Abstract
The hypothalamic decapeptide, GnRH, is the gatekeeper of mammalian reproductive development and function. Activation of specific, high-affinity cell surface receptors (GnRH receptors) on gonadotropes by GnRH triggers signal transduction cascades to stimulate the coordinated synthesis and secretion of the pituitary gonadotropins FSH and LH. These hormones direct gonadal steroidogenesis and gametogenesis, making their tightly regulated production and secretion essential for normal sexual maturation and reproductive health. FSH and LH are glycoprotein heterodimers comprised of a common α-subunit and a unique β-subunit (FSHβ and LHβ, respectively), which determines the biological specificity of the gonadotropins. The unique β-subunit is the rate-limiting step for the production of the mature gonadotropins. Therefore, FSH synthesis is regulated at the transcriptional level by Fshb gene expression. The overarching goal of this review is to expand our understanding of the mechanisms and pathways underlying the carefully orchestrated control of FSH synthesis and secretion by GnRH, focusing on the transcriptional regulation of the Fshb gene. Identification of these regulatory mechanisms is not only fundamental to our understanding of normal reproductive function but will also provide a context for the elucidation of the pathophysiology of reproductive disorders and infertility to lead to potential new therapeutic approaches.
Mammalian reproductive function is regulated by the hypothalamic–pituitary–gonadal axis through multiple interconnected feedback loops (1, 2). The hypothalamic neuropeptide, GnRH, is a hormone synthesized in hypothalamic neurons and released in a pulsatile manner into the hypophyseal portal vascular system to act on gonadotrope cells in the anterior pituitary. It binds to its high-affinity G protein–coupled receptor (GPCR), GnRH receptor (GnRHR), on the cell surface of the gonadotropes, stimulating signaling pathways that primarily regulate the synthesis and release of the gonadotropins FSH and LH (3). These gonadotropins, in turn, are secreted into the bloodstream, acting at the ovaries and the testes.
FSH and LH regulate critical aspects of mammalian sexual maturation and reproductive function, including gametogenesis, steroidogenesis, and ovulation (4). FSH is essential for normal human reproductive function of both sexes; its deficiency can cause absent or incomplete pubertal development in women and a relatively normal pubertal development but azoospermia in men, with infertility resulting in both women and men (5, 6). In mice, FSH is essential for female fertility but not for males. Female mice deficient in FSH are infertile and have a block in folliculogenesis prior to the antral stage, whereas male mice are fertile but with impaired reproductive function owing to reduced sperm count (7). Alternatively, LH is essential for normal gonadal function and pubertal development in both humans and mice, as its absence results in hypogonadism and infertility in both sexes and in both humans and mice (8–10).
Both FSH and LH are dimeric glycoproteins comprised of a common α-subunit (αGSU, encoded by the CGA gene) and a unique β-subunit, FSHβ and LHβ, respectively (11). The αGSU is produced at high levels basally and is less tightly regulated by GnRH (5). The distinct β-subunits confer the biological specificity of the gonadotropins, and their synthesis is the rate-limiting step for the production of the mature hormones (12, 13). Therefore, FSH and LH synthesis is regulated at the level of β-subunit transcription.
GnRH is released intermittently to produce pulses, and variations in the frequency of pulsatile GnRH release have differential effects on gonadotropin synthesis and release. FSH is preferentially stimulated at low GnRH pulse frequencies, whereas LH is preferentially stimulated at high GnRH pulse frequencies. Likewise, low GnRH pulse frequencies favor Fshb gene expression (14, 15), whereas high GnRH pulse frequencies favor Lhb gene expression (16, 17).
There are several other hormones of the hypothalamic–pituitary–gonadal axis that regulate gonadotropin synthesis and secretion, for example, activins, inhibins, follistatin, sex steroids, pituitary adenylate cyclase–activating polypeptide, and bone morphogenetic proteins, to name a few, but these are beyond the scope of this review (18). Herein, we focus on the regulation of Fshb transcription by GnRH, an important regulator of FSH in most mammalian species.
GnRH-Stimulated Signaling Pathways That Regulate Fshb Gene Expression
The GnRHR is a member of the seven-transmembrane GPCR family (19, 20). It is suggested that three forms of GnRHR (GnRHR1, GnRHR2, and GnRHR3) are expressed in most vertebrates, with distinct functions and distributions (21). However, only two types are expressed in mammals (22). The type I GnRHR, which is >80% identical among humans, rats, sheep, cows, and pigs (23), is predominant in the gonadotrope, but in humans it has been shown to also be expressed in extrapituitary normal reproductive tissues, such as breast, endometrium, ovaries, and prostate, as well as in tumors derived from these tissues (24). Alternatively, the type II GnRHR is fully functional in monkeys and pigs but absent or silent in humans, chimpanzees, cows, sheep, and mice (23). For the purposes of this review, we focus on the mammalian type I GnRHR.
Signaling by the type I GnRHR includes interaction with several heterotrimeric G proteins (25). Studies in the gonadotrope-derived LβT2 cell line and in primary rat pituitary cultures have shown that the GnRHR can couple with both Gαq/11 and Gαs proteins (26–28). Expression of Gαi and Gα12/13 has been reported in LβT2 cells as well, but without a clear effect on regulation of gonadotropin gene expression (27). Alternatively, studies in other cell types, including αT3-1, CHO-K1, and COS-7 cells, demonstrated that GnRHR interacts exclusively with the Gαq/11 to initiate multiple signaling pathways (29, 30). Therefore, the cell model that is being used needs to be taken into consideration when studying GnRHR signaling (25).
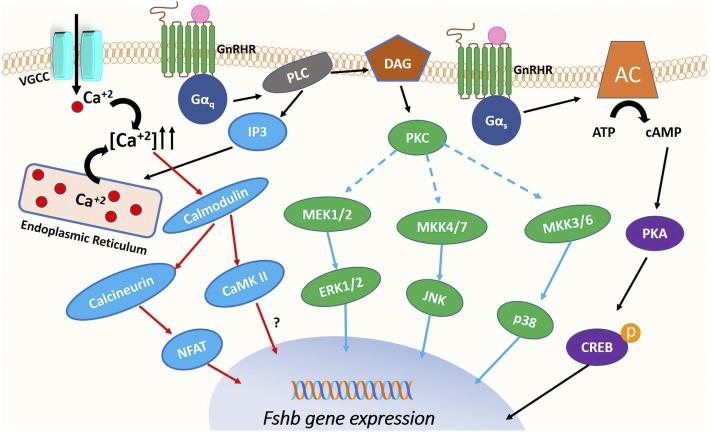
The interaction of the GnRHR with Gαq/11 activates phospholipase Cβ. This leads to activation of second messengers, inositol trisphosphate (IP3) and diacylglycerol (DAG), via cleavage of phosphatidylinositol 4,5-bisphosphate (1, 25, 31). IP3 mobilizes Ca2+ from endoplasmic reticulum stores, which in turn stimulates several calcium-dependent cascades. Ca2+ mobilization and DAG formation lead to activation of protein kinase C (PKC), which primarily activates the MAPK family (32, 33). The activation of Gαs typically activates adenylyl cyclase and consequently activates the cAMP/protein kinase A (PKA) signaling pathway (25). However, it has been demonstrated that the cAMP/PKA pathway can also be activated via Gαq/11 in gonadotropes upon GnRH stimulation (34).
Taken together, GnRHR has been shown to couple to multiple G proteins and initiate different signaling pathways. The diverse signaling via multiple G proteins may contribute to a link between GnRH pulse frequency and selective G protein activation in gonadotropes to preferentially stimulate FSH or LH synthesis and secretion. In this section, we focus on calcium-mediated signaling pathways, MAPK cascades, and the cAMP/PKA pathway and their roles in Fshb expression.
Calcium
The binding of GnRH to its receptor on the cell surface of gonadotropes mediates an increase in intracellular calcium concentrations. This increase can be mediated by calcium influx through l-type voltage-gated calcium channels (35) and by mobilization of calcium from intracellular stores (31, 36, 37) (Fig. 1). Intracellular calcium mobilization occurs through IP3 formation and is sensitive to desensitization via downregulation of IP3 receptors (38–40). The increase in intracellular calcium is essential for gonadotropin subunit gene expression and gonadotropin secretion (33). Studies in rat pituitary cells demonstrated that blockade of calcium channels attenuated the increase in Cga (encoding αGSU), Fshb, and Lhb gene expression (4, 25). Additionally, perifusion studies in rat primary cells with the calcium channel activator BayK8644 revealed a similar effect on gonadotropin gene expression as GnRH pulses. Low-frequency pulses preferentially stimulated Fshb whereas high-frequency pulses favored Lhb (41).
Figure 1.
GnRH-stimulated signaling pathways that regulate Fshb gene expression. When GnRH binds to its receptor (GnRHR), the receptor is activated and interacts either with Gαq/11 or Gαs to initiate intracellular signaling pathways that subsequently stimulate Fshb gene expression. The activation of Gαq/11 activates phospholipase C (PLC)β, which, in turn, results in generation of IP3 and DAG. IP3 mobilizes Ca2+ from endoplasmic reticulum stores. Further increase of intracellular Ca2+ is mediated by calcium influx through L-type voltage gated calcium channels (VGCC). The rise in calcium concentration stimulates several calcium-dependent cascades, including the calmodulin/calcineurin/NFAT pathway and the calmodulin/CaMK II pathway, resulting in increased Fshb gene expression. DAG formation leads to activation of PKC, which in turn activates the mitogen-activated protein kinase family (ERK1/2, JNK, p38) to further stimulate Fshb gene transcription. The activation of Gαs activates adenylyl cyclase (AC) and consequently activates the cAMP/PKA signaling pathway, which in turn leads to CREB phosphorylation and enhanced Fshb transcription. CaMK II, calcium/calmodulin-dependent kinase II; CREB, cAMP response element binding protein; MEK, MAPK kinase; NFAT, nuclear factor of activated T cells.
There are many Ca2+-regulated proteins that mediate transcriptional effects of GnRH in the gonadotropes. These include calmodulin, calcium/calmodulin-dependent kinase II (CaMK II), the calmodulin-dependent phosphatase calcineurin, and the Ca2+-dependent transcription factor nuclear factor of activated T-cells (NFAT) (42) (Fig. 1, “blue” pathway). It has been demonstrated in αT3-1 cells that calmodulin activation by elevated calcium levels is required for ERK signaling (43). More recently, Mugami et al. (44), performing studies in αT3-1 and LβT2 cells, showed that increased intracellular calcium levels are also necessary for the activation of certain PKC isoforms and of p38, indicating crosstalk among Ca2+ signaling and MAPK cascades to stimulate gonadotropin gene expression. CaMK II is a common mediator of calcium signaling in many cell types (45) and is also activated in gonadotropes (43, 46). Studies in primary rat pituitary cells demonstrated that a single pulse of GnRH can induce a rapid and transient increase in CaMK II activation and that inhibition of CaMK II attenuated GnRH-induced Cga, Fshb, and Lhb expression (47). However, CaMK II activation by GnRH is unrelated to GnRH pulse frequency (48), making its role as a GnRH pulse frequency decoder in the gonadotrope controversial. Another mediator of calcium action is the protein phosphatase calcineurin (49). NFAT is a proposed target of calcineurin to mediate GnRH-induced gonadotropin gene expression. In vitro studies in LβT2 and HeLa cells demonstrated that NFAT is indeed upregulated by GnRH stimulation (50, 51). Studies in the αT3-1 gonadotrope-derived cell line suggested a possible role for calcineurin in GnRH-mediated derepression of Fshb expression via activation of Nur77 (52). Because NFAT is a known activator of Nur77 (50), the calcineurin/NFAT signaling pathway may contribute to GnRH-induced Fshb expression. In support of this hypothesis, studies in LβT2 cells using cyclosporin A, calcineurin-specific inhibitor, and a small interfering RNA specific for NFAT revealed a reduction of the expression of Fos, Jun, and Atf3, immediate-early genes important for GnRH-mediated expression of Cga and Fshb (53). Pnueli et al. (51) and Armstrong et al. (54) developed live cell imaging readouts based on translocation of NFAT and ERK2 into the nucleus to study calcineurin/NFAT and MAPK kinase (MEK)/ERK activation, respectively, following GnRH stimulation. They demonstrated in LβT2 cells and in HeLa cells that pulsatile GnRH causes nuclear translocation of calcineurin/NFAT and MEK/ERK in a dose- and frequency-dependent manner. However, NFAT translocation was slower in onset and reversible compared with ERK2 translocation, and it lacked desensitization (51, 54). These results suggest that, although NFAT may contribute to mediating GnRH action, it is not the genuine decoder of GnRH pulse frequency in vitro. However, mathematical models developed from the same group suggested that NFAT and ERK pathways may decode GnRH pulse frequency when they act in a cooperative manner. Further studies are needed to explore experimentally the effects of NFAT on Fshb expression and its synergy with MAPK cascades, especially in vivo (42, 55, 56).
MAPK cascades
Similar to many other GPCRs, GnRHR mediates activation of several MAPK cascades in gonadotropes to induce gonadotropin subunit transcription, including ERK1 and ERK2 (MAPK1/3), JNKs (MAPK8/9), and p38 (MAPK14) (2, 4, 16, 18, 25, 57–60). These activated MAPKs translocate to the nucleus to initiate the transcriptional activation of Fshb (61) (Fig. 1, “green” pathway).
ERK1/2 signaling has been most strongly implicated in GnRH-induced Fshb expression and has been studied extensively. In vitro studies in LβT2 cells demonstrated that a low pulse frequency of GnRH leads to more rapid and sustained activation of ERK1/2, compared with a high GnRH pulse frequency, and results in increased levels of nuclear phosphorylated ERK (62). Additionally, in vivo studies conducted in adult castrated, testosterone-replaced male rats (to suppress endogenous GnRH) revealed that pulsatile GnRH stimulates ERK1/2 phosphorylation and, similarly to the results in vitro in LβT2 cells, low GnRH pulse frequency leads to a greater increase of ERK1/2 activation (48, 63). That ERK1/2 phosphorylation follows a pattern similar to Fshb expression upon GnRH stimulation suggests that ERK1/2 signaling may mediate GnRH-induced Fshb transcription. Indeed, pharmacologic inhibition of MEK1, the kinase that activates ERK1/2, attenuated GnRH-induced ovine, murine, rat, and human Fshb/FSHB promoter activities in LβT2 cells (18, 48, 62, 64–67). Furthermore, in a perifusion paradigm, MEK1/2 inhibition in LβT2 cells reduced GnRH stimulation of Fshb at both low and high GnRH pulse frequencies (68). Collectively, these results suggest an important role of ERK1/2 in GnRH-induced Fshb expression in vitro. However, the role of ERK signaling in Fshb expression in vivo is controversial. Mice deficient in ERK1/2 in the pituitary showed minimal changes in Fshb expression and circulating levels of FSH, despite more marked effects on Lhb expression and LH secretion, which lead to anovulatory infertility in ERK-deficient females. The male mice demonstrated normal reproductive function and gonadal histology (69). More recently, the same group developed gonadotrope-specific ERK1/2 knockout mice using a gonadotrope-specific Cre driver, the GnRHR–internal ribosomal entry site–Cre (GRIC) mouse (70). In this case, the GRIC-mediated ERK deletion resulted in reduced Fshb and Lhb expression and a blunted rise of FSH and LH secretion upon castration in both sexes, with a greater effect in females. The female mice were infertile and anovulatory, consistent with the previous mouse model, but the male mice were subfertile (70). Despite the differences between the two models, these studies demonstrate that ERK signaling is definitively essential for normal reproductive function in female mice and that the observed infertility in these two mouse models may be due to deficiency in LH rather than FSH. The FSH levels, although reduced, were sufficient for folliculogenesis. Perhaps other GnRH-mediated signaling pathways may compensate for ERK1/2 deficiency to maintain adequate FSH levels.
GnRH also activates the JNK and p38 branches of the MAPK pathway, both in vitro and in vivo (16, 44, 64, 71). Studies using dominant-negative mutants of JNK and p38 demonstrated reduced GnRH-stimulated ovine Fshb promoter activity (64). Alternatively, studies in LβT2 cells and in rat primary pituitary cells showed that inhibition of JNK and p38 had no effect on GnRH induction of Fshb expression, whereas JNK inhibition had some effect on basal levels of Fshb (16). Collectively, these data suggest that GnRH can stimulate JNK and p38 branches of MAPK in vitro but their exact roles in GnRH-mediated Fshb expression may vary among different species and need to be studied further.
cAMP/PKA signaling
The cAMP/PKA cascade has been implicated in GnRH-induced Fshb expression as well (13) (Fig. 1, “purple” pathway). Increased cAMP production upon GnRH stimulation has been reported in rat pituitary cells, in LβT2 cells, and in many heterologous cell lines, including HeLa, GH3, and COS-7 cells (17, 72, 73). Studies in LβT2 cells demonstrated that the GnRHR couples with Gαs protein to activate cAMP production (26). However, another group using the same cell line showed that PKCδ and PKCε can also stimulate cAMP production via activation of adenylate cyclase 5 and 7. This finding indicates that this pathway can also be activated via Gαq/11 (34). Tsutsumi et al. (74) studied the dynamics of cAMP and PKA responses in LβT2 cells, using fluorescence resonance energy transfer reporters, and they demonstrated that pulsatile GnRH stimulation, regardless of the GnRH pulse frequency, induces intracellular pulses of cAMP and PKA, which are rapid and transient. Many other studies, both in vitro and in rat pituitary cell cultures, have shown that PKA activity can be increased via GnRH stimulation in gonadotropes (75–77). However, studies exploring the role of PKA in Fshb expression are limited. Our group demonstrated that PKA can mediate stimulation of Fshb but not Lhb, at both high and low GnRH pulse frequencies in LβT2 cells. More interestingly, PKA activity was increased to a greater extent at low GnRH pulse frequencies, similar to Fshb expression, implicating PKA as an important mediator of GnRH-induced FSHβ synthesis in vitro (75).
Transcriptional Regulation of Fshb
The signaling cascades described affect both Cga and Fshb gene expression. As previously mentioned, αGSU is produced in excess, regardless of GnRH pulse frequency (14, 15). Therefore, the production of the distinct β-subunit controls FSH synthesis. In this section, we focus on transcription factors that are activated by GnRH to mediate Fshb expression.
During the last few decades, especially after the development of the LβT2 cell line, which expresses both Fshb and Lhb (78–80), significant advances have been made in the characterization of the Fshb gene promoter, providing much information about the regulation of Fshb gene expression upon GnRH stimulation. We (81) and others (66, 67) have identified a binding site in the proximal Fshb gene promoter that confers GnRH responsiveness, which is highly conserved in humans (67). This binding site contains a partial cAMP response element (CRE)/activator protein 1 (AP1) element.
CRE binding protein
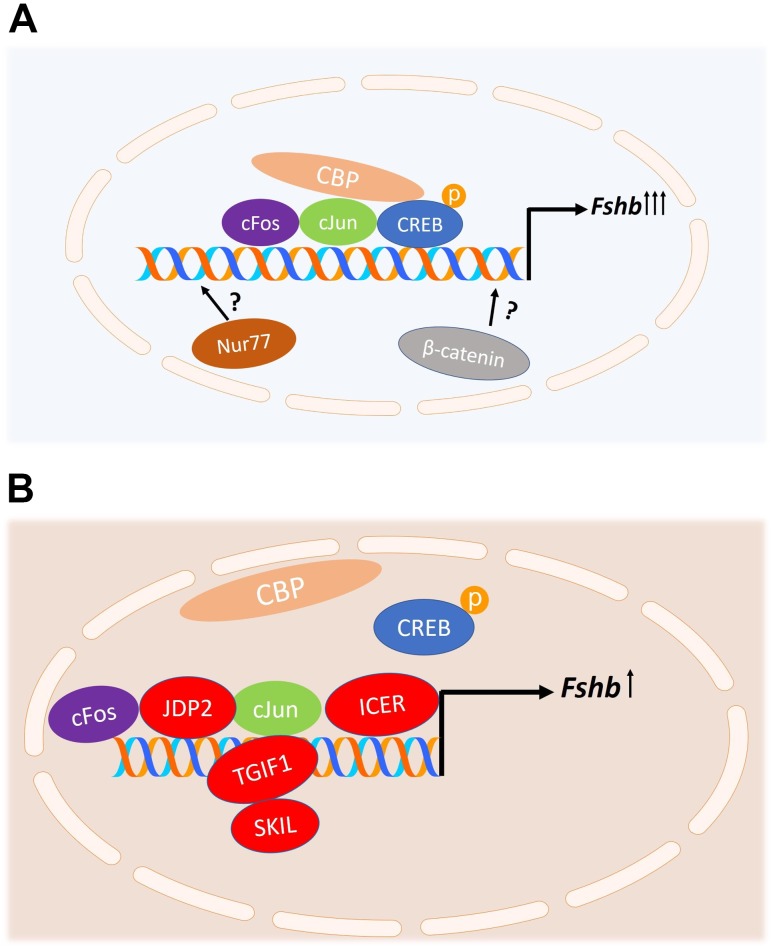
Our group focused on the CRE half-site and its role in GnRH-induced rat and mouse Fshb transcription. We showed in vitro that GnRH stimulates CRE-binding protein (CREB) phosphorylation, which results in recruitment of the histone acetyltransferase CREB-binding protein to the promoter and enhanced Fshb gene transcription (17, 81) (Fig. 2A). Furthermore, mutation of the CRE site within the Fshb promoter resulted in the loss of preferential GnRH stimulation at low GnRH pulse frequencies (82). We further explored the role of CREB in a perifusion paradigm. Using the LβT2 cell line, we demonstrated that preferential Fshb expression by pulsatile GnRH at low GnRH pulse frequencies is dependent on phosphorylated CREB. CREB phosphorylation is also increased at low GnRH pulse frequencies, and, furthermore, inhibition of PKA attenuated both CREB phosphorylation and Fshb expression (75). These findings support the hypothesis that CREB plays a major role in decoding GnRH pulse frequency and regulating FSH synthesis in vitro. Targeted gonadotropic-specific deletion of CREB in a mouse model will more accurately assess its physiologic impact in vivo.
Figure 2.
Proposed model of regulation of Fshb transcription by GnRH. (A) Stimulation of Fshb transcription by GnRH. The phosphorylated CREB protein binds to the homologous CRE-AP1 site of the Fshb gene promoter and recruits the histone acetyltransferase CREB-binding protein (CBP), stimulating Fshb transcription. GnRH-induced activation of MAPKs and Ca2+-dependent pathways stimulates the expression of cFos, FosB, cJun, and JunB, and a combination of these factors binds to the AP1 site in the Fshb promoter to stimulate Fshb transcription. Other mechanisms for GnRH stimulation of Fshb promoter involving the nuclear orphan receptor Nur77 and β-catenin have been proposed but are yet incompletely understood. (B) Repression of Fshb transcription. At high GnRH pulse frequencies, repressors are induced and compete with CREB and/or AP1 proteins for the same binding sites on the Fshb gene promoter. Inducible cAMP early repressor (ICER) attenuates GnRH-induced Fshb expression by antagonizing the binding of CREB to the CRE site and thus preventing the recruitment of CBP. SKIL and TGIF1 bind to the Fshb promoter and inhibit GnRH-mediated Fshb expression by attenuating the actions of cFos and cJun. c-JUN dimerization protein 2 (JDP2) is a novel repressor of GnRH-induced Fshb expression. JDP2 binds to the AP1 site of the Fshb promoter, complexing with cJun and hence preventing cFos-cJun dimerization.
AP1 family members
Other groups comprehensively studied the role of the AP1 site in GnRH-induced Fshb transcription. AP1 is a heterodimeric transcription factor comprised of Fos and Jun intermediate-early gene family members, which are rapidly and transiently activated by variety of growth factors, cytokines, neurotransmitters, and hormonal signals in most cell types (83, 84). Transcriptome analyses revealed that AP1 members are strongly induced by GnRH in LβT2 cells (85, 86) and in primary rat gonadotropes (87). Specifically, GnRH-induced activation of MAPKs, including ERK1/2, JNK, and p38, stimulates the expression of cFos, FosB, cJun, and JunB, and a combination of these factors binds to the AP1 site in the Fshb promoter to stimulate Fshb transcription (66, 88–90) (Fig. 2A). Additionally, it has been demonstrated that the AP1 members interact with factors involved in basal expression of Fshb, such as NF-Y in the mouse and USF-1 in the rat, to integrate GnRH responsiveness (66, 81, 91). Calcium signaling has also been implicated in GnRH-induced expression of cFos in vitro. Activation of CaMK II leads to phosphorylation of serum response factor to induce cFos and hence regulate Fshb gene expression in LβT2 cells (92). Other in vitro studies demonstrated a possible role of estrogen receptor α and β-catenin in FosB and cJun induction by GnRH (93, 94). The importance of cFos and cJun in normal reproductive function has been demonstrated in vivo as well. A recent study revealed that cFos knockout mice had impaired spermatogenesis and ovulation and reduced gonadotropins, in both mRNA levels and serum hormone levels, which did not recover after GnRH treatment (95). Consistent with these results, a previous study reported that cFos-deficient mice had small ovaries with atretic follicles (96), a phenotype similar to that of FSHβ knockout mice (7), suggesting the necessity of cFos for Fshb transcription. Alternatively, cJun knockout mice had impaired spermatogenesis in males and reduced numbers of corpora lutea in females, but unaffected FSH levels, making the role of cJun in the GnRH regulation of Fshb expression less clear. Compensation by JunB may explain this unexpected finding (97). That cFos and cJun protein levels are increased to a greater extent at high GnRH pulse frequencies, which is inconsistent with the preferential Fshb expression at low GnRH pulse frequencies (98), calls into question whether the AP1 proteins make a major contribution in decoding GnRH pulse frequency in the gonadotropes.
β-Catenin
β-Catenin (CTTNNB1), a key mediator of the Wnt developmental signaling pathway, has also been implicated in GnRH induction of the gonadotropin β-subunit (99, 100) (Fig. 2A). Studies in LβT2 cells demonstrated that GnRH can increase nuclear β-catenin levels via a JNK pathway. β-Catenin regulation of Fshb does not involve a direct transcriptional mechanism, but rather β-catenin induces Fshb expression through its cofactor, breast cancer metastasis suppressor 1-like (101). Although in vitro studies suggest a role for β-catenin in the regulation of Fshb expression, the role in gonadotropes in vivo is less clear. Gonadotrope-specific β-catenin knockout mice are fertile, with normal reproductive phenotypes and normal FSH levels. In contrast, male mice with a gain-of-function Ctnnb1 mutation, which prevents β-catenin degradation, demonstrated impaired Fshb expression and FSH secretion (102).
Nur77
Another mechanism for GnRH regulation of the murine Fshb promoter involving the nuclear orphan receptor Nur77 has been proposed (Fig. 2A). The Nr4a1 gene, encoding Nur77, belongs to a subfamily of nuclear receptors that includes two other members, Nurr1 [nuclear receptor subfamily 4, group A, member 2 (Nr4a20)] and Nor-1 [nuclear receptor subfamily 4, group A, member 3 (Nr4a3)] (103, 104). Studies in αT3-1 murine gonadotrope cells demonstrated that the expression of Fshb is initially repressed by histone deacetylase activity, and GnRH stimulation can overcome this repression by targeting Nur77 (52). A recent transcriptome analysis revealed high induction of Nr4a1 gene upon GnRH stimulation (86). In vitro studies in LβT2 cells demonstrated that GnRH transiently activates Nur77 and that this activation occurs predominantly via the cAMP-PKA signaling pathway, similar to CREB phosphorylation (105). Other groups have shown that Nr4a1 may be induced by GnRH via the calcium/PKC/ERK–dependent signaling pathway (106). In vivo studies indicated that Nur77-deficient mice were fertile, suggesting that compensation by other proteins may occur (107). Detailed reproductive phenotyping of these mice was not performed. The mechanism by which Nur77 regulates Fshb expression is still incompletely understood, and further studies are needed to determine whether Nur77 binds to the Fshb promoter and to validate its physiological relevance in vivo.
Repressors
To explain the relatively lower induction of Fshb at high GnRH pulse frequencies, several groups studied potential repressors that may be highly expressed at high GnRH pulse frequencies and compete with either CREB or AP1 proteins for the same binding sites on the FSHβ promoter. Indeed, our group identified the inducible cAMP early repressor (ICER) as a potential repressor of GnRH-mediated Fshb induction. Using LβT2 cells in our perifusion paradigm, we showed that ICER is preferentially induced by GnRH at high pulse frequencies and attenuates GnRH-induced Fshb expression by antagonizing the binding of CREB to the CRE site of the Fshb promoter (82) (Fig. 2B). More recently, we demonstrated that ICER induction by pulsatile GnRH is mediated by ERK1/2 signaling pathway (68). Using a MEK1/2 inhibitor, ICER expression was attenuated at high GnRH pulse frequency in vitro. Surprisingly, inhibition of ICER did not result in greater induction of Fshb expression at a high GnRH pulse frequency, likely the result of simultaneous inhibition of cFos and cJun induction via MEK1/2 inhibition (68). SKIL and TGIF1 repressors reduce Fshb expression by binding to the Fshb promoter and attenuating the actions of cFos and cJun (Fig. 2B). These repressors are also preferentially stimulated at high GnRH pulse frequencies (98). Although these in vitro studies are promising, the importance of these repressors has not yet been examined in vivo. More recently, Jonak et al. (108) identified c-JUN dimerization protein 2 (JDP2) as a novel repressor of GnRH-mediated Fshb induction. JDP2 binds to the AP1 site of the Fshb promoter, complexing with cJun and thereby preventing cJun-cFos dimerization (Fig. 2B). JDP2 is known to recruit histone deacetylase 3 to repress transcription via chromatin modification (109, 110). Additionally, JDP2 serves as negative regulator of Fshb transcription indirectly by reducing cJun expression (108). In vivo studies demonstrated that JDP2-null male mice are fertile with no adverse effects on reproductive function, whereas female mice revealed significant changes. JDP2-null female mice have early puberty, as indicated by early vaginal opening, increased litter size, and early reproductive senescence. JDP2-null females have also higher serum levels of FSH and estradiol (108). The GnRH pulse frequency dependence of this repressor is yet to be determined.
Posttranscriptional Regulation of Fshb: The Role of Microtranscriptome
Although transcriptional regulation of Fshb has been extensively studied, little is known about the posttranscriptional regulation. The miRNAs are a family of small (21- to 25-nucleotide) noncoding RNAs that regulate gene expression at a posttranscriptional level (111). They act by hybridizing with complementary regions in the RNA-induced silencing complex of the target mRNA. This interaction mediates transcript degradation or translational inhibition (112).
Yuen et al. (113) and Godoy et al. (114) investigated the microtranscriptome in LβT2 cells, identifying a large number of expressed miRNAs. Using multiple assays, they identified two miRNAs, miR-132 and miR-212, that are highly upregulated by GnRH (113, 114). Both miR-132 and miR-212 are localized within the first intron of a noncoding gene. This intron is highly conserved among species (115). Conversely, miR-125b was among the most repressed miRNAs upon GnRH stimulation (113, 114). Therefore, these miRNAs may serve as potential modulators of GnRH action. Studies in rat pituitary cells revealed that blocking the action of both miR-132 and miR-212 significantly reduced GnRH-stimulated FSH synthesis and secretion (116). Consistently, overexpression of both miRNAs in LβT2 cells resulted in higher Fshb expression (116). The same group recently showed that miR-125b prevents GnRH-induced FSH synthesis and secretion and thus serves as a repressor (117). In vivo studies of mice with gonadotrope-specific deletion of Dicer, an endoribonuclease important for the synthesis of miRNAs, led to suppressed gonadotropin synthesis and secretion and infertility in both male and female mice (118, 119). During the last decade, the miRNA pathway has been implicated in GnRH-regulated expression of FSH and is a field that definitely needs to be further explored.
Conclusions and Future Directions
Reproduction is a tightly regulated function that is crucial to the perpetuation of species. The pituitary gonadotropins FSH and LH play essential roles in the mammalian reproductive process to control fertility by directing steroidogenesis and gametogenesis. Befitting their important roles in endocrine physiology, the synthesis and secretion of the gonadotropins are under complex regulation by GnRH, gonadal sex steroids, and gonadal and other peptide hormones.
GnRH is a potent regulator of Fshb and Lhb transcription. Activation of GnRHR on gonadotropes by GnRH triggers multiple distinct signal transduction cascades, including calcium-dependent signaling pathways, MAPK cascades, and the cAMP/PKA pathway. Each pulse of GnRH induces transcription of immediate-early genes that leads to induction of Fshb and Lhb expression.
In recent decades, there have been immense advances in our understanding of the transcriptional regulation of the genes encoding FSH. The development of the murine gonadotrope-derived cell line, LβT2 cells, has benefited these studies, as previously there was no available homologous cell line to study Fshb gene regulation. Analyses of the Fshb promoter have identified a binding site in the proximal Fshb gene promoter region that confers GnRH responsiveness and contains a partial CRE/AP1 site. In vitro studies implicated CREB and AP1 proteins in GnRH-induced Fshb transcription. Studies in mice support a role for cFos in Fshb transcription and reproductive function in vivo (95). Targeted gonadotrope-specific deletion of CREB in a mouse model will further elucidate the role of CREB in decoding GnRH pulse frequency and regulating Fshb expression in vivo. Recently, two novel mechanisms for GnRH regulation of the murine Fshb promoter, involving the nuclear orphan receptor Nur77 and β-catenin, have been proposed and merit further investigation. Furthermore, several repressors, including ICER, SKIL, and TGIF1, have been identified that are highly expressed at high GnRH pulse frequencies and compete with either CREB or AP1 proteins for the same binding sites in the Fshb promoter. More recently, JDP2 was identified as a negative regulator of FSHβ synthesis and its role was analyzed both in vitro and in vivo. However, its GnRH pulse frequency–dependent expression and activity have not yet been determined. Additionally, some groups have recently focused on the posttranscriptional regulation of FSH, describing the role of miRNAs in the regulation of FSH synthesis and secretion.
In summary, important strides have been made in deciphering the molecular mechanisms of GnRH-induced Fshb transcription. The use of gonadotrope-specific targeted deletions in mice, studies with physiologically relevant pulsatile GnRH stimulation, and the potential development of new gonadotrope cell models will help to further expand our knowledge to answer the major question of clinical importance in reproductive physiology and pathophysiology: how does the gonadotrope decode GnRH pulse frequency to differentially control FSH and LH?
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants R01 HD019938 and R01 HD082314 (to U.B.K.) and T32 DK007529 (to G.A.S.), and by a Ferring Research Institute Innovation grant (to U.B.K.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AP1
activator protein 1
- CaMK II
calcium/calmodulin-dependent kinase II
- CRE
cAMP response element
- CREB
CRE-binding protein
- DAG
diacylglycerol
- GnRHR
GnRH receptor
- GPCR
G protein–coupled receptor
- GRIC
GnRHR–internal ribosomal entry site–Cre
- ICER
inducible cAMP early repressor
- JDP2
c-JUN dimerization protein 2
- IP3
inositol trisphosphate
- MEK
MAPK kinase
- NFAT
nuclear factor of activated T-cells
- PKA
protein kinase A
- PKC
protein kinase C
References
- 1. Brown JL, Roberson M. Novel insights into gonadotropin-releasing hormone action in the pituitary gonadotrope. Semin Reprod Med. 2017;35(2):130–138. [DOI] [PubMed] [Google Scholar]
- 2. Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 2010;31(3):322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18(1):46–70. [DOI] [PubMed] [Google Scholar]
- 4. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33(3):559–584. [DOI] [PubMed] [Google Scholar]
- 5. Coss D. Regulation of reproduction via tight control of gonadotropin hormone levels. Mol Cell Endocrinol. 2018;463:116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSHβ subunit gene is highly conserved. Mol Hum Reprod. 2005;11(8):601–605. [DOI] [PubMed] [Google Scholar]
- 7. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. [DOI] [PubMed] [Google Scholar]
- 8. Huhtaniemi I. Mutations along the pituitary-gonadal axis affecting sexual maturation: novel information from transgenic and knockout mice. Mol Cell Endocrinol. 2006;254–255:84–90. [DOI] [PubMed] [Google Scholar]
- 9. Huhtaniemi I, Ahtiainen P, Pakarainen T, Rulli SB, Zhang FP, Poutanen M. Genetically modified mouse models in studies of luteinising hormone action. Mol Cell Endocrinol. 2006;252(1–2):126–135. [DOI] [PubMed] [Google Scholar]
- 10. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101(49):17294–17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009;16(4):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11(1):177–199. [DOI] [PubMed] [Google Scholar]
- 13. Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2014;385(1–2):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128(1):509–517. [DOI] [PubMed] [Google Scholar]
- 15. Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125(2):917–923. [DOI] [PubMed] [Google Scholar]
- 16. Haisenleder DJ, Burger LL, Walsh HE, Stevens J, Aylor KW, Shupnik MA, Marshall JC. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology. 2008;149(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don’t, and why you should care. Fertil Steril. 2010;93(8):2465–2485. [DOI] [PubMed] [Google Scholar]
- 19. Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15(4):462–499. [DOI] [PubMed] [Google Scholar]
- 20. Re M, Pampillo M, Savard M, Dubuc C, McArdle CA, Millar RP, Conn PM, Gobeil F Jr, Bhattacharya M, Babwah AV. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One. 2010;5(7):e11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Troskie B, Illing N, Rumbak E, Sun YM, Hapgood J, Sealfon S, Conklin D, Millar R. Identification of three putative GnRH receptor subtypes in vertebrates. Gen Comp Endocrinol. 1998;112(3):296–302. [DOI] [PubMed] [Google Scholar]
- 22. Morgan K, Millar RP. Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol. 2004;139(3):191–197. [DOI] [PubMed] [Google Scholar]
- 23. Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25(2):235–275. [DOI] [PubMed] [Google Scholar]
- 24. Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275(22):5479–5495. [DOI] [PubMed] [Google Scholar]
- 25. Naor Z, Huhtaniemi I. Interactions of the GnRH receptor with heterotrimeric G proteins. Front Neuroendocrinol. 2013;34(2):88–94. [DOI] [PubMed] [Google Scholar]
- 26. Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both Gq/11 and Gs proteins in gonadotropin-releasing hormone receptor-mediated signaling in LβT2 cells. J Biol Chem. 2002;277(35):32099–32108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi SG, Jia J, Pfeffer RL, Sealfon SC. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J Biol Chem. 2012;287(25):21550–21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci USA. 2003;100(5):2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grosse R, Schmid A, Schöneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to Gq/11 proteins. J Biol Chem. 2000;275(13):9193–9200. [DOI] [PubMed] [Google Scholar]
- 30. Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992;6(10):1673–1681. [DOI] [PubMed] [Google Scholar]
- 31. Stojilkovic SS, Bjelobaba I, Zemkova H. Ion channels of pituitary gonadotrophs and their roles in signaling and secretion. Front Endocrinol (Lausanne). 2017;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. [DOI] [PubMed] [Google Scholar]
- 33. Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30(1):10–29. [DOI] [PubMed] [Google Scholar]
- 34. Larivière S, Garrel G, Simon V, Soh JW, Laverrière JN, Counis R, Cohen-Tannoudji J. Gonadotropin-releasing hormone couples to 3′,5′-cyclic adenosine-5′-monophosphate pathway through novel protein kinase Cδ and -ε in LβT2 gonadotrope cells. Endocrinology. 2007;148(3):1099–1107. [DOI] [PubMed] [Google Scholar]
- 35. Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem. 1999;274(42):29796–29804. [DOI] [PubMed] [Google Scholar]
- 36. Durán-Pastén ML, Fiordelisio T. GnRH-induced Ca2+ signaling patterns and gonadotropin secretion in pituitary gonadotrophs. functional adaptations to both ordinary and extraordinary physiological demands. Front Endocrinol (Lausanne). 2013;4:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stojilkovic SS. Molecular mechanisms of pituitary endocrine cell calcium handling. Cell Calcium. 2012;51(3–4):212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McArdle CA, Willars GB, Fowkes RC, Nahorski SR, Davidson JS, Forrest-Owen W. Desensitization of gonadotropin-releasing hormone action in αT3-1 cells due to uncoupling of inositol 1,4,5-trisphosphate generation and Ca2+ mobilization. J Biol Chem. 1996;271(39):23711–23717. [DOI] [PubMed] [Google Scholar]
- 39. McArdle CA, Davidson JS, Willars GB. The tail of the gonadotrophin-releasing hormone receptor: desensitization at, and distal to, G protein-coupled receptors. Mol Cell Endocrinol. 1999;151(1–2):129–136. [DOI] [PubMed] [Google Scholar]
- 40. McArdle CA, Franklin J, Green L, Hislop JN. Signalling, cycling and desensitisation of gonadotrophin-releasing hormone receptors. J Endocrinol. 2002;173(1):1–11. [DOI] [PubMed] [Google Scholar]
- 41. Haisenleder DJ, Workman LJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin subunit transcriptional responses to calcium signals in the rat: evidence for regulation by pulse frequency. Biol Reprod. 2001;65(6):1789–1793. [DOI] [PubMed] [Google Scholar]
- 42. Pratap A, Garner KL, Voliotis M, Tsaneva-Atanasova K, McArdle CA. Mathematical modeling of gonadotropin-releasing hormone signaling. Mol Cell Endocrinol. 2017;449:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol. 2005;19(9):2412–2423. [DOI] [PubMed] [Google Scholar]
- 44. Mugami S, Kravchook S, Rahamim-Ben Navi L, Seger R, Naor Z. Differential roles of PKC isoforms (PKCs) and Ca2+ in GnRH and phorbol 12-myristate 13-acetate (PMA) stimulation of p38MAPK phosphorylation in immortalized gonadotrope cells. Mol Cell Endocrinol. 2017;439:141–154. [DOI] [PubMed] [Google Scholar]
- 45. Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol Reprod. 2006;74(6):993–998. [DOI] [PubMed] [Google Scholar]
- 46. Melamed P, Savulescu D, Lim S, Wijeweera A, Luo Z, Luo M, Pnueli L. Gonadotrophin-releasing hormone signalling downstream of calmodulin. J Neuroendocrinol. 2012;24(12):1463–1475. [DOI] [PubMed] [Google Scholar]
- 47. Haisenleder DJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription: evidence for the involvement of calcium/calmodulin-dependent kinase II (Ca/CAMK II) activation in rat pituitaries. Endocrinology. 2003;144(7):2768–2774. [DOI] [PubMed] [Google Scholar]
- 48. Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79(5):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Natarajan K, Ness J, Wooge CH, Janovick JA, Conn PM. Specific identification and subcellular localization of three calmodulin-binding proteins in the rat gonadotrope: spectrin, caldesmon, and calcineurin. Biol Reprod. 1991;44(1):43–52. [DOI] [PubMed] [Google Scholar]
- 50. Pnueli L, Luo M, Wang S, Naor Z, Melamed P. Calcineurin mediates the gonadotropin-releasing hormone effect on expression of both subunits of the follicle-stimulating hormone through distinct mechanisms. Mol Cell Biol. 2011;31(24):5023–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J Biol Chem. 2009;284(51):35746–35757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lim S, Luo M, Koh M, Yang M, bin Abdul Kadir MN, Tan JH, Ye Z, Wang W, Melamed P. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27(11):4105–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Binder AK, Grammer JC, Herndon MK, Stanton JD, Nilson JH. GnRH regulation of Jun and Atf3 requires calcium, calcineurin, and NFAT. Mol Endocrinol. 2012;26(5):873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency? J Biol Chem. 2010;285(32):24360–24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garner KL, Voliotis M, Alobaid H, Perrett RM, Pham T, Tsaneva-Atanasova K, McArdle CA. Information transfer via gonadotropin-releasing hormone receptors to ERK and NFAT: sensing GnRH and sensing dynamics. J Endocr Soc. 2017;1(4):260–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsaneva-Atanasova K, Mina P, Caunt CJ, Armstrong SP, McArdle CA. Decoding GnRH neurohormone pulse frequency by convergent signalling modules. J R Soc Interface. 2012;9(66):170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dobkin-Bekman M, Naidich M, Pawson AJ, Millar RP, Seger R, Naor Z. Activation of mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol Cell Endocrinol. 2006;252(1-2):184–190. [DOI] [PubMed] [Google Scholar]
- 58. Kanasaki H, Purwana I, Oride A, Mijiddorj T, Miyazaki K. Extracellular signal-regulated kinase (ERK) activation and mitogen-activated protein kinase phosphatase 1 induction by pulsatile gonadotropin-releasing hormone in pituitary gonadotrophs. J Signal Transduct. 2012;2012:198527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ando H, Hew CL, Urano A. Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression. Comp Biochem Physiol B Biochem Mol Biol. 2001;129(2–3):525–532. [DOI] [PubMed] [Google Scholar]
- 60. Caunt CJ, Finch AR, Sedgley KR, McArdle CA. Seven-transmembrane receptor signalling and ERK compartmentalization. Trends Endocrinol Metab. 2006;17(7):276–283. [DOI] [PubMed] [Google Scholar]
- 61. Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31(5):268–275. [DOI] [PubMed] [Google Scholar]
- 62. Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LβT2 cells. Endocrinology. 2005;146(12):5503–5513. [DOI] [PubMed] [Google Scholar]
- 63. Haisenleder DJ, Cox ME, Parsons SJ, Marshall JC. Gonadotropin-releasing hormone pulses are required to maintain activation of mitogen-activated protein kinase: role in stimulation of gonadotrope gene expression. Endocrinology. 1998;139(7):3104–3111. [DOI] [PubMed] [Google Scholar]
- 64. Bonfil D, Chuderland D, Kraus S, Shahbazian D, Friedberg I, Seger R, Naor Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2004;145(5):2228–2244. [DOI] [PubMed] [Google Scholar]
- 65. Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. p38 Mitogen-activated protein kinase is critical for synergistic induction of the FSHβ gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol. 2007;21(12):3071–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279(1):152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. Activator protein-1 and Smad proteins synergistically regulate human follicle-stimulating hormone β-promoter activity. Endocrinology. 2008;149(11):5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thompson IR, Ciccone NA, Zhou Q, Xu S, Khogeer A, Carroll RS, Kaiser UB. GnRH pulse frequency control of Fshb gene expression is mediated via ERK1/2 regulation of ICER. Mol Endocrinol. 2016;30(3):348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23(7):1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brown JL, Xie J, Brieño-Enriquez MA, Sones J, Angulo CN, Boehm U, Miller A, Toufaily C, Wang Y, Bernard DJ, Roberson MS. Sex- and age-specific impact of ERK loss within the pituitary gonadotrope in mice. Endocrinology. 2018;159(3):1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHβ-subunit promoter. Endocrinology. 2002;143(3):1018–1025. [DOI] [PubMed] [Google Scholar]
- 72. Perrett RM, McArdle CA. Molecular mechanisms of gonadotropin-releasing hormone signaling: integrating cyclic nucleotides into the network. Front Endocrinol (Lausanne). 2013;4:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cohen-Tannoudji J, Avet C, Garrel G, Counis R, Simon V. Decoding high gonadotropin-releasing hormone pulsatility: a role for GnRH receptor coupling to the cAMP pathway? Front Endocrinol (Lausanne). 2012;3:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LβT2 gonadotrope cells. J Biol Chem. 2010;285(26):20262–20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thompson IR, Ciccone NA, Xu S, Zaytseva S, Carroll RS, Kaiser UB. GnRH pulse frequency-dependent stimulation of FSHβ transcription is mediated via activation of PKA and CREB. Mol Endocrinol. 2013;27(4):606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garrel G, Simon V, Thieulant ML, Cayla X, Garcia A, Counis R, Cohen-Tannoudji J. Sustained gonadotropin-releasing hormone stimulation mobilizes the cAMP/PKA pathway to induce nitric oxide synthase type 1 expression in rat pituitary cells in vitro and in vivo at proestrus. Biol Reprod. 2010;82(6):1170–1179. [DOI] [PubMed] [Google Scholar]
- 77. Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol. 2009;23(7):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10(4):439–450. [DOI] [PubMed] [Google Scholar]
- 79. Graham KE, Nusser KD, Low MJ. LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J Endocrinol. 1999;162(3):R1–R5. [DOI] [PubMed] [Google Scholar]
- 80. Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology. 2001;142(6):2284–2295. [DOI] [PubMed] [Google Scholar]
- 81. Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol Endocrinol. 2008;22(8):1908–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30(4):1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kakar SS, Winters SJ, Zacharias W, Miller DM, Flynn S. Identification of distinct gene expression profiles associated with treatment of LβT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene. 2003;308:67–77. [DOI] [PubMed] [Google Scholar]
- 84. Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9(2):240–246. [DOI] [PubMed] [Google Scholar]
- 85. Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276(50):47195–47201. [DOI] [PubMed] [Google Scholar]
- 86. Ruf-Zamojski F, Fribourg M, Ge Y, Nair V, Pincas H, Zaslavsky E, Nudelman G, Tuminello SJ, Watanabe H, Turgeon JL, Sealfon SC. Regulatory architecture of the LβT2 gonadotrope cell underlying the response to gonadotropin-releasing hormone. Front Endocrinol (Lausanne). 2018;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yuen T, Choi SG, Pincas H, Waring DW, Sealfon SC, Turgeon JL. Optimized amplification and single-cell analysis identify GnRH-mediated activation of Rap1b in primary rat gonadotropes. Mol Cell Endocrinol. 2012;350(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol. 2002;16(3):419–434. [DOI] [PubMed] [Google Scholar]
- 89. Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol. 2005;19(10):2624–2638. [DOI] [PubMed] [Google Scholar]
- 90. Lindaman LL, Yeh DM, Xie C, Breen KM, Coss D. Phosphorylation of ATF2 and interaction with NFY induces c-Jun in the gonadotrope. Mol Cell Endocrinol. 2013;365(2):316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314(2):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ely HA, Mellon PL, Coss D. GnRH induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol Endocrinol. 2011;25(4):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen J, An BS, Cheng L, Hammond GL, Leung PC. Gonadotropin-releasing hormone-mediated phosphorylation of estrogen receptor-α contributes to fosB expression in mouse gonadotrophs. Endocrinology. 2009;150(10):4583–4593. [DOI] [PubMed] [Google Scholar]
- 94. Salisbury TB, Binder AK, Grammer JC, Nilson JH. GnRH-regulated expression of Jun and JUN target genes in gonadotropes requires a functional interaction between TCF/LEF family members and β-catenin. Mol Endocrinol. 2009;23(3):402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xie C, Jonak CR, Kauffman AS, Coss D. Gonadotropin and kisspeptin gene expression, but not GnRH, are impaired in cFOS deficient mice. Mol Cell Endocrinol. 2015;411:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71(4):577–586. [DOI] [PubMed] [Google Scholar]
- 97. Jonak CR, Lainez NM, Boehm U, Coss D. GnRH receptor expression and reproductive function depend on JUN in the GnRH receptor-expressing cells. Endocrinology. 2018;159(3):1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-β gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25(8):1387–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gardner S, Maudsley S, Millar RP, Pawson AJ. Nuclear stabilization of β-catenin and inactivation of glycogen synthase kinase-3β by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol. 2007;21(12):3028–3038. [DOI] [PubMed] [Google Scholar]
- 100. Salisbury TB, Binder AK, Nilson JH. Welcoming β-catenin to the gonadotropin-releasing hormone transcriptional network in gonadotropes. Mol Endocrinol. 2008;22(6):1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang Q, Chikina M, Zaslavsky E, Pincas H, Sealfon SC. β-Catenin regulates GnRH-induced FSHβ gene expression. Mol Endocrinol. 2013;27(2):224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Boerboom D, Kumar V, Boyer A, Wang Y, Lambrot R, Zhou X, Rico C, Boehm U, Paquet M, Céleste C, Kimmins S, Bernard DJ. β-Catenin stabilization in gonadotropes impairs FSH synthesis in male mice in vivo. Endocrinology. 2015;156(1):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao N, Li X, Feng Y, Han J, Feng Z, Li X, Wen Y. The nuclear orphan receptor Nur77 alleviates palmitate-induced fat accumulation by down-regulating G0S2 in HepG2 Cells. Sci Rep. 2018;8(1):4809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104. Martínez-González J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65(3):609–618. [DOI] [PubMed] [Google Scholar]
- 105. Hamid T, Malik MT, Millar RP, Kakar SS. Protein kinase A serves as a primary pathway in activation of Nur77 expression by gonadotropin-releasing hormone in the LβT2 mouse pituitary gonadotroph tumor cell line. Int J Oncol. 2008;33(5):1055–1064. [PubMed] [Google Scholar]
- 106. Bliss SP, Navratil AM, Xie J, Miller A, Baccarini M, Roberson MS. ERK signaling, but not c-Raf, is required for gonadotropin-releasing hormone (GnRH)-induced regulation of Nur77 in pituitary gonadotropes. Endocrinology. 2012;153(2):700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science. 1995;269(5223):532–535. [DOI] [PubMed] [Google Scholar]
- 108. Jonak CR, Lainez NM, Roybal LL, Williamson AD, Coss D. c-JUN dimerization protein 2 (JDP2) is a transcriptional repressor of follicle-stimulating hormone β (FSHβ) and is required for preventing premature reproductive senescence in female mice. J Biol Chem. 2017;292(7):2646–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jin C, Li H, Murata T, Sun K, Horikoshi M, Chiu R, Yokoyama KK. JDP2, a repressor of AP-1, recruits a histone deacetylase 3 complex to inhibit the retinoic acid-induced differentiation of F9 cells. Mol Cell Biol. 2002;22(13):4815–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jin C, Kato K, Chimura T, Yamasaki T, Nakade K, Murata T, Li H, Pan J, Zhao M, Sun K, Chiu R, Ito T, Nagata K, Horikoshi M, Yokoyama KK. Regulation of histone acetylation and nucleosome assembly by transcription factor JDP2. Nat Struct Mol Biol. 2006;13(4):331–338. [DOI] [PubMed] [Google Scholar]
- 111. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation [published correction appears in Nat Rev Genet. 2004;5(8):631]. Nat Rev Genet. 2004;5(7):522–531. [DOI] [PubMed] [Google Scholar]
- 112. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–234. [DOI] [PubMed] [Google Scholar]
- 113. Yuen T, Ruf F, Chu T, Sealfon SC. Microtranscriptome regulation by gonadotropin-releasing hormone. Mol Cell Endocrinol. 2009;302(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Godoy J, Nishimura M, Webster NJ. Gonadotropin-releasing hormone induces miR-132 and miR-212 to regulate cellular morphology and migration in immortalized LβT2 pituitary gonadotrope cells. Mol Endocrinol. 2011;25(5):810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wanet A, Tacheny A, Arnould T, Renard P. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 2012;40(11):4742–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lannes J, L’Hôte D, Garrel G, Laverrière JN, Cohen-Tannoudji J, Quérat B. Rapid communication: a microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol Endocrinol. 2015;29(3):364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lannes J, L’hôte D, Fernandez-Vega A, Garrel G, Laverrière JN, Cohen-Tannoudji J, Quérat B. A regulatory loop between miR-132 and miR-125b involved in gonadotrope cells desensitization to GnRH [published correction appears in Sci Rep. 2016;6:34676]. Sci Rep. 2016;6(1):31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang H, Graham I, Hastings R, Gunewardena S, Brinkmeier ML, Conn PM, Camper SA, Kumar TR. Gonadotrope-specific deletion of Dicer results in severely suppressed gonadotropins and fertility defects. J Biol Chem. 2015;290(5):2699–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang H, Hastings R, Miller WL, Kumar TR. Fshb-iCre mice are efficient and specific Cre deleters for the gonadotrope lineage. Mol Cell Endocrinol. 2016;419:124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]