in different lineages.
Supplementary Material
Acknowledgements
We thank David Linz and Eduardo Zattara for helpful comments on earlier drafts of this manuscript. We are grateful to Teiya Kijimoto and Eduardo Zattara for their assistance with molecular work, Christy Bergeon Burns and the CISAB laboratory for critical help with qRT-PCR troubleshooting, and Kayla Copper for her help in collecting morphometric data.
Ethics
All animal procedures adhered to the ABS Guidelines for the Use of Animals in Research. Animals were kept in the best possible conditions based on the biology of this species. The study involved a common species of insect for which no review by an institutional or governmental regulatory body was required.
Data accessibility
All data are available either as the electronic supplementary material or deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.35g4j2q [42].
Authors' contributions
S.C. and A.P.M. designed the study. S.C. performed the molecular work and morphometric measurements. S.C. and A.P.M. analysed the data and drafted the manuscript.
Competing interests
We have no competing interests.
Funding
Support for this study was provided by National Science Foundation (grant nos IOS 1120209 and 1256689) to A.P.M. as well as a grant from the John Templeton Foundation. The opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the National Science Foundation or John Templeton Foundation.
References
- 1.Andersen LH, Kristensen TN, Loeschcke V, Toft S, Mayntz D. 2010. Protein and carbohydrate composition of larval food affects tolerance to thermal stress and desiccation in adult Drosophila melanogaster. J. Ins. Phys. 56, 336–340. ( 10.1016/j.jinsphys.2009.11.006) [DOI] [PubMed] [Google Scholar]
- 2.Simpson SJ, Raubenheimer D. 2011. The nature of nutrition: a unifying framework. Aust. J. Zool. 59, 350 ( 10.1071/ZO11068) [DOI] [Google Scholar]
- 3.Kind KL, Moore VM, Davies MJ. 2006. Diet around conception and during pregnancy: effects on fetal and neonatal outcomes. Reprod. Biomed. Online 12, 532–541. ( 10.1016/S1472-6483(10)61178-9) [DOI] [PubMed] [Google Scholar]
- 4.Beldade P, Mateus AR, Keller RA. 2011. Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 20, 1347–1363. ( 10.1111/j.1365-294X.2011.05016.x) [DOI] [PubMed] [Google Scholar]
- 5.Emlen DJ, Warren I, Johns A, Dworkin I, Lavine LC. 2012. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337, 860–864. ( 10.1126/science.1224286) [DOI] [PubMed] [Google Scholar]
- 6.Warren IA, Gotoh H, Dworkin IM, Emlen DJ, Lavine LC. 2013. A general mechanism for conditional expression of exaggerated sexually-selected traits. BioEssays 35, 889–899. ( 10.1002/bies.201300031) [DOI] [PubMed] [Google Scholar]
- 7.Parzer H, Polly D, Moczek AP. 2018. The evolution of relative trait size and shape: insights from the genitalia of dung beetles. Dev. Genes Evol. 228, 83–93. (doi:10.10.1007/s00427-018-0602-2) [DOI] [PubMed] [Google Scholar]
- 8.Eberhard WG. 2009. Static allometry and animal genitalia. Evolution 63, 48–66. (doi:10.10.1111/j.1558-5646.2008.00528.x) [DOI] [PubMed] [Google Scholar]
- 9.Cheng LY, Bailey AP, Leevers SJ, Ragan TJ, Driscoll PC, Gould AP. 2011. Anaplastic lymphoma kinase spares organ growth during nutrient restriction in Drosophila. Cell 146, 435–447. ( 10.1016/j.cell.2011.06.040) [DOI] [PubMed] [Google Scholar]
- 10.Polilov AA, Makarova AA. 2017. The scaling and allometry of organ size associated with miniaturization in insects: a case study for Coleoptera and Hymenoptera. Sci. Rep. 7, 43095 ( 10.1038/srep43095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shingleton AW, Frankino WA, Flatt T, Nijhout HF, Emlen DJ. 2007. Size and shape: the developmental regulation of static allometry in insects. BioEssays 29, 536–548. ( 10.1002/bies.20584) [DOI] [PubMed] [Google Scholar]
- 12.Mirth CK, Frankino WA, Shingleton AW. 2016. Allometry and size control: what can studies of body size regulation teach us about the evolution of morphological scaling relationships? Curr. Opin. Insect Sci. 13, 93–98. ( 10.1016/j.cois.2016.02.010) [DOI] [PubMed] [Google Scholar]
- 13.Emlen DJ, Nijhout HF. 2000. The development and evolution of exaggerated morphologies in insects. Annu. Rev. Entomol. 45, 661–708. ( 10.1146/annurev.ento.45.1.661) [DOI] [PubMed] [Google Scholar]
- 14.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213–221. ( 10.1016/S0960-9822(01)00068-9) [DOI] [PubMed] [Google Scholar]
- 15.Grewal SS. 2009. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int. J. Biochem. Cell Biol. 41, 1006–1010. ( 10.1016/j.biocel.2008.10.010) [DOI] [PubMed] [Google Scholar]
- 16.Tang HY, Smith-Caldas MSB, Driscoll MV, Salhadar S, Shingleton AW. 2011. FOXO regulates organ-specific phenotypic plasticity in Drosophila. PLoS Genet. 7, e1002373 ( 10.1371/journal.pgen.1002373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shingleton AW, Das J, Vinicius L, Stern DL. 2005. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 3, e289 ( 10.1371/journal.pbio.0030289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu HJ, et al. 2015. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519, 464–467. ( 10.1038/nature14286) [DOI] [PubMed] [Google Scholar]
- 19.Fawcett MM, et al. 2018. Manipulation of insulin signaling phenocopies evolution of a host-associated polyphenism. Nat. Commun. 9, 1699 ( 10.1038/s41467-018-04102-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moczek AP. 2002. Allometric plasticity in a polyphenic beetle. Ecol. Entomol. 27, 58–67 ( 10.1046/j.0307-6946.2001.00385.x) [DOI] [Google Scholar]
- 21.Kijimoto T, Moczek AP, Andrews J. 2012. Diversification of doublesex function regulates morph-, sex-, and species-specific expression of beetle horns. Proc. Natl Acad. Sci. USA. 109, 20 526–20 531. ( 10.1073/pnas.1118589109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kijimoto T, Moczek AP. 2016. Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle. Proc. Natl Acad. Sci. USA 113, 5982–5987. ( 10.1073/pnas.1601505113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moczek AP, Nagy LM. 2005. Diverse developmental mechanisms contribute to different levels of diversity in horned beetles. Evol. Dev. 7, 75–185. ( 10.1111/j.1525-142X.2005.05020.x) [DOI] [PubMed] [Google Scholar]
- 24.Snell-Rood EC, Moczek AP. 2012. Insulin signaling as a mechanism underlying developmental plasticity: the role of FOXO in a nutritional polyphenism. PLoS ONE 7, e34857 ( 10.1371/journal.pone.0034857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moczek AP. 2006. A matter of measurements: challenges and approaches in the comparative analysis of static allometries. Am. Nat. 167, 606–611. ( 10.1086/501075) [DOI] [PubMed] [Google Scholar]
- 26.Nijhout HF, Emlen DJ. 1998. Competition among body parts in the development and evolution of insect morphology. Proc. Natl Acad. Sci. USA 95, 3685–3689. ( 10.1073/pnas.95.7.3685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parzer HF, Moczek AP. 2008. Rapid antagonistic coevolution between primary and secondary sexual characters in horned beetles. Evolution 62, 2423–2428. ( 10.1111/j.1558-5646.2008.00448.x) [DOI] [PubMed] [Google Scholar]
- 28.Zattara E, et al. 2016. Onthophagus taurus genome assembly 1.0. Ag Data Commons. See 10.15482/USDA.ADC/1255156. [DOI]
- 29.Ledón-Rettig CC, Zattara EE, Moczek AP. 2017. Asymmetric interactions between doublesex and sex- and tissue-specific target genes mediate sexual dimorphism in beetles. Nat. Commun. 8, 14593 ( 10.1038/ncomms14593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pespeni MH, Ladner JT, Moczek AP. 2017. Signals of selection in conditionally expressed genes in the diversification of three horned beetles species. J. Evol. Biol. 30, 1644–1657. ( 10.1111/jeb.13079) [DOI] [PubMed] [Google Scholar]
- 31.Xu H-J, Zhang C-X. 2017. Insulin receptors and wing dimorphism in rice planthoppers. Phil. Trans. R. Soc. B 372, 20150489 ( 10.1098/rstb.2015.0489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sang M, Li C, Wu W, Li B. 2016. Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene 585, 196–204. ( 10.1016/j.gene.2016.02.034) [DOI] [PubMed] [Google Scholar]
- 33.Puig O, Marr MT, Ruhf ML, Tjian R. 2003. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17, 2006–2020. ( 10.1101/gad.1098703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puig O, Tjian R. 2005. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 19, 2435–2446 ( 10.1101/gad.1340505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emlen DJ, Hunt J, Simmons LW. 2005. Evolution of sexual dimorphism and male dimorphism in the expression of beetle horns: phylogenetic evidence for modularity, evolutionary lability, and constraint. Am. Nat. 166, S42–S68. ( 10.1086/444599) [DOI] [PubMed] [Google Scholar]
- 36.Arrow GH. 1951. Horned beetles. The Hague, The Netherlands: Junk. [Google Scholar]
- 37.Grimaldi D, Engel MS. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 38.Colombani J, et al. 2005. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310, 667–670. ( 10.1126/science.1119432) [DOI] [PubMed] [Google Scholar]
- 39.Sheng Z, Xu J, Bai H, Zhu F, Palli SR. 2011. Juvenile hormone regulates Vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum . J. Biol. Chem. 286, 41 924–41 936. ( 10.1074/jbc.M111.269845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herboso L, et al. 2015. Ecdysone promotes growth of imaginal discs through the regulation of Thor in D. melanogaster. Sci. Rep. 5, 1–14. ( 10.1038/srep12383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotoh H, et al. 2014. Developmental link between sex and nutrition; doublesex regulates sex-specific mandible growth via juvenile hormone signaling in stag beetles. PLoS Genet. 10, e1004098 ( 10.1371/journal.pgen.1004098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casasa S, Moczek AP.2018. Data from: Insulin signaling's role in mediating tissue-specific nutritional plasticity and robustness in the horn-polyphenic beetle Onthophagus taurus. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Casasa S, Moczek AP.2018. Data from: Insulin signaling's role in mediating tissue-specific nutritional plasticity and robustness in the horn-polyphenic beetle Onthophagus taurus. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available either as the electronic supplementary material or deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.35g4j2q [42].