Abstract
Global GH receptor–null or knockout (GHRKO) mice have been extensively studied owing to their unique phenotype (dwarf and obese but remarkably insulin sensitive and long-lived). To better understand the influence of adipose tissue (AT) on the GHRKO phenotype, we previously generated fat-specific GHRKO (FaGHRKO) mice using the adipocyte protein-2 (aP2) promoter driving Cre expression. Unlike global GHRKO mice, FaGHRKO mice are larger than control mice and have an increase in white AT (WAT) mass and adipocyte size as well as an increase in brown AT mass. FaGHRKO mice also have an unexpected increase in IGF-1, decrease in adiponectin, no change in insulin sensitivity or liver triglyceride content, and a decreased lifespan. Extensive analysis of the aP2 promoter/enhancer by multiple laboratories has revealed expression in nonadipose tissues, confounding interpretation of results. In the current study, we used the adiponectin promoter/enhancer to drive Cre expression, which better targets mature adipocytes, and generated a new line of adipocyte-specific GHRKO (AdGHRKO) mice. AdGHRKO mice have an increase in adipocyte size and WAT depot mass in all depots except male perigonadal, a WAT accumulation pattern similar to FaGHRKO mice. Likewise, adiponectin levels and WAT fibrosis are decreased in both tissue-specific mouse lines. However, unlike FaGHRKO mice, AdGHRKO mice have no change in IGF-1 levels, improved glucose homeostasis, and reduced liver triglycerides. Thus, AdGHRKO mice should be valuable for future studies assessing the contribution of adipocyte GHR signaling in long-term health and lifespan.
GH is involved in many diverse processes such as somatic growth, metabolism, immunity, reproduction, and aging. To study the pleiotropic effects of GH, the global GH receptor (GHR) gene-disrupted or knockout (GHRKO) mouse was created in 1997 (1). GHRKO mice are GH insensitive with elevated GH and significantly decreased IGF-1, resulting in dwarfism. Although GHRKO mice are obese throughout life (2) and glucose intolerant, they are surprisingly healthy with enhanced insulin sensitivity, resistance to cancer and diabetes, and a marked increase in lifespan (3, 4).
White adipose tissue (WAT) is recognized as a vital player in maintaining energy balance, nutrient status, and metabolic homeostasis; thus, the obese phenotype of the long-lived GHRKO mice has garnered significant attention. WAT accumulation in GHRKO mice is dramatically increased throughout life (2) and is depot-dependent with preferential enlargement of the subcutaneous (SC) vs visceral depots (5). As might be expected, GHRKO mice have increases in adipocyte size in the SC depots as well as life-long increases in total and high-molecular-mass adiponectin and normal to elevated levels of leptin and resistin (6). Importantly, note that the positive correlation of adiponectin with these other two AT-derived hormones is unusual, as high adiponectin levels are more commonly associated with lower leptin and resistin levels (7). Furthermore, GHRKO mice have reduced preadipocyte senescence burden (8), lower levels of circulating inflammatory cytokines (9, 10), and increased adipogenesis potential of the mesenchymal stem cells isolated from the SC depot (11). Collectively, these data suggest that GHRKO mice have a “healthy” expansion of SC WAT mass. Interestingly, removal of visceral depots in GHRKO mice results in impaired insulin sensitivity and reduced serum adiponectin, suggesting potential benefits of other fat depots in these exceptionally long-lived mice (10).
To better understand the direct effects of GH on WAT, we previously generated fat-specific GHRKO (FaGHRKO) mice using the adipocyte protein-2 (aP2) transcriptional regulatory region (promoter/enhancer) driving Cre expression (12). These mice are obese with a more uniform increase in WAT and adipocyte size (all depots enlarged except the male perigonadal depot) and with enlargement in brown AT (BAT) in both sexes. Furthermore, unlike GHRKO mice, they show no improvement in insulin sensitivity, have increased circulating IGF-1 levels, and are significantly longer (nasal–anal length) than their littermate controls (12). The adipokine secretion pattern is also distinct from global GHRKO mice with significant decreases in adiponectin despite comparable increases in leptin. More recently, it was reported that WAT fibrosis, a hallmark of AT dysfunction, is decreased in FaGHRKO mice (13).
Although many laboratories have used the aP2 promoter/enhancer to generate fat-specific mouse lines, evidence from multiple laboratories indicates that its expression is “leaky” in nonadipose tissues or cells such as macrophages, endothelial cells, brain tissue (including hypothalamus), embryonic tissue, and other tissues (14, 15). This ectopic expression confounds interpretation of results when using it to generate adipose-specific gene disruption/deletion in mice and may help explain the unexpected results described for FaGHRKO mice. Fortunately, an alternative transcriptional regulatory region, the adiponectin (adipoq) promoter/enhancer, is available to generate transgenic mouse lines with adipocyte specific expression. Comparison of Cre expression between aP2 and adipoq promoters/enhancers indicates that the adipoq is far more specific to mature adipocytes compared with aP2 (14). Therefore, in the current study we used the adiponectin promoter/enhancer to drive Cre expression and generated a new line of adipocyte-specific GHR knockout (AdGHRKO) mice. Using protocols identical to those used previously, we are also able to compare our results with those previously published for FaGHRKO mice.
Materials and Methods
AdGHRKO mouse production
Mice with floxed GHR (GHRflox/flox) were created by the Knockout Mouse Project as previously described (12, 16, 17). Mice expressing adipocyte-specific Cre under the control of adiponectin promoter/enhancer, B6;FVB-Tg(Adipoq-cre)1Evdr/J (stock no. 010803), were purchased from The Jackson Laboratory (Bar Harbor, ME). Adipocyte-specific GHRKO mice were generated by breeding GHRflox/flox mice with B6;FVB-Tg(Adipoq-cre)1Evdr/J mice. Male and female Cre-expressing mice were used as breeders with no differences observed in offspring.
In the current study, 34 mice (6 female AdGHRKO mice, 8 female floxed littermate controls, 9 male AdGHRKO mice, and 11 male floxed littermate controls) were used to collect all measurements. Mice were housed three to four per cage and given ad libitum access to water and standard laboratory chow (ProLab RMH 3000; Lab Supply, Fort Worth, TX). The cages were maintained in a temperature-controlled room and exposed to a 14-hour light/10-hour dark cycle. All procedures were approved by the Ohio University Institutional Animal Care and Use Committee.
Quantitative PCR
Whole tissue was homogenized with Precellys 24 dual tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). RNA was purified from the homogenate using a Qiagen RNeasy mini kit (Qiagen, Chatsworth, CA). The concentration and integrity of purified RNA was determined with a Thermo NanoDrop 2000c and Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). A Qiagen QuantiTect reverse transcription kit was used for cDNA synthesis. Real-time PCR was performed using a Qiagen QuantiTect SYBR Green PCR kit with a Bio-Rad iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA). Besides runs with the primers targeting GHR, multiple reference genes (EEF2, RPS3, B2M, ACTB, HPRT, EiF3F, and RPL38) were used for each tissue as previously described (12). To determine the most stable reference genes for each tissue and to analyze all quantitative PCR results, qbase+ software with the geNorm analysis was used (Biogazelle, Zwijnaarde, Belgium). Primers used are provided in Table 1.
Table 1.
Primer Sequences
| Primers | Sequences (5′→3′) |
|---|---|
| GHR, forward | GCCTGGGGACAAGTTCTTCTGGA |
| GHR, reverse | TGCAGCTTGTCGTTGGCTTTCCC |
| EEF2, forward | CGGTACTTTGATCCGGCCA |
| EEF2, reverse | TAGTGGCGTCGAACACCTTG |
| RPS3, forward | ATCAGAGAGTTGACCGCAGTT |
| RPS3, reverse | AATGAACCGAAGCACACCATA |
| B2M, forward | CTGGTCTTTCTATATCCTGGCT |
| B2M, reverse | CATGTCTCGATCCCAGTAGAC |
| ACTB, forward | CAGCTTCTTTGCAGCTCCTT |
| ACTB, reverse | CACGATGGAGGGGAATACAG |
| HPRT, forward | ATCAGTCAACGGGGGACATA |
| HPRT, reverse | AGAGGTCCTTTTCACCAGCA |
| EIF3F, forward | TACGAACGCCGCAACGAGGG |
| EIF3F, reverse | TGGCACCGAAAAGCAGTTGGTGA |
| RPL38, forward | CGCGTCGCCATGCCTCGGAA |
| RPL38, reverse | ACTTGGCATCCTTCCGCCGGG |
Body composition
Body composition of AdGHRKO mice and controls was measured using a nuclear magnetic resonance imaging Bruker Minispec (Bruker Corporation, Woodlands, TX). Measurements were obtained monthly starting at 1 month of age and ending at 6 months of age.
Glucose and insulin tolerance tests
For the glucose tolerance test (GTT), mice were fasted overnight for 12 hours, and the 0 time point was collected at 0900 hours. Each mouse was given an IP injection of 15% glucose at 0.01 mL/g body weight. For the insulin tolerance test (ITT), mice were fasted for 6 hours, and the 0 time point was collected at 1500 hours. Each mouse was given 0.050 U/mL at 0.01 mL/g body weight of recombinant human insulin (Humulin-R; Eli Lilly & Co., Indianapolis, IN). Blood glucose measurements for both GTTs and ITTs were collected using OneTouch Ultra glucometers and test strips (Lifescan, Milpitas, CA). For both GTTs and ITTs, blood was collected at 0, 15, 30, 45, 60, and 90 minutes by cutting ∼1 mm from the tip of the tail.
Blood measurements
Serum measurements were performed from samples collected at 6 months of age. C-peptide, IL-6, resistin, leptin, monocyte chemoattractant protein 1, and TNF-α were measured using a mouse metabolic magnetic bead panel (catalog no. MMHMAG-44K; MilliporeSigma, Burlington, MA). Fibroblast growth factor 21, follistatin-related protein 1, myostatin, osteocrin/musculin, and osteonectin were measured using a mouse myokine magnetic bead panel (catalog no. MMYOMAG-74K; MilliporeSigma). Adiponectin was measured using a mouse high-molecular-weight and total adiponectin ELISA kit (catalog no. 47-ADPMS-E01; ALPCO, Salem, NH). IGF-1 levels were measured using a mouse/rat IGF-1 ELISA kit (catalog no. 22-IG1MS-E01; ALPCO). Insulin was measured using a mouse ultrasensitive ELISA kit (catalog no. 80-INSMSU-E01; ALPCO). Nonesterified free fatty acids (NEFAs) were measured using HR series NEFA-HR (2) (catalog nos. 999-34691, 995-34791, 991-34891, 993-35191, 276-76491; FUJIFILM Wako Diagnostics U.S.A. Corporation, Mountain View, CA). All kits were completed according to the manufacturers’ instructions.
Tissue collection and histology
Mice were dissected starting at 0900 hours following an overnight 12-hour fast. Tissues were collected, weighed, and stored at −80°C and/or prepared for histology as previously described (12). A portion of liver as well as SC and perigonadal AT were fixed in 10% formalin and embedded in paraffin. Sections were then stained with hematoxylin and eosin for cell sizing and picrosirius red (PSR) for determination of collagen content and overall fibrosis. PSR, hydroxyproline, and adipocyte cell size were determined as previously described (13).
Biochemical analysis of liver
Frozen liver tissue was thawed and lipid was extracted using the KOH in ethanol extraction procedure previously described (18). Extracted lipids were assayed using a triglycerides (GPO) reagent set (catalog no. T7532; Pointe Scientific, Canton, MI).
Statistical analysis
Statistics were performed using SPSS version 17.0 (IBM, Chicago, IL). A two-tailed unpaired Student t test was used to assess the difference between groups. For comparison of longitudinal data, including body weight, fat mass, and lean mass over time, repeated measures ANOVA was used. Differences were considered significant at P < 0.05.
Results
AdGHRKO generation, tissue GHR levels, circulating GH, and IGF-1
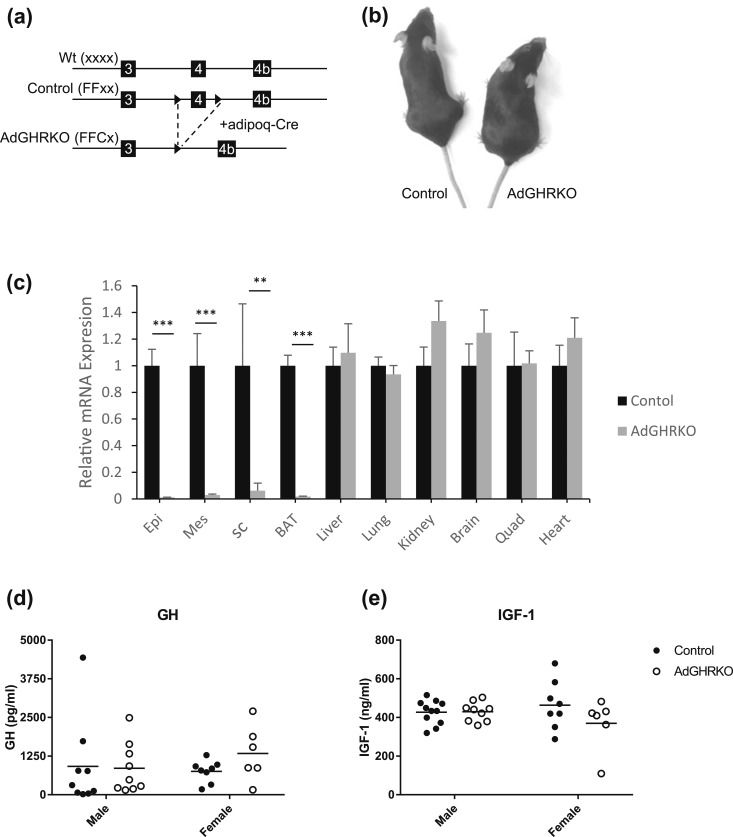
AdGHRKO mice (FFCx) and floxed littermate controls (FFxx) were generated by breeding conditional floxed GHRflox/flox mice to B6;FVB-Tg(Adipoq-cre)1Evdr/J mice [Fig. 1(a) and 1(b)]. GHR expression was significantly decreased in AT (including WAT and BAT) of the AdGHRKO mice [Fig. 1(c)]. Serum levels of GH and IGF-1 were not significantly different between AdGHRKO and control mice [Fig. 1(d) and 1(e)].
Figure 1.
Generation and characterization of AdGHRKO mice. (a) AdGHRKO mice were generated by crossing mice with a floxed exon 4 of the GHR to transgenic mice that express Cre recombinase under the control of the adiponectin promoter/enhancer (adipoq-cre). Solid arrowheads depict LoxP sites. (b) Photograph of female control and AdGHRKO mice. (c) GHR mRNA expression levels in various tissues from AdGHRKO mice (gray bars) vs floxed controls (black bars). (d and e) Serum GH and IGF-1 levels in AdGHRKO mice (●) and floxed controls (○). Values are represented as mean ± SEM. **P < 0.01; ***P < 0.001. Wt, wild type.
Body length, weight, and body composition
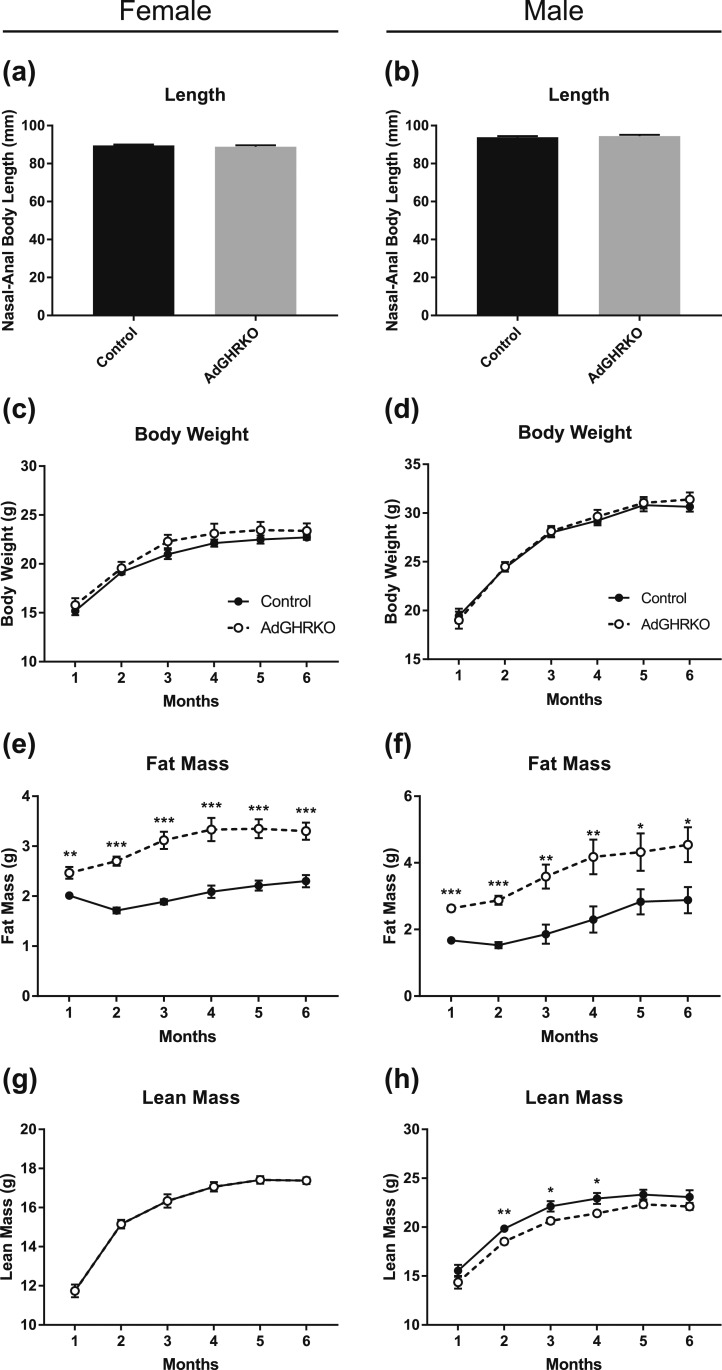
There was no difference in nasal–anal body length between AdGHRKO and control mice at 6 months of age [Fig. 2(a) and 2(b)]. Likewise, there were no significant differences in body weight between AdGHRKO mice and controls during the 6 months of measurement [Fig. 2(c) and 2(d)]. Fat mass was significantly increased in both AdGHRKO sexes throughout the analysis [Fig. 2(e) and 2(f)]; by 6 months of age, male and female AdGHRKO mice had a 158% and 144% increase in fat mass, respectively. Female AdGHRKO mice showed no differences in lean mass [Fig. 2(g)]; however, male AdGHRKO mice had decreased lean mass (93% of controls) in months 2 to 4 of the analysis [Fig. 2(h)].
Figure 2.
Length and body composition of AdGHRKO mice. Nasal–anal lengths were measured in both (a) female and (b) male mice. Thirty-four mice (n = 6 to 10 per group) were used monthly to measure body composition up to 6 mo of age when they were dissected. (c and d) Body weight, (e and f) fat mass, and (g and h) lean mass are shown for males and females over time. Values are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
AT depot mass and cell size
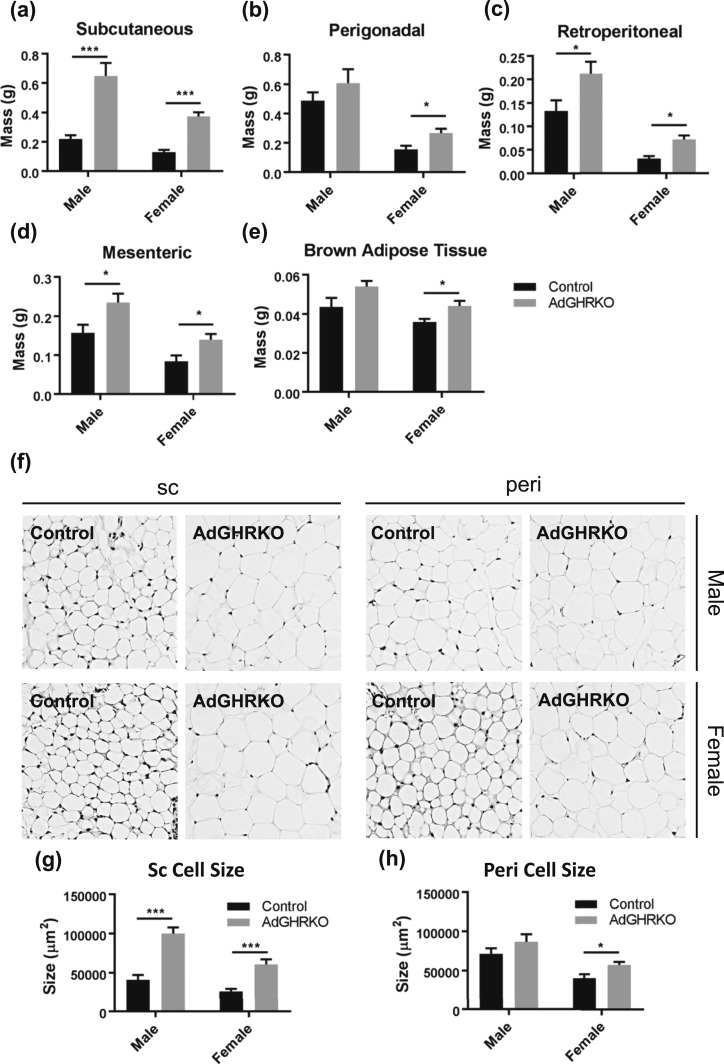
WAT mass was significantly increased in all four depots for AdGHRKO female mice and in all depots except perigonadal in AdGHRKO males compared with controls [Fig. 3(a)–3(d)]. The SC depot was most significantly impacted by GHR removal with male and female AdGHRKO mice having a 195% and 186% increase, respectively. Female AdGHRKO mice had significantly increased BAT (22%); however, the increase in BAT in male AdGHRKO mice did not reach statistical significance (P = 0.082) [Fig. 3(e)].
Figure 3.
AdGHRKO mice have increased depot-specific fat mass and cell size. (a) SC, (b) perigonadal, (c) retroperitoneal, (d) mesenteric, and (e) BAT are shown for male and female AdGHRKO mice and controls. (f) Hematoxylin and eosin staining of SC (left panels) and perigonadal (right panels) depots are shown at original magnification of ×400. Mean adipocyte size is shown for (g) SC and (h) perigonadal depots. Values are represented as mean ± SEM (n = 6 to 10 per group). *P < 0.05; ***P < 0.001.
The increase in total AT mass appears mainly due to an increase in adipocyte size. That is, male and female AdGHRKO mice had adipocytes that were 45% or 35% larger, respectively, than littermate controls in the SC depot [Fig. 3(f) and 3(g)]. Whereas perigonadal cell size was increased in female AdGHRKO mice compared with controls, adipocyte size was unchanged in male perigonadal WAT similar to the results in WAT mass [Fig. 3(f) and 3(h)].
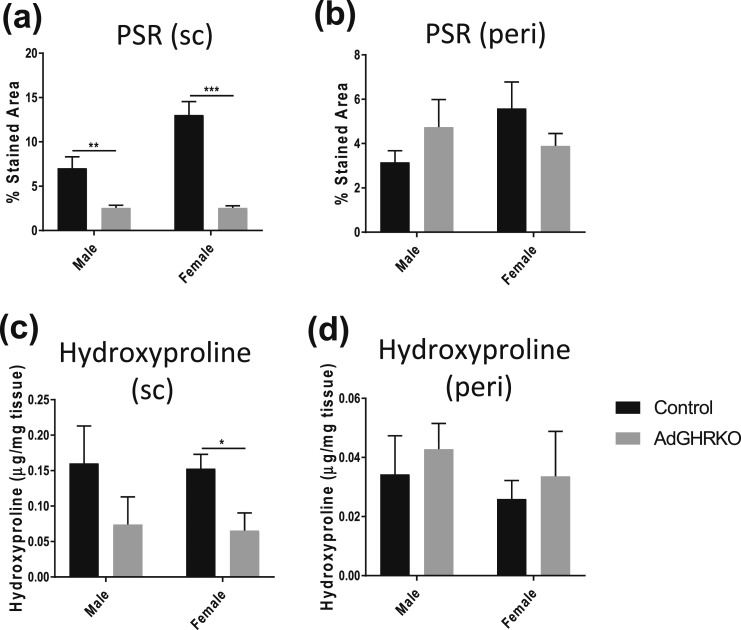
AT fibrosis
As fibrosis has been linked to AT health and to GH action, collagen deposition was quantified via PSR staining and hydroxyproline quantification. There was a significant decrease in PSR-stained area in male and female AdGHRKO SC depots [Fig. 4(a)]. Likewise, hydroxyproline content was decreased in females, and although the trend was for decreased levels in males, it did not reach statistical significance [Fig. 4(c)]. No differences were seen in collagen content by either method in the perigonadal depots in the AdGHRKO mice [Fig. 4(b) and 4(d)].
Figure 4.
AT fibrosis is reduced in SC depots of AdGHRKO mice. PSR staining was quantified in (a) SC and (b) perigonadal depots. Hydroxyproline content of (c) SC and (d) perigonadal depots was measured in both males and females. Values are represented as mean ± SEM (n = 6 to 10 per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Glucose homeostasis
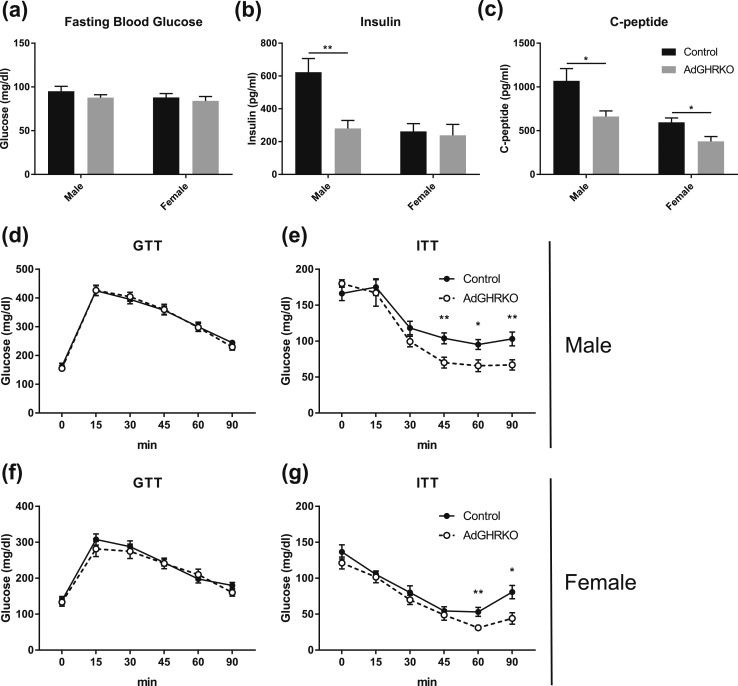
Male and female AdGHRKO mice had overall improved glucose homeostasis. Despite normal fasting blood glucose in AdGHRKO mice compared with controls [Fig. 5(a)], total insulin was dramatically and significantly reduced in male AdGHRKO mice but not in female AdGHRKO mice [Fig. 5(b)]. However, both male and female AdGHRKO mice had significantly reduced serum C-peptide [Fig. 5(c)]. GTTs showed no difference in AdGHRKO mice compared with controls [Fig. 5(d) and 5(f)]. However, AdGHRKO mice of both sexes were significantly more insulin sensitive as determined by an ITT [Fig. 5(e) and 5(g)].
Figure 5.
AdGHRKO mice show increased insulin sensitivity at 6 mo of age. (a) Fasting blood glucose, (b) serum insulin, and (c) C-peptide are shown. GTTs were performed at 6 mo of age in (d) males and (f) females. ITTs were performed at 6 mo of age in (e) males and (g) females. Mice were fasted for 12 h for GTTs and 6 h for ITTs. Values are represented as mean ± SEM (n = 6 to 11 per group). *P < 0.05; **P < 0.01.
Organ mass and liver triglyceride content
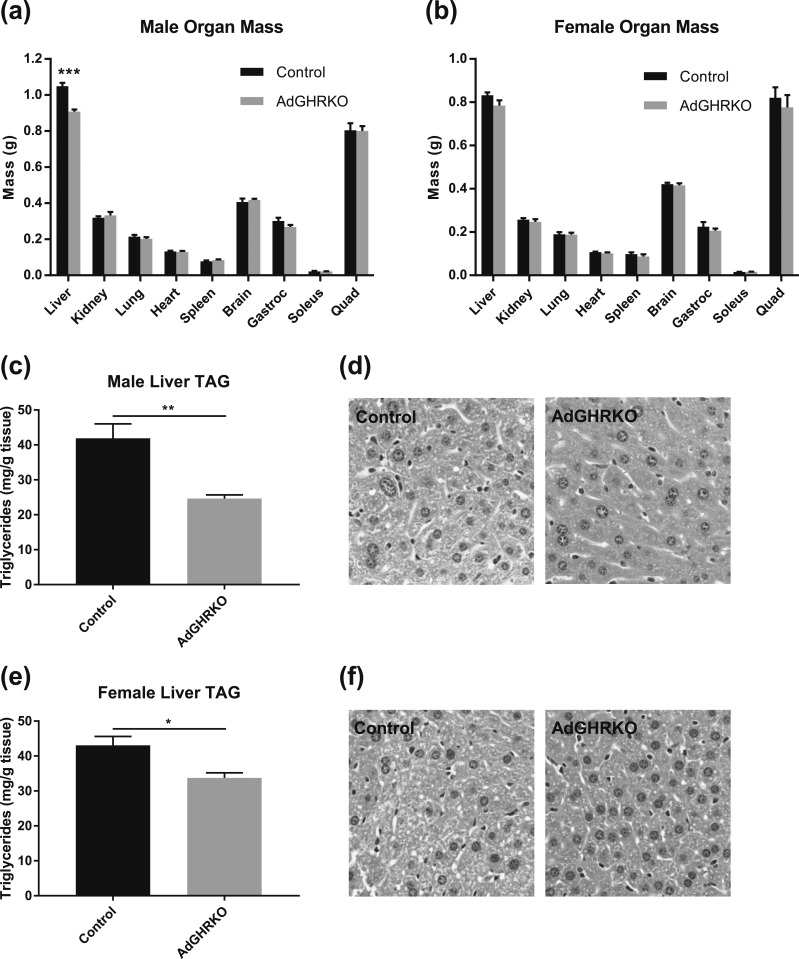
There was no change in kidney, lung, heart, spleen, brain, quadriceps, gastrocnemius, or soleus mass in either sex of AdGHRKO mice compared with controls [Fig. 6(a) and 6(b)]. In fact, the only tissue other than AT that was significantly different between AdGHRKO mice and littermate controls was the liver mass of male AdGHRKO mice, which was significantly decreased [Fig. 6(a)]. Differences in liver weight between female AdGHRKO mice and control mice did not reach significance (P = 0.097) [Fig. 6(b)]. This decrease in mass may be due, in part, to the reduction (59% of controls) in total liver triglycerides seen in the males [Fig. 6(c) and 6(d)]. However, there was also a significant reduction in liver triglycerides of female AdGHRKO mice [Fig. 6(e) and 6(f)] despite no significant difference in liver mass [Fig. 6(b)].
Figure 6.
Organ mass and liver triglyceride (TAG) content. A total of 34 mice were dissected at 6 mo of age. The weight of liver, kidney, lung, heart, spleen, brain, quadriceps femoris (quad), gastrocnemius (gastroc), and soleus are shown for (a) males and (b) females. (c and e) Quantitative and (d and f) histological measures of liver TAG are shown for males and females. Liver images are shown at original magnification of ×400. Values are represented as mean ± SEM (n = 6 to 11 per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Serum measurements
Many circulating factors that have been associated with adipocyte and/or GH function or regulation were measured and were not significantly different between AdGHRKO mice and controls regardless of sex (Table 2). Surprisingly, despite the significant increase in fat mass, serum levels of NEFA and leptin were unchanged in both sexes of AdGHRKO mice. Selected molecules were altered in male and female AdGHRKO mice, such as resistin, which was significantly decreased by 44% and 41%, respectively, and adiponectin, which was decreased by 34% and 28%, respectively. Interestingly, osteocrin/musculin was dramatically reduced in female AdGHRKO mice, whereas there was no difference between male AdGHRKO mice and controls in this circulating factor.
Table 2.
Serum Measurements in AdGHRKO and Control Male and Female Mice
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Control | AdGHRKO | P Value | Control | AdGHRKO | P Value | |
| NEFA | 0.81 ± 0.06 | 0.78 ± 0.04 | 0.747 | 0.78 ± 0.05 | 0.79 ± 0.04 | 0.846 |
| Leptin | 6267 ± 1456 | 6845 ± 1538 | 0.789 | 2309 ± 310 | 2890 ± 690 | 0.417 |
| Resistin | 11,341 ± 1174 | 6391 ± 418 | 0.002a | 15,149 ± 1752 | 8907 ± 911 | 0.014b |
| Adiponectin | 21,530 ± 1216 | 14,150 ± 1544 | 0.001a | 34,403 ± 1647 | 24,801 ± 2317 | 0.005a |
| MCP1 | 192 ± 64 | 122 ± 31 | 0.366 | 194 ± 43 | 106 ± 54 | 0.215 |
| TNFa | 56 ± 21 | 26 ± 10 | 0.241 | 45 ± 15 | 25 ± 17 | 0.385 |
| IL-6 | 125 ± 28 | 102 ± 16 | 0.493 | 111 ± 14 | 87 ± 43 | 0.562 |
| FGF21 | 71 ± 26 | 29 ± 11 | 0.154 | 61 ± 18 | 41 ± 10 | 0.390 |
| FSTL1 | 68 ± 0 | 191 ± 78 | 0.130 | 143 ± 56 | 96 ± 19 | 0.494 |
| Myostatin | 297 ± 152 | 83 ± 34 | 0.186 | 160 ± 140 | 15 ± 1 | 0.392 |
| OSTCRN | 42 ± 5 | 31 ± 4 | 0.100 | 54 ± 4 | 27 ± 3 | 6 × 10−5c |
| SPARC | 19,240 ± 1997 | 17,970 ± 1748 | 0.639 | 17,493 ± 1056 | 20,918 ± 1999 | 0.130 |
Values are represented in pg/mL (or mmol/L for NEFA) as mean ± SEM (n = 6 to 11 per group). Measurements made at 6 mo of age.
Abbreviations: FGF21, fibroblast growth factor 21; FSTL1, follistatin-related protein 1; MCP1, monocyte chemoattractant protein 1; OSTCRN, osteocrin/musclin; SPARC, osteonectin; TNFa, TNF-α.
P < 0.01.
P < 0.05.
P < 0.001.
Discussion
The first available and most commonly used transcriptional regulatory region to target gene deletion in AT is from the mouse aP2 gene, which encodes fatty acid–binding protein-4 (Fabp4). Indeed, we previously reported the phenotype of fat-specific GHR deletion (FaGHRKO) in mice using this promoter/enhancer (12). More recent studies have demonstrated that the aP2 promotor is promiscuous with Fabp4 expressed in other cell types, such as macrophages (19) and endothelial cells (20), in the adrenal medulla and neurons throughout the central nervous system (21), in nonadipose tissue during embryonic development (22), and in even other metabolic tissues such as muscle and liver (15, 23). Furthermore, adipocytes are a heterogeneous population of cells and selected adipose precursor cells do not express Fabp4 (15), which would also skew findings and complicate interpretation when attributing the phenotype with this promoter/enhancer to fat tissue or adipocytes. Importantly for the GH/IGF-1 axis, Fabp4 has been shown to be highly expressed in the hypothalamus (24). Altered hypothalamic expression is especially concerning in a model involving GH because reduced GHR in the hypothalamus would result in blunted feedback inhibition and result in elevated GH and IGF-1, which likely explains the alteration in IGF-1 levels observed in FaGHRKO mice (12). Thus, the current study provides the initial characterization of a novel mouse line, AdGHRKO mice, in which GHR deletion was more selectively targeted to adipocytes using the adipoq promoter/enhancer to drive Cre expression. We employed similar methods and mice of identical ages to allow us to compare the phenotype previously reported for FaGHRKO mice with the novel mice reported in the present study. Overall, the novel AdGHRKO mice have an AT profile remarkably similar to the previously reported FaGHRKO mice but with important differences in several metabolic parameters.
GH is well established to promote lipolysis, to inhibit lipogenesis, to alter proliferation/differentiation of preadipocytes, and, more recently, to alter other characteristics of AT such as its immune cell composition (25), degree of fibrosis (13), and adipokine secretion profile (6, 26). Additionally, data reveal that GH’s effects are not uniform across all depots with greater impacts in SC AT reported in mice (10, 27) and in visceral depots in clinical studies (28–30). Thus, one would expect dramatic differences in WAT that may be depot-dependent in not only global GHR disruption (GHRKO mice) but also with AT-targeted GHR disruption (either FaGHRKO or AdGHRKO). A summary and comparison of the phenotype of GHRKO, FaGHRKO, and AdGHRKO mice are provided in Table 3. Indeed, all three mouse lines have the expected increase in relative fat mass, although it is important to recognize that there are extreme differences in body size among these lines compared with controls (GHRKO mice weigh significantly less, FaGHRKO mice weigh significantly more, and AdGHRKO mice have no significant difference in weight relative to controls). As expected, not all depots are equally impacted in any of these mouse lines. In GHRKO mice, the SC depot is the only depot consistently enlarged with larger adipocytes, whereas perigonadal and mesenteric fat pads are proportional to the size of the animal (2, 5). In contrast, tissue-specific deletion of GHR results in enlargement of all WAT depots except male perigonadal in FaGHRKO mice (12) and in all except male perigonadal and male BAT in AdGHRKO mice. Other features of WAT explored in this study, including adipocyte size and collagen deposition, again show a very similar pattern between AdGHRKO and FaGHRKO mice with both enlarged adipocytes (12) and reduced fibrosis (13), and with the difference more pronounced in the SC depot. Thus, the pattern of fat deposition and the basic characteristics of WAT are strikingly similar between the two tissue-specific mouse lines even with the use of a different promoter/enhancer to direct Cre expression. Despite these similarities, we cannot exclude the likely possibility that other molecular or cellular differences exist between AdGHRKO and FaGHRKO AT that were not addressed in this initial characterization study.
Table 3.
Phenotypic Comparison of GHRKO, FaGHRKO, and AdGHRKO Mouse Lines
| GHRKO | FaGHRKO | AdGHRKO | |
|---|---|---|---|
| GH | ↑ | ↔ | ↔ |
| IGF-1 | ↓ | ↑♂, ↔♀ | ↔ |
| Body length | ↓ | ↑ | ↔ |
| Percentage body fat | ↑ | ↑ | ↑ |
| Liver triglycerides | ↑ | ↔ | ↓ |
| Glucose homeostasis | |||
| Glucose | ↔↓ | ↔ | ↔ |
| Insulin/C-peptide | ↓ | ↔ | ↓ |
| Glucose tolerance | Impaired | ↔ | ↔ |
| Insulin sensitivity | Enhanced | ↔ | Enhanced |
| Adipokines | |||
| Leptin | ↔↑ | ↔♂, ↑♀ | ↔ |
| Resistin | ↑ | ↔ | ↓ |
| Adiponectin | ↑ | ↓♂, ↔♀ | ↓ |
| SC WAT | |||
| Depot weight | ↑ | ↑ | ↑ |
| Adipocyte size | ↑ | ↑ | ↑ |
| Fibrosis | nr | ↓ | ↓ |
| Perigonadal WAT | |||
| Depot weight | ↔ | ↔♂, ↑♀ | ↔♂, ↑♀ |
| Adipocyte size | ↔ | ↑ | ↔♂, ↑♀ |
| Fibrosis | nr | ↔ | ↔ |
| Lifespan | ↑ | ↓♂, ↔♀ | nr |
↑ indicates increased; ↓, decreased; ↔, unchanged; ↔↓, unchanged or decreased; and ↔↑ indicates unchanged or increased.
Abbreviation: nr, not reported in the literature.
Despite similarities in mass and gross morphology of AT between AdGHRKO and FaGHRKO mice, there are important differences that likely influence the health of AdGHRKO mice. In AdGHRKO mice, there were no unexpected changes to the GH/IGF-1 axis, with GH levels, IGF-1 levels, and body length being similar to controls. FaGHRKO mice had elevated circulating IGF-1, at least in males, a trend for increased GH in both sexes, and they were significantly longer with an increase in body weight (12). Furthermore, despite obesity, AdGHRKO mice had decreased circulating insulin and enhanced insulin sensitivity, which is consistent with global GHRKO mice (3). Both male and female FaGHRKO mice exhibit no alteration in measures of glucose homeostasis. Because GH has diabetogenic (anti-insulin) activity, global removal of GH action results in greatly enhanced insulin sensitivity with reduced insulin; likewise, removal of GH action in AT should also improve insulin sensitivity and reduce insulin levels. Thus, it appears that AdGHRKO mice and not FaGHRKO mice adhere to this paradigm. Finally, both male and female AdGHRKO mice have reduced liver triglycerides, which might be anticipated with somatotropic dysfunction or with alterations in the capacity for lipid storage in AT. This observation is distinct from what is observed for liver triglycerides in FaGHRKO mice (no change) (12) and in the global GHRKO mice (no change or slight elevation depending on the particular study) (2) and is discussed in more detail below. Finally, there are distinct patterns in adipokines and cytokines between AdGHRKO and FaGHRKO mice, which is discussed in detail later. Based on our current understanding of the role of each of these individual characteristics on lifespan, one would anticipate that the AdGHRKO mice may benefit from improved healthspan and longevity unlike FaGHRKO mice that have a reduced lifespan. Most likely, the “leaky” nature of the aP2 promoter used for the FaGHRKO mouse line resulted in perturbations to the GH/IGF-1 axis in other tissues, making fat-specific interpretations with FaGHRKO mice difficult.
Additional comparisons can be drawn from other mouse lines that target adipocytes using the adipoq-Cre promoter and that explore downstream effectors of GH action. For example, mice with adipose-specific knockout of STAT5 using adipoq-Cre promoter (STAT5Adipoq) share many features with AdGHRKO mice, including increased body fat, which was more prominent in the SC region, slightly but nonsignificant reduction in liver triglycerides, and enhanced insulin sensitivity with no change to glucose tolerance (31). A characteristic feature of STAT5Adipoq mice is normal stimulated lipolysis but reduced capacity for basal lipolysis in WAT via reduction in adipose triglyceride lipase and its coactivator (CGI-1) accompanied by impairment in lipid mobilization. Upon prolonged fasting, these mice are able to compensate for the reduced basal lipolysis in AT by enhanced lipid mobilization from the liver. Another mouse line, adipose-specific knockout of JAK2 (JAK2A) in mice, have no change in circulating GH or IGF-1, but again with increased body fat and a preferential enlargement of the inguinal SC depot (32). These mice also have markedly improved whole-body insulin sensitivity and reduced lipolytic capacity in SC WAT mass (33). Although lipolysis was not assessed in the current study, these mice do provide a foundation for future exploration.
A decrease of GH action often results in the ectopic storage of triglyceride in the liver, whereas increased GH action decreases triglyceride storage. This phenomenon has been seen in patients with GH deficiency (34), which can be reversed upon treatment with recombinant hGH (35) and in patients with acromegaly in which treatment results in an increase in intrahepatic lipids (36). Likewise, mice with decreased GH action, such as GH antagonist transgenic and global GHRKO mice, have a significant or at least a trend toward increased liver triglycerides, whereas mice with elevated GH action and mice treated with exogenous GH have decreased liver triglycerides (2, 18, 37, 38). Interestingly, AdGHRKO mice had a decrease in total liver triglycerides, which may also be responsible for the decrease in total liver mass observed. We hypothesize this reduction in hepatic triglycerides accumulation is due to the combined effect of (i) the lack of insulin-antagonizing and lipolytic effects of GH on adipocytes, allowing WAT to act as a ”sink” for the excess lipids; (ii) the enhanced whole-body insulin sensitivity in these mice, most likely resulting in improved insulin sensitivity of the liver; and (iii) the antilipogenic actions of normal GH levels on hepatocytes (39). Collectively, these would prevent significant lipid accumulation in the liver. Note that insulin resistance is strongly correlated with hepatic steatosis (40). In accordance with this concept, JAK2A mice have been reported to have no change or a nonsignificant decline in liver triglycerides but enhanced hepatic insulin sensitivity (33). These authors theorized that a paracrine regulator derived from AT and caused by the GH’s anti-insulin action on WAT mediates hepatic insulin sensitivity, highlighting a crosstalk between adipose and liver. Developing an additional mouse line with GHR disrupted in both liver and adipose and comparing it to single tissue disruption may provide additional insight.
As adipokines are secretory products of AT, the levels in circulation should reflect the metabolic health and status of this tissue. The AdGHRKO mice have dramatically increased levels of fat mass with enlarged adipocytes but improved insulin sensitivity; this trend is similar to the phenotype of the global GHRKO mice (3). Despite these similarities, their circulating adipokine pattern is distinct. The global GHRKO mice have high to normal levels of leptin as well as both increased resistin and adiponectin in circulation (5, 6). In contrast, both sexes of AdGHRKO mice have significantly decreased levels of adiponectin and resistin, and surprisingly, despite the dramatic increase in fat mass, leptin levels are unchanged. Thus, AdGHRKO mice have divergent serum levels of these major adipokines when compared with the global GHRKO as well as levels in circulation that do not match the anticipated secretion pattern that would be anticipated with increased insulin sensitivity or an obese state. Circulating adipokine levels of AdGHRKO mice are also distinct from the FaGHRKO mice in which leptin is increased, resistin unchanged, and adiponectin decreased in only males (12). Interestingly, these three adipokines have been shown to directly regulate the secretion of GH from the pituitary in vitro and are generally decreased with increased GH action (41). However, we were unable to detect a change in either GH or IGF-1 in serum in AdGHRKO mice. Of note, JAK2A mice, introduced above, have a similar adipokine profile with no change in leptin and decreased adiponectin (32). The only other serum measurement that was significantly different was a decrease in musclin (also called osteocrin) for female AdGHRKO. While research on musclin/osteocrin is scarce, there is evidence that this myokine is induced with exercise and promotes insulin resistance (42, 43). Although two groups initially identified this peptide as a bone-derived (osteocrin) (44) or a muscle-derived myokine (musclin) (45), there is expression in BAT (45). Notably, only female AdGHRKO mice had significantly decreased levels of musclin and only AdGHRKO mice had altered levels of BAT mass. No previous reports have studied this hormone with respect to the GH/IGF-1 axis, but our data would suggest that further exploration is warranted.
In summary, AdGHRKO mice have a unique phenotype likely to provide important insights of how GH influences AT, fat metabolism, and healthspan. That is, they have increased adiposity due to increased cell size, reduced WAT fibrosis, and a larger proportion of excess WAT accumulating in the SC depot. Despite similar general features of WAT between FaGHRKO and AdGHRKO mice, only AdGHRKO mice have better metabolic health as determined by their improved glucose homeostasis, more similar to the global GHRKO mice, and decreased levels of liver triglycerides as well as a trend toward decreased inflammatory cytokines. Collectively, this phenotype suggests that AdGHRKO mice have better overall metabolic health than do FaGHRKO mice and a metabolic profile more comparable to the long-lived GHRKO mice. Because of the improved specificity of the adiponectin promoter/enhancer used, this novel mouse line will provide a more accurate picture of the role of GH on AT and contribution of AT to the phenotype of long-lived GHRKO mice.
Acknowledgments
Financial Support: This work was supported in part by a Baker Grant, the AMVETS and the Diabetes Institute at Ohio University, National Institutes of Health/National Institute on Aging Grant AG059779, and by the State of Ohio’s Eminent Scholar Program, including a gift from Milton and Lawrence Goll.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AdGHRKO
adipocyte-specific GH receptor knockout
- aP2
adipocyte protein-2
- AT
adipose tissue
- BAT
brown adipose tissue
- Fabp4
fatty acid–binding protein-4
- FaGHRKO
fat-specific GH receptor knockout
- GHR
GH receptor
- GHRKO
GH receptor knockout
- GTT
glucose tolerance test
- ITT
insulin tolerance test
- JAK2A
adipose-specific knockout of JAK2
- NEFA
nonesterified free fatty acid
- PSR
picrosirius red
- SC
subcutaneous
- WAT
white adipose tissue
References
- 1. Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94(24):13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O’Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. [DOI] [PubMed] [Google Scholar]
- 4. List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev. 2011;32(3):356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–318. [DOI] [PubMed] [Google Scholar]
- 6. Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216(3):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18(1):37–45. [DOI] [PubMed] [Google Scholar]
- 8. Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY). 2014;6(7):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19(3):187–197. [DOI] [PubMed] [Google Scholar]
- 10. Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y, Salmon AB, Hughes LF, Liberati T, Boparai R, Kopchick JJ, Westbrook R. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olarescu NC, Berryman DE, Householder LA, Lubbers ER, List EO, Benencia F, Kopchick JJ, Bollerslev J. GH action influences adipogenesis of mouse adipose tissue-derived mesenchymal stem cells. J Endocrinol. 2015;226(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Householder LA, Comisford R, Duran-Ortiz S, Lee K, Troike K, Wilson C, Jara A, Harberson M, List EO, Kopchick JJ, Berryman DE. Increased fibrosis: a novel means by which GH influences white adipose tissue function. Growth Horm IGF Res. 2018;39:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA, Smyth G, Rourk M, Cederquist C, Rosen ED, Kahn BB, Kahn CR. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62(3):864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krueger KC, Costa MJ, Du H, Feldman BJ. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports. 2014;3(6):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. List EO, Berryman DE, Ikeno Y, Hubbard GB, Funk K, Comisford R, Young JA, Stout MB, Tchkonia T, Masternak MM, Bartke A, Kirkland JL, Miller RA, Kopchick JJ. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR−/− mice. Aging (Albany NY). 2015;7(7):500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52(8):1647–1655. [DOI] [PubMed] [Google Scholar]
- 19. Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J Biol Chem. 2005;280(13):12888–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23(11):3865–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martens K, Bottelbergs A, Baes M. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS Lett. 2010;584(5):1054–1058. [DOI] [PubMed] [Google Scholar]
- 22. Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/fatty acid binding protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15(5):647–653. [DOI] [PubMed] [Google Scholar]
- 23. Mullican SE, Tomaru T, Gaddis CA, Peed LC, Sundaram A, Lazar MA. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol. 2013;27(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonson P, Matic M, Portwood N, Kuiper RV, Bryzgalova G, Gao H, Windahl SH, Humire P, Ohlsson C, Berggren PO, Gustafsson JA, Dahlman-Wright K. aP2-Cre-mediated inactivation of estrogen receptor alpha causes hydrometra. PLoS One. 2014;9(1):e85581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benencia F, Harshman S, Duran-Ortiz S, Lubbers ER, List EO, Householder L, Al-Naeeli M, Liang X, Welch L, Kopchick JJ, Berryman DE. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology. 2015;156(5):1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olarescu NC, Ueland T, Godang K, Lindberg-Larsen R, Jørgensen JO, Bollerslev J. Inflammatory adipokines contribute to insulin resistance in active acromegaly and respond differently to different treatment modalities. Eur J Endocrinol. 2013;170(1):39–48. [DOI] [PubMed] [Google Scholar]
- 27. Berryman DE, Henry B, Hjortebjerg R, List EO, Kopchick JJ. Developments in our understanding of the effects of growth hormone on white adipose tissue from mice: implications to the clinic. Expert Rev Endocrinol Metab. 2016;11(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freda PU, Shen W, Heymsfield SB, Reyes-Vidal CM, Geer EB, Bruce JN, Gallagher D. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab. 2008;93(6):2334–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bengtsson BA, Edén S, Lönn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tölli J, Sjöström L, Isaksson OG. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76(2):309–317. [DOI] [PubMed] [Google Scholar]
- 30. Johannsson G, Mårin P, Lönn L, Ottosson M, Stenlöf K, Björntorp P, Sjöström L, Bengtsson BA. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82(3):727–734. [DOI] [PubMed] [Google Scholar]
- 31. Kaltenecker D, Mueller KM, Benedikt P, Feiler U, Themanns M, Schlederer M, Kenner L, Schweiger M, Haemmerle G, Moriggl R. Adipocyte STAT5 deficiency promotes adiposity and impairs lipid mobilisation in mice. Diabetologia. 2017;60(2):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nordstrom SM, Tran JL, Sos BC, Wagner KU, Weiss EJ. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol Endocrinol. 2013;27(8):1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corbit KC, Camporez JP, Tran JL, Wilson CG, Lowe DA, Nordstrom SM, Ganeshan K, Perry RJ, Shulman GI, Jurczak MJ, Weiss EJ. Adipocyte JAK2 mediates growth hormone-induced hepatic insulin resistance. JCI Insight. 2017;2(3):e91001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ichikawa T, Hamasaki K, Ishikawa H, Ejima E, Eguchi K, Nakao K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52(6):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takahashi Y, Iida K, Takahashi K, Yoshioka S, Fukuoka H, Takeno R, Imanaka M, Nishizawa H, Takahashi M, Seo Y, Hayashi Y, Kondo T, Okimura Y, Kaji H, Kitazawa R, Kitazawa S, Chihara K. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132(3):938–943. [DOI] [PubMed] [Google Scholar]
- 36. Reyes-Vidal CM, Mojahed H, Shen W, Jin Z, Arias-Mendoza F, Fernandez JC, Gallagher D, Bruce JN, Post KD, Freda PU. Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J Clin Endocrinol Metab. 2015;100(8):2946–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150(3):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berryman DE, Lubbers ER, Magon V, List EO, Kopchick JJ. A dwarf mouse model with decreased GH/IGF-1 activity that does not experience life-span extension: potential impact of increased adiposity, leptin, and insulin with advancing age. J Gerontol A Biol Sci Med Sci. 2014;69(2):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takahashi Y. The role of growth hormone and insulin-like growth factor-i in the liver. Int J Mol Sci. 2017;18(7):1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuzaka T, Shimano H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J Diabetes Investig. 2011;2(3):170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarmento-Cabral A, Peinado JR, Halliday LC, Malagon MM, Castaño JP, Kineman RD, Luque RM. Adipokines (leptin, adiponectin, resistin) differentially regulate all hormonal cell types in primary anterior pituitary cell cultures from two primate species. Sci Rep. 2017;7(1):43537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Huo X, Pang XF, Zong ZH, Meng X, Liu GL. Musclin inhibits insulin activation of Akt/protein kinase B in rat skeletal muscle. J Int Med Res. 2008;36(3):496–504. [DOI] [PubMed] [Google Scholar]
- 43. Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, Stepniak E, Walsh SA, Acevedo MR, Perez-Terzic CM, Hodgson-Zingman DM, Zingman LV. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci USA. 2015;112(52):16042–16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas G, Moffatt P, Salois P, Gaumond MH, Gingras R, Godin E, Miao D, Goltzman D, Lanctôt C. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J Biol Chem. 2003;278(50):50563–50571. [DOI] [PubMed] [Google Scholar]
- 45. Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, Kitamura T, Shimomura I. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279(19):19391–19395. [DOI] [PubMed] [Google Scholar]