Abstract
Infrared (IR) thermography, where temperature measurements are made with IR cameras, has proven to be a very useful and widely used tool in biological science. Several thermography parameters are critical to the proper operation of thermal cameras and the accuracy of measurements, and these must usually be provided to the camera. Failure to account for these parameters may lead to less accurate measurements. Furthermore, the failure to provide information of parameter choices in reports may compromise appraisal of accuracy and replicate studies. In this review, we investigate how well biologists report thermography parameters. This is done through a systematic review of biological thermography literature that included articles published between years 2007 and 2017. We found that in primary biological thermography papers, which make some kind of quantitative temperature measurement, 48% fail to report values used for emissivity (an object's capacity to emit thermal radiation relative to a black body radiator), which is the minimum level of reporting that should take place. This finding highlights the need for life scientists to take into account and report key parameter information when carrying out thermography, in the future.
Keywords: thermography, thermal camera, infrared, emissivity, temperature measurement
1. Introduction
Temperature is an important biological variable. It is a key influence on living organisms [1–8], and temperature can also be used as an indicator for metabolic activity [7,9–11], disease, injury and stress [12–16]. Temperature of organisms has been measured using thermocouples [17–19] or thermistors [20,21], though use of thermographic cameras has increased dramatically in recent years with improvement of the technology [14,22]. Thermographic cameras detect the radiation from all objects hotter the absolute zero, usually in the human invisible ‘thermal infrared band', wavelength range of 2–14 µm. These radiation measurements, along with thermography parameters that are input into the camera, can be used to estimate the temperature of an object. The main thermography parameter is the target object's emissivity, which is its capacity to radiate infrared (IR) radiation relative to a black body radiator at the same temperature. Other parameters used are information about the environment in which measurements are taking place: IR reflections, distance between camera and target, environmental temperature and environmental humidity [22–24]. Thermography has a number of benefits when compared with other temperature measurement methods such as thermocouples [25,26]. Firstly, in contrast to thermocouples and thermistors with individual contact points, it is easier with thermal cameras to measure the changes of temperature with high spatial resolution, across a target or simultaneously in several targets [14,27–29]. Secondly, it responds quickly to changes allowing monitoring of subjects that are moving or might change temperature quickly [27,30]. Lastly, and possibly most importantly to biologists, it is non-contact [22,23,25]; this is important because attempting contact measurements with biological subjects may disturb or damage the subject, or in more delicate applications disrupt temperature distributions. Using a non-contact technique also means temperature measurements can be made on more distant targets [31–33].
Infrared thermography is a valuable tool for biologists and has been widely applied for temperature measurements [14,22,25,26,29,34]. However, doubt has been expressed over how well biologists understand and use these tools [22]. Understanding of how thermal cameras estimate the temperature of objects requires an understanding of the thermography parameters that must be entered into the camera. Here, we will discuss these parameters and assess how they are reported in the biological literature using a systematic literature review. Correct reporting is important, as it is both vital for ensuring repeatability of a thermographic study, and allows a reader to evaluate the correctness of a reported result. By reviewing how often thermographic parameters are reported, we can evaluate how well life scientists appear to understand thermography. Based on our findings, we will provide advice for biological thermographers and highlight common mistakes that can be easily avoided in future work.
2. Background information
2.1. Principles of thermography
All objects of a temperature above absolute zero emit electromagnetic radiation. Increased temperature leads to increased levels of radiation [35,36]. This radiation is usually within the thermal IR band, which is invisible to humans and has wavelength ranges between 0.8 and 14 µm [22–24]. However, once heated to a certain point, objects will begin to radiate more in the shorter wavelengths, including in the light spectrum visible to humans. Thermal cameras are equipped with IR-transmitting optics and arrays of sensors that are sensitive to portions of the thermal IR band [22–24]. The sensor readings are converted to radiometric units and colour-coded to generate false colour images that allow us to visualize thermal IR radiation that cannot be seen by the human eye. Most commercially available thermal cameras are sensitive to either mid-wave IR (2–5 µm) or long-wave IR (8–14 µm) [22–24]. These restrictions of wavelengths cameras are sensitive to are of the wavelengths of expected thermal radiation and those that provide high transmission (see below) through the atmosphere and camera optics [22–24].
The thermal radiation emitted by an object (Wobj) is dependent on the object's temperature (Tobj, measured in K) in accordance with the Stefan–Boltzmann formula [35,36]:
| 2.1 |
where σ is the Stefan–Boltzmann constant (ca 5.67 × 10−8 W m−2 K−4) and ɛ is the emissivity of the object. Emissivity is the capacity of an object to emit thermal radiation relative to a black body at the same temperature. A black body is a theoretical body which is non-transmissive and non-reflective, in other words completely absorbs any kind of incident electromagnetic radiation. Emissivity is represented as a fraction between 0 and 1, and black bodies have an ɛ of 1.
A thermal camera detects electromagnetic waves in the thermal IR band, and just like a regular human-visible light camera does not distinguish between emitted and reflected radiation. Like human-visible light, thermal radiation has to be transmitted through the atmosphere. Furthermore, the atmosphere itself emits thermal IR radiation [22–24]. Thus, when imaging a non-transmissive object through the air, the total radiation Wtot entering a thermal camera will be the sum of the emitted radiation of the object (Wobj), the amount of radiation reflected off the object (Wref) and the amount of radiation emitted by the atmosphere (Watm):
| 2.2 |
This means that the radiation-based image viewed through the camera does not necessarily indicate the focal object's temperature, and that some level of calibration of the raw radiation image is needed to account for these additional sources of radiation [24]. This uncalibrated thermal image is known as ‘apparent temperature'. Wobj, Wref and Watm are each influenced by the transmissivity of the atmosphere between the object and camera, τatm, and can be calculated by:
| 2.3 |
| 2.4 |
| 2.5 |
where Tx refers to the temperature of x (x being the object, the environment or reflections). Note that the emissivity of the atmosphere equals (1 − τatm), as objects can either emit, transmit or reflect radiation [23] and the atmosphere is non-reflective within the thermal IR band. Equations (2.3)–(2.5) can be substituted into equation (2.2) to give,
| 2.6 |
which can be reorganized
| 2.7 |
to give temperature estimates of the object of interest.
The calculation in equation (2.7) is normally carried out by the camera itself, or related software (e.g. FLIR tools [37]) after the image has been captured [24]. Equation (2.7) identifies several parameter inputs required by the camera, or software, to accurately measure the temperature of the object. These must be applied to images before measurements of temperature are taken from them, using the camera or related software. However, several of these parameter inputs are dependent on the time of image capture. Thus, although they can be applied to images afterwards, they must be measured at the time of thermograph capture. A checklist summary of the requirements for obtaining the most accurate thermographic temperature measurements and how the required timings influence protocol, is provided in table 1. The best quality thermographic measurements require accurate estimates of these parameter inputs in addition to correct use of camera optics in terms of image focus [23,24].
Table 1.
A checklist for accurate thermographic temperature measurements. The six aspects needed for accurate thermographic temperature measurements are listed, as well as where the timing of such aspects should be considered in experimental protocols. Note that the requirements, although all contributing to maximizing accuracy, do not influence accuracy equally. This checklist assumes thermography is not being carried out through a thermal IR transmissive window. It is very unlikely that researchers conducting biological thermography would need to use a transmissive window, but if this is the case further considerations must be made (see [24]).
| aspect | ideal requirements | timing considerations |
|---|---|---|
| quality thermographic image capture | a well-focused, unobscured image of target organism or tissues | Thermograph image focus and content cannot be altered after capture. Image contrast and appearance in terms of temperature scales can be altered and are not important for temperate measurements, although they can aid with obtaining good image focus. |
| emissivity (ɛ) estimate | a measurement of emissivity from the same object being thermographed | Emissivity can normally be applied to images after capture. It does not necessarily need to be known at the time of image capture but needs to be obtained and applied to an image before measurements are taken from images. |
| reflected temperature (Tref) measurement | a measurement of reflected temperature off the thermography target | Measurement of reflected temperature should be made simultaneously with each thermographic image capture. More practically, mirrors require Tref measurements to be made immediately after image capture. Reflected temperature can then normally be applied to the relevant thermograph images after capture. |
| environmental temperature (Tenv) measurements | a measurement of the temperature of the environment where the thermal image was captured | Should be made simultaneously with image capture. Environmental temperature can then normally be applied to the relevant thermograph images after capture. |
| environmental relative humidity (rh) measurements | a measurement of the relative humidity of the environment where the thermal image was captured | Measurements should be made simultaneously with image capture. Environmental relative humidity can then normally be applied to the relevant thermograph images after capture. This is used by the camera or software to calculate τatm. |
| distance between the camera and thermography target (d) | a measure of distance between the camera and thermography target | This should be either controlled, and therefore known, or measured after image capture. As long as positions are noted, this measurement does not need to occur right away. This is used by the camera or software to calculate τatm. |
2.2. Emissivity
Object emissivity, ɛ, alternatively called ‘emittance’, ‘emission' or ‘emission coefficient', is a proportion (bound between 0 and 1) that represents the capacity of an object to radiate thermal IR radiation relative to a black body at the same temperature [22–24]. An emissivity of 1 treats the target object as a black body. Objects with high emissivity have temperatures that align closely with apparent temperature, while the total radiation entering a thermal camera (Wtot) when observing a low emissivity object will be influenced more strongly by reflected IR radiation (equation (2.6)).
Emissivity can be measured using several methods, usually involving comparing the radiation from the object with that of a known emissivity of the same temperature [24]. This can be achieved by coating part of the object in something of known emissivity and heating the object evenly. Here, a true measurement of the object temperature can be made with the thermal camera, and the emissivity parameter can then be adjusted until matching estimates of temperature are achieved on the uncoated parts of the object [10,38,39]. Often such coating is difficult on biological subjects, and heating live subjects evenly can be difficult and unethical. Although estimates could be carried out using dead subjects, where suitable and ethically obtainable [22,40]. Alternatively, if the objects' temperature is known through another temperature measurement method, emissivity can be calculated by rearranging equation (2.7) [38,41–43].
Inaccurate estimates of emissivity have the largest influence on the accuracy of temperature measurements [22,23]. As seen in equation (2.7), changing emissivity changes the portion of Wtot taken to be from the object itself as opposed to from other sources and can lead to misjudgements in the contribution of reflections to Wtot relative to the object radiation. Emissivity has a direct effect on the temperature the object is estimated to have when emitting a given amount of radiation. Therefore, information on emissivity of the object is key for thermographic measurements.
Emissivity is normally high in biological tissues, approximately 0.9 or higher (e.g. [22,25]). This has the benefit that the impacts on inaccurate emissivity measurements are reduced when compared to low emissivity objects (see equation (2.7)). An inaccurate but still high emissivity value, assuming the target's true emissivity is, in fact, high, will cause smaller levels of inaccuracy then similar inaccuracy in low emissivity targets [22,23]. However, such impacts are not removed entirely. Emissivity is primarily influenced by the object's composition, and this can vary across different biological tissues. Emissivity can also be influenced by object properties such as geometry and surface structure [24]. As these can differ across and between different types of biological subjects [44,45], it is advised that when appropriate sources for emissivity values are not available, emissivity is measured on the tissues to be thermographed or estimated based on sources on a similar tissue.
2.3. Reflected temperature
Reflected temperature (Tref) is an estimate of the level of background radiation reflected off the thermography target object [22–24], and is frequently expressed as a temperature value. Reflected temperature can also be referred to as ‘reflected apparent temperature', ‘background radiation’, ‘reflected radiation from ambient sources'. Also, confusingly, simply ‘ambient' or ‘background temperature' can be used to describe reflected temperature [46–49]. Such terms for reflected temperature can be easily confused with environmental temperature (Tenv), and should be discouraged. It should be clearly stated what information is used to estimate reflected and environmental temperature in calculations. This is especially true as environmental temperature can be used as a reasonable estimate of reflected temperature [23].
There are several ways this value can be estimated alongside thermographic measurements. A mirrored surface [23,46], preferably a multidirectional mirror [38,43], placed on a plane with the thermography target can be used to measure Tref. Here Tref is taken as the average apparent temperature of the mirror (achieved by setting the camera's emissivity to 1 and distance to 0). Practically speaking this normally involves taking a second thermograph of the target with the mirror placed in frame alongside it in the same plane immediately after measurements are taken. Tref can then be calculated and applied to the initial image [38,46]. Alternatively, the environmental temperature is often a reasonable estimate of reflected temperature [23], as long as no sources of a large amount of light or heat are near the object. Such sources of heat and light may lead to reflected temperature differing from environmental temperature. Efforts can be taken to minimize sources of reflected temperature, such as shielding and repositioning the camera; however, an accurate measure of reflected temperature value still has to be entered into the camera, and how reflected temperature was estimated should still be reported. Reflected temperature should be measured simultaneously or immediately following thermographic measurements, as changes in conditions or positioning of objects can alter reflected temperature, as noted in table 1.
Inaccurate estimates of reflected temperature can lead to misjudgement of the amount of radiation coming from the target object and other sources. However, biological tissues have a high emissivity, so the contribution of reflected temperature to Wtot is usually small [22] within biological applications (see equation (2.7)). Usually, the best estimate of Tref is achieved by measuring it along with each thermograph using a multidirectional mirror. This can be easier with stationary targets unlikely to move, such as plants. Similarly, in laboratory conditions multidirectional mirrors can be installed in such a way that Tref measurements are taken simultaneously with target measurements (as in [43]). The use of mirrors and constant measurement of reflected temperature can be impractical in some experiments. Biological targets, particularly wild animals can be disturbed by the addition of mirrors or may be too distant or be too fast moving. In such instances, the environmental temperature should be used as an estimate for reflected temperature [22].
2.4. Other environmental parameters
Besides reflected temperature, environmental temperature, Tenv, and the transmissivity of the atmosphere, τatm, are also specified in equation (2.7), and require entry into the thermographic camera. Environmental temperature allows the camera to account for the radiation emitted by the air between the camera and the target. Transmissivity of the atmosphere, τatm, accounts for how well that radiation travels through the air between the camera and target. Transmissivity of the atmosphere is normally estimated by the camera using the distance of the target from the camera, d, and the percentage relative humidity of the environment, rh. Usually, both values are entered into the camera which then computes τatm. Environmental temperature, environmental humidity and camera distance are easily estimated using standard measurement tools. To maximize accuracy these should also be measured simultaneously with thermography measurements, as noted in table 1. However, τatm is typically very close to 1 [23,24]. Consequently, the effects of changes in these parameters are normally very small. In most instances, the accuracy in these measurements has little effect on thermography data. Therefore, these parameters are often not measured alongside each thermograph, and an appropriate value is chosen for calculations [23]. Such practices have the advantage of saving time with minimal effects on accuracy. The potential exceptions to this are in extreme scenarios such as very hot or humid environments, or where measurements are being taken over a long distance. In such cases, these inputs should be measured.
3. Impacts of parameter omission
Above, we have discussed the thermographic parameters needed to accurately estimate temperature using thermal cameras, and the relative importance of the values chosen for these parameters. Emissivity estimated from the same kinds of tissue can vary [44,50], which means that the chosen emissivity value will have a drastic impact on the accuracy of thermographic measurements. Accuracy of measurement is also affected by the extent to which reflected temperature and other environmental parameters are accounted for [24]: whether they are measured; if so how they are measured; and, if not, what value was assumed for calculations. For this reason, when thermographic temperature measurements are made, the values used for emissivity should be included in reports as a minimum standard for accurate reporting, preferably alongside the method by which reflected temperature was accounted for. Assuming that thermography has been carried out correctly, the failure to provide this parameter information represents an incomplete methodology, and potentially misrepresents the accuracy of the thermographic measurements made. This limits the reader's ability to evaluate the choice of parameters, and compromises comparable replicate studies, as experimenters repeating a methodology will need to make an increasing number of assumptions about the methodologies of previous studies. Such assumptions may include: the value of emissivity used in estimates and if or how environmental parameters were monitored and adjusted for. If environmental parameters like Tref, Tenv, rh and d were not adjusted during the experiment, repeat experimenters will also have to assume the values used for calculations if they are not provided. This need to assume parameter choices will impact on the usefulness of studies where the replication of the described methods is expected. These include standardized monitoring studies such as those screening injury [14], disease [51–53] or stress [12,13,32,54–57].
We assessed the frequency in which key thermography parameters are reported in the recent primary biological literature, through a systematic literature review, aiming to evaluate how well thermography is understood and reported by biologists. A lack of inclusion of thermography parameters could be the result of two different scenarios. Firstly, the thermographic camera was used correctly, with parameters adjusted appropriately, but the detail of their adjustment was not provided in the published methodology. Alternatively, the thermographic camera could have been used incorrectly, and consequently, parameters are not adjusted or reported. Thus, a lack of information on the thermography parameters, especially emissivity, could indicate that thermography is not well understood by experimenters at some level.
4. Methods
4.1. Search criteria
Our literature search was carried out using the Web of Science core collection (Clarivate Analytics), limited to papers published between 2007 and 2017, with the final search taking place on 17th December 2017. This comparably recent search was chosen to allow us to focus our assessments of how biologists are using thermography currently, and to minimize the effects changes in the technology might have on the reporting of methods and applications. The following search terms were used: ‘[infrared OR infra-red OR infra red] AND [thermograph* OR thermal imag* OR camera]' (‘*' denoting derivations of the word, so ‘thermal imag*’ includes derivations such as ‘thermal image' and ‘thermal imaging’).
The search was then refined further to include only publications in at least one of the following 23 Web of Science Categories: agriculture dairy animal science, agriculture multidisciplinary, agronomy, behavioural sciences, biology, biophysics, ecology, entomology, evolutionary biology, fisheries, forestry, horticulture, marine-freshwater biology, ornithology, physiology, plant sciences, psychology, psychology applied, psychology biological, psychology experimental, psychology multidisciplinary, veterinary sciences and zoology. Full texts of all search results were searched for using University of Bristol library subscriptions and through Google Scholar. If publications could still not be found, and the paper could not be excluded based on the information in the abstract provided by Web of Science or linked sites alone (see exclusion criteria), the corresponding authors (where contact details provided) were contacted for copies of publications. Any publication that was not obtained through these methods was excluded. A summary of the Web of Science search history used in our literature search can be found in electronic supplementary material, S1.
4.2. Review process
Search results were examined in a chronological order by a biological scientist and qualified thermographer (M.J.M.H, Level 1 thermographer, IR training centre, awarded June 2015). Publications were checked for any criteria for exclusion (criteria detailed below), a process which left only primary biological science research papers that reported work using IR thermography in some way. These papers' methodology, how thermographic tools were employed, and the inclusion of thermographic parameters were assessed. Non-English language journals were assessed with aid of a native speaking translator if the journal could not be excluded based on the abstract alone (12 papers in total, translators are listed in acknowledgements). After completion of the full review process, all search results were worked through and assessed a second time to ensure confidence and consistency in our assessment.
4.3. Exclusion criteria
The search criteria used in this systematic review was deliberately broad to allow for the many ways thermal cameras might be described in publications, such as ‘thermal camera’ and ‘infrared camera'. This was done to minimize the chance of accidently excluding papers that genuinely use IR thermography. This accidental exclusion of relevant papers has been identified as a major issue in systematic reviews [58]. This has the consequence that many publications included in the Web of Science search results, were not primary biological science papers that used thermal imaging. The exclusion criteria applied to our search results are summarized in table 2.
Table 2.
A summary of the exclusion criteria applied to the results of our Web of Science search results. Each criterion for exclusion is given in the order they are applied. For each criterion, the publications that are still included, and those that are excluded, when the criteria are applied, are summarized. Also summarized here are the papers excluded from our analysis of emissivity reporting after the thermography methods assessment.
| order | exclusion criterion | included in assessment | excluded on this criterion |
|---|---|---|---|
| excluded from thermography methods assessment | |||
| 1 | not thermography | Publications that carry out thermography or images collected by infrared thermography. | Publications that do not use thermography in any way also excluded are theoretical studies on applications of thermography if studies do not make thermal imaging measurements. |
| 2 | not biological | Thermography is applied to a biological research application. | Thermography is applied to a non-biological application. |
| 3 | isolated abstract | Publications that are not isolated abstracts from conferences. Conference reports are retained if they have a methods section. | Isolated abstracts from conferences which have no featured section for reporting methods. |
| 4 | retracted article | Publications that have not been retracted by the publishing body at time of last search. | Articles that had been retracted by the publishing body for any reason at time of last search. |
| 5 | review | Article is a primary research paper. | Publication is a secondary research paper reporting or providing commentary on the findings of previous work (these publications are filed separately in electronic supplementary material, S2 for ease of reference). |
| excluded from statistical analysis after thermography assessment completed | |||
| 6 | quantitative–qualitative | Paper presents temperature measurements dependent on thermography or data that required thermographic temperature measurements for its calculation. Thus, should report thermography parameter information. | Paper uses thermal imaging in an application that does not involve measuring temperature and is dependent wholly on apparent temperature. Thus, reporting of thermography parameter information is not required to assess accuracy or repeat methods. |
Only publications which carried out thermography and reported data or images collected by IR thermography were included in our review, everything else was excluded as ‘not thermography'. These excluded works included those using non-thermal IR technologies, such as triggers and sensors [59–63], IR reflectance cameras [64–66], hyperspectral cameras [67] and the use of non-thermal IR devices for night vision [68–71]. Additionally, publications using ‘infrared thermometry' [72–74] as opposed to thermography were excluded (although IR thermometry tools do use the same principles for point measurements). Theoretical studies investigating applications of IR thermography [75–78], if such studies did not report any thermal imaging measurements, were also excluded.
This review aims to assess the use of IR cameras in the life sciences area. Thus, if the application of IR thermography did not appear to be biological, publications were also excluded as ‘not biological'. Such application treated as non-biological included the industrial preparation of baked goods [79], materials science [80–82], biomechanical surgery tool maintenance [83], assessment of building materials in agricultural management [84] and canal upkeep [85]. Biomechanical studies where temperatures of artificial replacements were only monitored outside the body, for example, in mechanical stress assessment [86], and studies where biological tissue mimics were employed instead of real biological targets [87,88] were likewise excluded as ‘not biological'.
Any isolated abstracts from conferences were excluded, as such summary articles typically do not normally provide detailed information on their methodology. Published conference reports were not excluded if they featured a methods section. Any retracted articles, at time of the search, were also excluded.
Lastly, review articles that either discussed IR thermography or thermography-dependent results were excluded, albeit for reference review articles were filed separately from other exclusions (see electronic supplementary material, S2).
4.4. Thermography methods assessment
Included publications were assessed to obtain data on how IR thermography was used and has been reported. The information extracted from each publication can be found in table 3. It was beyond the scope of this review to evaluate in each case how appropriate the parameters used were and how this influenced the value of the thermographic measurements taken within the study. This review process consequently focused on whether primary research papers provided the information needed to make such evaluations of parameter choice or repeat the study without having to assume parameter choice. For emissivity, a specific value used in measurements was required. Simply an acknowledgement that emissivity was input was deemed as insufficient as the actual value is needed for appraisal of papers. For environmental thermography parameters (Tref, Tenv, rh and d), indication that these were used in calculations was required. The method of Tref measurement was also monitored, and could either be a single quoted value used for the parameter at measurements or a continuous measurement alongside the thermography measurements, as both are acceptable [23]. The information listed in table 3 could be provided at any point in the paper main text, including within thermograph figures when information was not given in the text. The article main text was the focus of the publication search, and ‘supplementary' or ‘supplemental' text was only consulted for this information if the publication explicitly directed us to do so.
Table 3.
The information extracted from each publication during the thermography methods assessment. Each datapoint, the format of this datapoint and a description of this datapoint are given.
| datapoint | format | description |
|---|---|---|
| thermography target | category | The subject for the research involving thermography. |
| quantitative temperature values | Boolean y/n |
Whether the paper used thermal imaging for a qualitative or quantitative study. ‘y' if quantitative, ‘n’ if qualitative. |
| emissivity, ɛ, value given | Boolean y/n |
An indicator of whether the ɛ value(s) used are given in the publication. |
| ɛ value(s) | value | The ɛ value(s) used in the study. n/a if the ɛ value(s) are not given. |
| ɛ value(s) measured or referenced | category | An indicator of the source for the ɛ value(s) used. If emissivity was measured by the researchers this is indicated here. If emissivity was taken from an existing source that source is indicated. n if ɛ value(s) are given but no indication of measurement or source is given, n/a if the ɛ value(s) are not given. |
| Tref considered | Boolean y/n |
An indicator that the publication accounts for reflected temperature (Tref) in temperature measurements in any way. n if this information is not given or if the study merely attempted to minimize reflection. |
| Tref method | category | How reflected temperature (Tref) was accounted for. If the reflected temperature value is assumed to be ambient this method is listed as ‘assumed to be ambient’. n if publication gives a value for reflected temperature but gives no detail. n/a if reflected temperature is not accounted for. |
| Tenv considered | Boolean y/n |
An indicator of whether the environmental temperature was measured or estimated alongside thermal imaging. |
| rh considered | Boolean y/n |
An indicator of whether environmental relative humidity was measured or estimated alongside thermal imaging. |
| d considered | Boolean y/n |
An indicator of whether camera distance was measured or estimated alongside thermal imaging |
| camera manufacturer model | category | The manufacturer and model of the thermal camera(s) used in the publication. n if this information is not given. |
Throughout the review, we aimed to give authors the benefit of the doubt where possible. If a study indicated at any point in the paper that the environmental factors (Tenv, rh and d) in the sampling area were known, it was assumed they were input into the camera. This could be simply mentioning that these parameters were measured in the thermography sampling area. If the camera was mounted in a fixed position relative to the target it was assumed that distance had been measured and input. As several thermography parameters can be referred to by various names (listed previously), any of these were acceptable. As reflected temperature, Tref, is sometimes referred to as ‘ambient temperature' [46–49], if a study referred to environmental temperature as ‘ambient temperature' it was assumed that this value was also used for reflected temperature unless stated otherwise. However, a note was made of instances where this assumption was made (table 3). For each piece of information noted in table 3, page locations within the relevant publication were noted in each publication (using the page numbers on the version accessed).
Not all applications of thermal cameras involve measurements of temperature, for example, thermal cameras can be used to spot animals at long distances or in the dark [33,89–91]. In such non-quantitative or ‘qualitative’ applications, data are dependent only on apparent temperature [24]. Consequently, thermography parameter information is not required to assess accuracy or repeat methods of qualitative studies. It is thus important in our assessment of biological thermography publications to evaluate whether thermal imaging was used in a quantitative manner or not (table 3), as this will determine whether failing to report parameters affects study accuracy or repeatability. A publication was determined to be a quantitative study if it presented temperature data dependent on thermal imaging. This thermography-dependent temperature data could be presented graphically, or as quoted temperature values, or as a thermograph with temperature scales. If the paper presented data that required temperature measurements for its calculation, such as plant water stress index [32,54,56], such papers were viewed as quantitative. Studies deemed qualitative use IR thermal imaging but do not measure temperature values.
Each paper was assigned a biological field based on the subject of research in each study. These biological fields are listed in table 4. This also allowed assessment of whether certain biological research disciplines are more likely to fail to report IR thermography parameters when they are required (in quantitative studies). The number of quantitative studies that failed and succeeded in reporting emissivity, the minimum level of parameter reporting for thermographic temperature measurements (see above), was calculated for each biological field. The association between emissivity reporting and biological field was assessed using a χ2 test using R v. 3.4.1 [92]. It was deemed acceptable for wholly qualitative studies to not include parameter information [24]; thus, any qualitative publications were not included in this analysis (as described in table 2).
Table 4.
The biological fields assigned to papers based on the subject of the thermography research. A description of the research subjects of papers in each field is also provided.
| biological field | thermography research subjects |
|---|---|
| agricultural animals | animals used in agricultural practice, such as cows, goats, sheep and pigs |
| birds and poultry | birds and poultry, includes chickens, turkeys and their eggs |
| earth and soil | ground, rock or soil when measured within biological studies |
| humans/medical | humans, including sports science, medical and psychological studies |
| insects | any insects |
| mammals | mammals, excluding humans and agricultural animals |
| plants | any plants, including crop science |
| reptiles and amphibians | any reptiles and amphibians |
| other | any subject not covered in the above biological fields |
5. Results
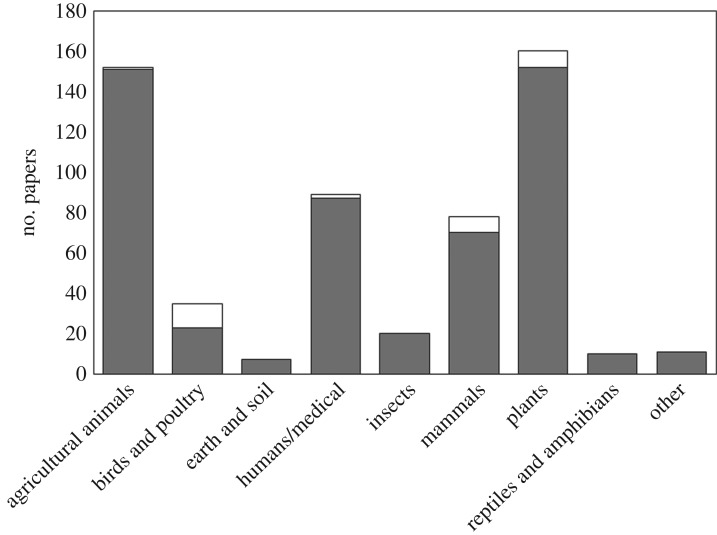
The search yielded a total of 1219 search results. Exclusions accounted for most of this number. 575 publications were excluded in total: 466 ‘not thermography'; 35 ‘not biological’; 36 isolated abstracts; 1 retracted; and 37 that were not obtained by the authors and could not be otherwise excluded. This left 562 primary biological publications which employed IR thermography and a further 82 reviews featuring IR thermography. Of these 562 primary publications, 531 (94.48%) were deemed to use quantitative temperature measurements in some way, leaving 31 (5.52%) wholly qualitative studies. The frequency of quantitative and qualitative papers in each biological field is presented in figure 1.
Figure 1.
The frequency of thermography papers within each biological field, as categorized in table 4. Quantitative and qualitative papers are indicated by shading: quantitative papers, darker grey shading; qualitative papers, clear white.
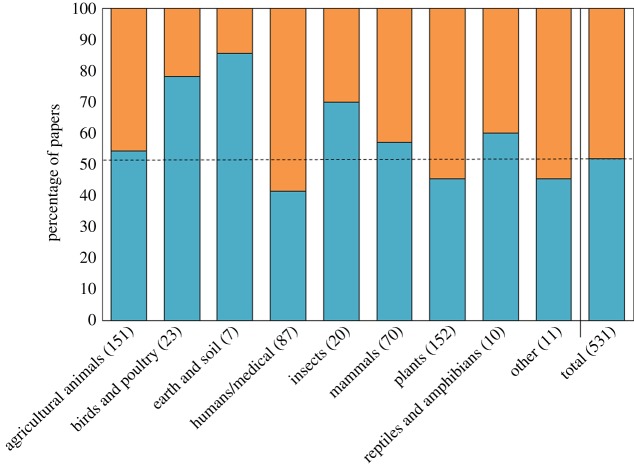
Of the 531 quantitative papers, where camera parameter inputs are necessary for accurate temperature measurements, 52.0% of all quantitative studies provided emissivity values (276 publications) and 48.0% of all quantitative studies failed to provide emissivity values (255 publications). Figure 2 shows the percentage of quantitative papers in each biological field that report emissivity compared to all quantitative papers. χ2 analysis revealed a significant association between the biological field and reporting of emissivity (, p = 0.01). This association is largely due to papers in the ‘birds and poultry', ‘insects' and ‘earth and soil' biological fields reporting emissivity more frequently than expected and the ‘plants’ and ‘humans/medical' biological fields reporting emissivity less frequently than expected. Table 5 gives the frequencies of emissivity reporting across research fields alongside expected frequencies and Pearson residual used in our χ2 analysis. Of the 276 papers that provided emissivity values, 45.2% (126 publications) provided a source for that value choice and a further 5.4% (15 publications) measured the value within the study. A summary of emissivity values used in studies measuring similar targets, targets of the same research field, is given in table 6.
Figure 2.
The percentage reporting of emissivity within all quantitative papers (total) and different biological fields, as categorized in table 4. Lower blue bars indicate the percentage of papers that report emissivity, higher orange bars indicate the percentage of papers that fail to report emissivity. The dotted line indicates 51.98%, the percentage of all quantitative papers that report emissivity, allowing comparison of how frequency of reporting differs compared to the overall frequency. Numbers of quantitative papers in each biological field and the total are indicated in brackets.
Table 5.
The realized, expected and Pearson residual values of emissivity reporting used in χ² analysis of emissivity reporting within each biological field (biological field described in table 4). Realized frequency represents the actual observed values of emissivity reporting. Expected frequency represents the frequency of reporting expected if no effect of research field was present, given the size of the groups. Pearson residual values indicate the relative influence of the research field on a χ² analysis result.
| realized frequency |
expected frequency |
Pearson residuals |
||||
|---|---|---|---|---|---|---|
| emissivity value not given | emissivity value given | emissivity value not given | emissivity value given | emissivity value not given | emissivity value given | |
| agricultural animals | 69 | 82 | 73 | 78 | −0.413 | 0.397 |
| birds and poultry | 5 | 18 | 11 | 12 | −1.819 | 1.748 |
| earth and soil | 1 | 6 | 3 | 4 | −1.288 | 1.238 |
| humans/medical | 51 | 36 | 42 | 45 | 1.426 | −1.371 |
| insects | 6 | 14 | 10 | 10 | −1.163 | 1.118 |
| mammals | 30 | 40 | 34 | 36 | −0.624 | 0.599 |
| plants | 83 | 69 | 73 | 79 | 1.171 | −1.126 |
| reptiles and amphibians | 4 | 6 | 5 | 5 | −0.366 | 0.352 |
| other | 6 | 5 | 5 | 6 | 0.312 | −0.300 |
Table 6.
A summary of emissivity values reported by publications monitoring similar biological targets, targets of the same research field and for all studies.
| agricultural animals | birds and poultry | earth and soil | humans/ medical | insects | mammals | plants | reptiles and amphibians | other | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| mean ɛ value | 0.969 | 0.956 | 0.978 | 0.976 | 0.964 | 0.977 | 0.957 | 0.970 | 0.947 | 0.967 |
| standard deviation in ɛ | 0.018 | 0.033 | 0.020 | 0.007 | 0.009 | 0.015 | 0.038 | 0.020 | 0.059 | 0.026 |
| minimum ɛ value | 0.92 | 0.86 | 0.95 | 0.95 | 0.95 | 0.95 | 0.8 | 0.95 | 0.85 | 0.8 |
| maximum ɛ value | 1 | 1 | 1 | 0.98 | 0.97 | 1 | 1 | 1 | 1 | 1 |
Reflected temperature was only reported in 26.7% (142 publications) of all quantitative papers. Within papers that gave emissivity values, reflected temperature was reported in 41.7% (115 publications) of papers. However, in 52.2% (60 publications) of these papers reflected temperature information was not explicitly given but ‘assumed to be ambient'. In papers that failed to give emissivity, reflected temperature was reported in only 10.6% of papers (27 publications).
Environmental parameters associated less directly with IR thermography tended to be reported more frequently than emissivity and reflected temperature. With environmental temperature, environmental humidity and camera distance being reported in 81.2%, 51.6% and 66.7% of all quantitative papers respectively. Environmental temperature, environmental humidity and camera distance were reported more frequently in papers that gave emissivity values (89.9%, 60.9% and 80.1%, respectively) than those that did not (71.8%, 41.6% and 52.2%, respectively), but this difference between papers that report emissivity and those that did not was less stark than that seen in reflected temperature.
A list of the 1219 papers found in our search categorized into primary papers, reviews and exclusions as well as the data extracted from each paper can be found in electronic supplementary material, S2. A summary of the frequency of parameter reporting, broken down further by biological field, can be found in table 7.
Table 7.
A breakdown summary of primary thermography papers in our Web of Science search. Given are frequencies (and percentages where indicated) of publications at each level of our thermography reporting assessment. This is given for all primary thermography publications (total) and broken down by research field (as defined in table 4).
| agricultural animals | birds and poultry | earth and soil | humans/ medical | insects | mammals | plants | reptiles and amphibians | other | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| primary thermography papers: | ||||||||||
| qualitative studies | 1 | 12 | 0 | 2 | 0 | 8 | 8 | 0 | 0 | 31 |
| quantitative studies | 151 | 23 | 7 | 87 | 20 | 70 | 152 | 10 | 11 | 531 |
| research field total | 152 | 35 | 7 | 89 | 20 | 78 | 160 | 10 | 11 | 562 |
| of all quantitative studies: | ||||||||||
| number that: | ||||||||||
| ɛ value not given | 69 | 5 | 1 | 51 | 6 | 30 | 83 | 4 | 6 | 255 |
| ɛ value given | 82 | 18 | 6 | 36 | 14 | 40 | 69 | 6 | 5 | 276 |
| ɛ value referenced or measured | 25 | 9 | 2 | 19 | 13 | 19 | 47 | 3 | 3 | 140 |
| Tref measured | 50 | 13 | 2 | 19 | 5 | 24 | 27 | 2 | 0 | 142 |
| Tenv measured | 119 | 21 | 5 | 73 | 18 | 61 | 119 | 8 | 7 | 431 |
| rh measured | 90 | 12 | 3 | 43 | 8 | 30 | 83 | 2 | 3 | 274 |
| d measured | 125 | 13 | 5 | 48 | 5 | 51 | 98 | 3 | 6 | 354 |
| percentage that: | ||||||||||
| failed to give ɛ | 46% | 22% | 14% | 59% | 30% | 43% | 55% | 40% | 55% | 48% |
| gave ɛ | 54% | 78% | 86% | 41% | 70% | 57% | 45% | 60% | 45% | 52% |
| of quantitative studies that gave ɛ: | ||||||||||
| number that: | ||||||||||
| Tref measured | 34 | 11 | 2 | 17 | 5 | 19 | 25 | 2 | 0 | 115 |
| Tref measured but ‘assumed to be ambient' | 22 | 10 | 1 | 10 | 5 | 7 | 3 | 2 | 0 | 60 |
| Tenv measured | 72 | 18 | 5 | 33 | 12 | 38 | 61 | 5 | 4 | 248 |
| rh measured | 53 | 10 | 3 | 21 | 6 | 22 | 49 | 2 | 2 | 168 |
| d measured | 72 | 11 | 5 | 27 | 3 | 33 | 63 | 2 | 5 | 221 |
| of quantitative studies that failed to give ɛ: | ||||||||||
| number that: | ||||||||||
| Tref measured | 16 | 2 | 0 | 2 | 0 | 5 | 2 | 0 | 0 | 27 |
| Tenv measured | 47 | 3 | 0 | 40 | 6 | 23 | 58 | 3 | 3 | 183 |
| rh measured | 37 | 2 | 0 | 22 | 2 | 8 | 34 | 0 | 1 | 106 |
| d measured | 53 | 2 | 0 | 21 | 2 | 18 | 35 | 1 | 1 | 133 |
6. Discussion
Infrared thermography parameters are an important part of making accurate thermography measurements [22–25]. Failure to include this information represents incomplete reporting on methodologies and can compromise the value and utility of studies that depend on thermography. Furthermore, it can indicate some misunderstanding of parameter importance and the thermal imaging methods used. The systematic review of biological primary research papers presented above reveals that, of those which carried out some kind of quantitative thermographic measurements, 48% failed to give the emissivity values used. Although this significantly varied between different biological research fields, we note that a large portion of all fields failed to give any indication of emissivity. Reporting emissivity represents the minimum parameter information that quantitative papers ought to include. Reflected temperature, the other large contributor to accuracy of biological thermographic measurements, was reported less frequently than emissivity, in 26% of all quantitative papers. This value includes those where reporting was unclear but the descriptions suggest that ambient temperature was entered as reflected temperature in calculations. It appears that the true frequency of reflected temperature reporting is likely to be lower. These findings reveal biological literature to be quite poor at reporting basic thermography parameter information used in studies, and suggests that greater effort is needed on the part of authors to report key thermography parameters.
Environmental temperature (Tenv), relative humidity (rh) and camera distance (d) have little influence on the accuracy of temperature measurements [22,23]. Nevertheless, reporting of these environmental parameters is found more frequently than explicit statements of values for emissivity and reflected temperature. This tendency for papers to report these less critical parameters seems to be the result of two factors. Firstly, we assumed in our analysis that if these parameters were known they were entered into the camera. Secondly, there is often a biological reason to include monitoring of these environmental factors independent of their influence on thermography. This is especially true of environmental temperature, a key biological variable [1–8]. This means even without any knowledge of what parameters needed to be entered into the camera, and included in the report, it is likely authors would have monitored and reported these environmental parameters. This explains why many papers that failed to give emissivity and reflected temperature still gave environmental temperature and humidity (table 7). This, unfortunately, suggests the high inclusion frequency of these parameters is not indicative of understanding of thermography.
Without parameter information it is difficult to assess the accuracy of thermographic measurements within papers, or to tell if thermography was carried out correctly or not. A number of studies (10.6%) appear to give information on reflected temperature when emissivity information is not given [93–95], or mention that emissivity was input into the camera [53,93,96,97] or even measured [98] but provide no information on the value used. These suggest an understanding of thermography and the parameters involved, most probably indicating correct operation of thermal cameras but with incomplete reporting. However, many quantitative studies make use of thermal cameras but make no mention of emissivity or reflected temperature at all [55,57,99–101]. Camera models and sensitivities and the temperature ranges displayed in images are given but not thermography parameters. Camera specifications are useful for assessment of measurement accuracy, and at least the model of camera used should be reported. However, quoted accuracies of the camera only apply when the camera inputs are correct. Likewise, the temperature range applied to the image, while influencing the image seen by operators and in reports, does not influence the temperature measurements given [24]. Taken as a whole, the frequent failure to report thermographic parameter information is likely to be the result of a combination of both scenarios. In both cases, our ability to actually appraise the accuracy and repeatability of these studies is compromised. More worryingly, if no accounting for thermography parameters has been conducted, there is a strong possibility that these papers suffer from a larger level of inaccuracy in their measurements. As these two quite different causes of parameter omission cannot be easily distinguished and have quite different effects on the paper's validity and usefulness, it is critical that researchers report parameter information. At the very least, this will then confirm that these settings were taken into account when carrying out thermographic measurements.
We found a significant association between research field and emissivity reporting, although the level of reporting was not high in most research fields (figure 2). Research fields with a very large amount of quantitative thermography publications ‘plants' and ‘humans/medical' tended to report emissivity slightly less often than other fields, while smaller groups like ‘birds and poultry', ‘earth and soil' and ‘insects' reported emissivity more often. That said, the largest research field from our review, ‘agricultural animals', reported emissivity at about the average frequency. It is likely that existing publications, especially those in the same research field, set a precedent for authors and reviewers, that thermography parameter information does not need to be included in new publications. This may explain the lower frequency of parameter reporting in certain research fields. Such an explanation could be applied more generally to explain the low frequency of parameter reporting throughout biology. It is important that journals ask for this parameter information, at least emissivity, to be included in the future to prevent such a precedent continuing. As the research fields applied to this review are deliberately quite broad, further breakdowns of the research fields would perhaps reveal specific subdivisions more prone to parameter omission than others. However, no field reported emissivity with great frequency, with failure ranging from 20% to 60% of cases across fields. So, tendency to not include parameter information is likely to continue into subdivided fields to some extent.
While our systematic review suggests that an issue exists with thermography parameter reporting in biology, it does not necessarily give a full representation of how well biologists carry out thermography. Successfully reporting parameters such as emissivity does not guarantee thermography was carried out correctly. Other operation issues can still occur when parameter settings are input correctly. Furthermore, it was beyond the scope of our review to evaluate in each instance how applicable the values used for emissivity actually were, and instead our focus was upon whether such appraisals can be done based on the information reported. Consequently, it is possible that the values chosen were still inappropriate and result in inaccurate temperature measurements. However, most often biological tissues have an emissivity of approximately 0.9 [22,25], and this is supported by the values found in the review which range from 0.8 to 1. Although, our review confirms that estimates can vary even within similar applications (table 6). In papers where emissivity values are supported by measurements or a source which measures emissivity of the tissues thermographed, we can be more confident in the emissivity values chosen. For this reason, we strongly encourage authors to provide sources for emissivity values chosen. As certain biological targets can be hard to measure emissivity from, particularly when delicate or hard to access, papers providing information on biological tissue emissivity [38,41–44,102] should be encouraged as they will help biological thermographers make more informed parameter choices and be more precise in their measurements.
Our review treats all quantitative thermography as equally important to studies; we made no evaluation of how critical the temperature measurements were to the paper's findings (outside of assessing if the paper was qualitative and quantitative). It is possible that some papers may use thermography in such a minor way that authors felt parameter detail unnecessary. However, reporting parameter information represents a small addition to the methods. Furthermore, in such instances where the accuracy of measurements is less important, papers should still give the information on parameters, but perhaps need not worry for a precise estimate of emissivity or monitor environmental parameters with every measurement. Our review process did not penalize papers for applying these less accurate approaches if they reported the necessary information, consequently a less precise approach for less critical measurements was acceptable within our review.
Frequently, emissivity and other parameter values were provided within a thermograph figure with no mention of it in the main text [103–105]. Our review process counted this as reporting, as the information was indicative that parameters were adjusted, or at least are known. However, in such instances the value could easily be overlooked if the reader were not experienced with thermography. This is particularly likely when the thermography format is unusual, perhaps due to a less common camera manufacturer. Inclusion of parameters within the article text should be encouraged over inclusion within thermographs.
7. Conclusion
This study has highlighted a common tendency for biologists to omit information on critical thermographic parameters such as emissivity and reflected temperature in published primary literature. This omission suggests a lack of understanding of thermographical methods. More care should be taken to include parameter information in publications. This will improve clarity and confidence in measurements but also allow the assessment of the limitations of thermography in different types of biological studies. Fortunately, the addition of parameter information represents a small effort which can significantly improve the evaluation of reported research and awareness of the correct use of thermal cameras in biological studies. It is recommended as a minimum that the emissivity values should be given, preferably with sources or measurements supporting the parameter choice. Additionally, the method of assessing reflected temperature should be included as well.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the following for their aid in translation of non-English language publications: Gerardo Arias, Ulrike Bauer, Olivia Davis, Kate Maia, Zoe Nemec Venza, Burak Urgancioğlu and Gilda Varliero.
Data accessibility
All data are available in the electronic supplementary material.
Authors' contributions
M.J.M.H. and S.A.R. composed the literature search criteria. M.J.M.H. carried out systematic review itself. H.M.W. and S.A.R. supervised and were involved in acquisition of funding and resources. M.J.M.H wrote the original manuscript draft. All authors conceptualized the study, discussed the results and reviewed and edited the manuscript. All authors gave approval of the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
M.J.M.H. was supported by a Natural Environment Research Council studentship within the GW4+ Doctoral Training Partnership (NE/L002434/).
References
- 1.Gates DM. 1968. Transpiration and leaf temperature. Annu. Rev. Plant. Physiol. 19, 211– 238. ( 10.1146/annurev.pp.19.060168.001235) [DOI] [Google Scholar]
- 2.Levitt J. 1980. Responses of plants to environmetal stress, volume 1: chilling, freezing and high temperature stress. New York, NY: Academic Press. [Google Scholar]
- 3.Cossins A, Bowler K. 1987. The temperature biology of animals. London, UK: Chapman and Hall. [Google Scholar]
- 4.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251. ( 10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 5.Azad AK, Sawa Y, Ishikawa T, Shibata H. 2007. Temperature-dependent stomatal movement in tulip petals controls water transpiration during flower opening and closing. Ann. Appl. Biol. 150, 81–87. ( 10.1111/j.1744-7348.2006.00111.x) [DOI] [Google Scholar]
- 6.Rands SA, Whitney HM. 2008. Floral temperature and optimal foraging: is heat a feasible floral reward for pollinators? PLoS ONE 3, e2007 ( 10.1371/journal.pone.0002007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour RS, Ito Y, Onda Y, Ito K. 2009. Effects of floral thermogenesis on pollen function in Asian skunk cabbage Symplocarpus renifolius. Biol. Lett. 5, 568–570. ( 10.1098/rsbl.2009.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Ai H-L, Yu W-B, Wang H, Li D-Z. 2010. Flower heliotropism of Anemone rivularis (Ranunculaceae) in the Himalayas: effects on floral temperature and reproductive fitness. Plant Ecol. 209, 301–312. ( 10.1007/s11258-010-9739-4) [DOI] [Google Scholar]
- 9.Seymour RS, Schultze-Motel P. 1997. Heat-producing flowers. Endeavour 21, 125–129. ( 10.1016/S0160-9327(97)80222-0) [DOI] [Google Scholar]
- 10.Tattersall GJ, Milsom WK, Abe AS, Brito SP, Andrade DV. 2004. The thermogenesis of digestion in rattlesnakes. J. Exp. Biol. 207, 579–585. ( 10.1242/jeb.00790) [DOI] [PubMed] [Google Scholar]
- 11.Seymour RS, Maass E, Bolin JF. 2009. Floral thermogenesis of three species of Hydnora (Hydnoraceae) in Africa. Ann. Bot. 104, 823–832. ( 10.1093/aob/mcp168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marazziti D, Di Muro A, Castrogiovanni P. 1992. Psychological stress and body temperature changes in humans. Physiol. Behav. 52, 393–395. ( 10.1016/0031-9384(92)90290-I) [DOI] [PubMed] [Google Scholar]
- 13.Carere C, van Oers K. 2004. Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol. Behav. 82, 905–912. ( 10.1016/S0031-9384(04)00312-9) [DOI] [PubMed] [Google Scholar]
- 14.Ring EF, Ammer K. 2012. Infrared thermal imaging in medicine. Physiol. Meas. 33, R33–R46. ( 10.1088/0967-3334/33/3/R33) [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Alonso M, Miranda-de la Lama GC, Aguayo-Ulloa L, Ezquerro L, Villarroel M, Marín RH, Maria GA. 2015. Effect of postweaning handling strategies on welfare and productive traits in lambs. J. Appl. Anim. Welf. Sci. 18, 42–56. ( 10.1080/10888705.2014.941107) [DOI] [PubMed] [Google Scholar]
- 16.Duncan AE, Torgerson-White LL, Allard SM, Schneider T. 2016. An evaluation of infrared thermography for detection of bumblefoot (pododermatitis) in penguins. J. Zoo Wildl. Med. 47, 474–485. ( 10.1638/2015-0199.1) [DOI] [PubMed] [Google Scholar]
- 17.Gale J, Manes A, Poljakoff-Mayber A. 1970. A rapidly equilibrating thermocouple contact thermometer for measurement of leaf-surface temperatures. Ecology 51, 521–525. ( 10.2307/1935390) [DOI] [Google Scholar]
- 18.Brinnel H, Cabanac M. 1989. Tympanic temperature is a core temperature in humans. J. Therm. Biol. 14, 47–53. ( 10.1016/0306-4565(89)90029-6) [DOI] [Google Scholar]
- 19.Barnes BM. 1989. Freeze avoidance in a mammal: body temperatures below 0°C in an Arctic hibernator. Science 244, 1593–1595. ( 10.1126/science.2740905) [DOI] [PubMed] [Google Scholar]
- 20.Togawa T. 1985. Body temperature measurement. Clin. Phys. Physiol. Meas. 6, 83–108. ( 10.1088/0143-0815/6/2/001) [DOI] [PubMed] [Google Scholar]
- 21.Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrick R. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab. Anim. 32, 260–269. ( 10.1258/002367798780559329) [DOI] [PubMed] [Google Scholar]
- 22.Tattersall GJ. 2016. Infrared thermography: a non-invasive window into thermal physiology. Comp. Biochem. Phys. A 202, 78–98. ( 10.1016/j.cbpa.2016.02.022) [DOI] [PubMed] [Google Scholar]
- 23.Usamentiaga R, Venegas P, Guerediaga J, Vega L, Molleda J, Bulnes FG. 2014. Infrared thermography for temperature measurement and non-destructive testing. Sensors 14, 12 305–12 348. ( 10.3390/s140712305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer M, Möllman K-P. 2017. Infrared thermal imaging: fundamentals, research and applications. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 25.Kastberger G, Stachl R. 2003. Infrared imaging technology and biological applications. Behav. Res. Methods Instum. Comput. 35, 429–439. ( 10.3758/BF03195520) [DOI] [PubMed] [Google Scholar]
- 26.McCafferty DJ. 2007. The value of infrared thermography for research on mammals: previous applications and future directions. Mamm. Rev. 2007, 207–223. ( 10.1111/j.1365-2907.2007.00111.x) [DOI] [Google Scholar]
- 27.Rejšková A, Brom J, Pokorný J, Koreĉko J. 2010. Temperature distribution in light-coloured flowers and inflorescences of early spring temperate species measured by infrared camera. Flora 205, 282–289. ( 10.1016/j.flora.2009.05.001) [DOI] [Google Scholar]
- 28.Nääs IA, Romanini CEB, Neves DP, Nascimento GR, Vercellino RA. 2010. Broiler surface temperature distribution of 42 day old chickens. Sci. Agric. 67, 497–502. [Google Scholar]
- 29.Harrap MJM, Rands SA, Hempel de Ibarra N, Whitney HM. 2017. The diversity of floral temperature patterns, and their use by pollinators. eLife 6, e31262 ( 10.7554/eLife.31262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moe RO, Stubsjøen SM, Bohlin J, Bakken M. 2012. Peripheral temperature drop in response to anticipation and consumption of a signaled palatable reward in laying hens (Gallus domesticus). Physiol. Behav. 106, 527–533. ( 10.1016/j.physbeh.2012.03.032) [DOI] [PubMed] [Google Scholar]
- 31.Lenthe J-H, Oerke E-C, Dehne H-W. 2007. Digital infrared thermography for monitoring canopy health of wheat. Precis. Agric. 8, 15–26. ( 10.1007/s11119-006-9025-6) [DOI] [Google Scholar]
- 32.Bellvert J, Zarco-Tejada PJ, Girona J, Fereres E. 2014. Mapping crop water stress index in a ‘Pinot-noir’ vineyard: comparing ground measurements with thermal remote sensing imagery from an unmanned aerial vehicle. Precis. Agric. 15, 361–376. ( 10.1007/s11119-013-9334-5) [DOI] [Google Scholar]
- 33.Gillette GL, Reese KP, Connelly JW, Colt CJ, Knetter JM. 2015. Evaluating the potential of aerial infrared as a lek count method for prairie grouse. J. Fish Wildl. Manage. 6, 486–497. ( 10.3996/022015-JFWM-008) [DOI] [Google Scholar]
- 34.Wisniewski M, Glenn DM. 2008. Using infrared thermography to study freezing in plants. HortScience 43, 1648–1651. [Google Scholar]
- 35.Stefan J. 1879. Über die Beziehung zwischen der Wärmestrahlung und der Temperatur. Sitzungsber. Akad. Wiss. Math. Naturwiss. 79, 391–428. [Google Scholar]
- 36.Boltzmann L. 1884. Ableitung des Stefan'schen Gesetzes, betreffend die Abhängigkeit der Wärmestrahlung von der Temperatur aus der electromagnetischen Lichttheorie. Ann. Phys. 298, 291–294. ( 10.1002/andp.18842580616) [DOI] [Google Scholar]
- 37.FLIR Systems Inc. 2015. FLIR tools software version 5.11.16357.2007. Wilsonville, OR: FLIR Systems Inc. [Google Scholar]
- 38.Bulanon DM, Burks TF, Alchanatis V. 2008. Study on temporal variation in citrus canopy using thermal imaging for citrus fruit detection. Biosyst. Eng. 101, 161–171. ( 10.1016/j.biosystemseng.2008.08.002) [DOI] [Google Scholar]
- 39.Gallego B, Verdú J, Carrascal LM, Lobo JM. 2017. Thermal tolerance and recovery behaviour of Thorectes lusitanicus (Coleoptera, Geotrupidae). Ecol. Entomol. 42, 758–767. ( 10.1111/een.12447) [DOI] [Google Scholar]
- 40.Potts R, Clarke RM, Oldfield SE, Wood LK, Hempel de Ibarra N, Cresswell JE. 2018. The effect of dietary neonicotinoid pesticides on non-flight thermogenesis in worker bumble bees (Bombus terrestris). J. Insect Physiol. 104, 33–39. ( 10.1016/j.jinsphys.2017.11.006) [DOI] [PubMed] [Google Scholar]
- 41.Idso SB, Jackson RD, Ehrler WL, Mitchell ST. 1969. A method for determination of infrared emittance of leaves. Ecology 50, 899–902. ( 10.2307/1933705) [DOI] [Google Scholar]
- 42.Stabentheiner A, Schmaranzer S. 1987. Thermographic determination of body temperatures in honey bees and hornets: calibration and applications. Thermology 2, 563–572. [Google Scholar]
- 43.Lópes A, Molina-Aiz FD, Valera DL, Peña A. 2012. Determining the emissivity of the leaves of nine horticultural crops by means of infrared thermography. Sci. Hort. 137, 49–58. ( 10.1016/j.scienta.2012.01.022) [DOI] [Google Scholar]
- 44.Rubio E, Caselles V, Badenas C. 1997. Emissivity measurements of several soils and vegetation types in the 8-14μm wave band: analysis of two field methods. Remote Sens. Environ. 59, 490–521. ( 10.1016/S0034-4257(96)00123-X) [DOI] [Google Scholar]
- 45.Kevan PG, Lane MA. 1985. Petal microtexture is a tactile cue for bees. Proc. Natl Acad. Sci. USA 82, 4750–4752. ( 10.1073/pnas.82.14.4750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaňa R, Vass I. 2008. Thermoimaging as a tool for studying light-induced heating of leaves: correlation of heat dissipation with the efficiency of photosystem II photochemistry and non photochemical quenching. Environ. Exper. Bot. 64, 90–96. ( 10.1016/j.envexpbot.2008.02.006) [DOI] [Google Scholar]
- 47.Bowers S, Gandy S, Anderson B, Ryan P, Willard S. 2009. Assessment of pregnancy in the late-gestation mare using digital infrared thermography. Therigenology 72, 372–377. ( 10.1016/j.theriogenology.2009.03.005) [DOI] [PubMed] [Google Scholar]
- 48.Costa JM, Ortuño MF, Lopes CM, Chaves MM. 2011. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 39, 179–189. ( 10.1071/FP11156) [DOI] [PubMed] [Google Scholar]
- 49.Manier N, Bach AJE, Stewart IB, Costello JT. 2015. The effect of using different regions of interest on local and mean skin temperature. J. Therm. Biol. 49–50, 33–38. ( 10.1016/j.jtherbio.2015.01.008) [DOI] [PubMed] [Google Scholar]
- 50.Belloni M, Almeida Paz ICL, Nääs IA, Alves MCF, Garcia RG, Caldara FR, Seno LO. 2015. Productive, qualitative, and physiological aspects of layer hens fed with propolis. Brazil. J. P. Sci. 17, 467–472. ( 10.1590/1516-635X1704467-472) [DOI] [Google Scholar]
- 51.Hovinen M, Siivonen J, Taponen S, Hänninen L, Pastell M, Aisla A-M, Pyörälä S. 2008. Detection of clinical mastitis with the help of a thermal camera. J. Dairy Res. 91, 4592–4598. ( 10.3168/jds.2008-1218) [DOI] [PubMed] [Google Scholar]
- 52.Rainwater-Lovett K, Pacheco JM, Packer C, Rodriguez LL. 2009. Detection of foot-and-mouth disease virus infected cattle using infrared thermography. Vet. J. 180, 317–324. ( 10.1016/j.tvjl.2008.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson SR, Rao S, Hussey SB, Morley PS, Traub-Dargatz JL. 2011. Thermographic eye temperature as an index to body temperature in ponies. J. Equine Vet. Sci. 31, 63–66. ( 10.1016/j.jevs.2010.12.004) [DOI] [Google Scholar]
- 54.Alchanatuis V, Cohen Y, Cohen S, Moller M, Sprinstin M, Meron M, Tsipris J, Saranga Y, Sela E. 2010. Evaluation of different approaches for estimating and mapping crop water status in cotton with thermal imaging. Precis. Agric. 11, 27–41. ( 10.1007/s11119-009-9111-7) [DOI] [Google Scholar]
- 55.Kuraoka K, Nakamura K. 2011. The use of nasal skin temperature measurements in studying emotion in macaque monkeys. Physiol. Behav. 102, 347–355. ( 10.1016/j.physbeh.2010.11.029) [DOI] [PubMed] [Google Scholar]
- 56.Ballester C, Jiménez-Bello MA, Castel JR, Intrigliolo DS. 2013. Usefulness of thermography for plant water stress detection in citrus and persimmon trees. Agric. Forest Meteoro. 168, 120–129. ( 10.1016/j.agrformet.2012.08.005) [DOI] [Google Scholar]
- 57.Yarnell K, Hall C, Royle C, Waljker SL. 2015. Domesticated horses differ in their behavioural and physiological responses to isolated and group housing. Physiol. Behav. 143, 51–57. ( 10.1016/j.physbeh.2015.02.040) [DOI] [PubMed] [Google Scholar]
- 58.Gerstner K, Moreno-Mateos D, Gurevitch J, Beckmann M, Kambach S, Jones HP, Seppelt R. 2017. Will your paper be used in a meta-analysis? Make the reach of your research broader and longer lasting. Methods Ecol. Evol. 8, 777–784. ( 10.1111/2041-210X.12758) [DOI] [Google Scholar]
- 59.Larrucea SE, Brussard PF, Jaeger MM, Barrett RH. 2007. Cameras, coyotes, and the assumption of equal detectability. J. Wildl. Manage. 71, 1682–1689. ( 10.2193/2006-407) [DOI] [Google Scholar]
- 60.Braden AW, Lopez RR, Roberts CW, Silvy NJ, Owen CB, Frank PA. 2008. Florida Key deer Odocoileus virginianus clavium underpass use and movements along a highway corridor. Wild. Biol. 14, 155–163. ( 10.2981/0909-6396(2008)14%5B155:FKDOVC%5D2.0.CO;2) [DOI] [Google Scholar]
- 61.Li S, McShea WJ, Wang D, Shao L, Shi X. 2010. The use of infrared-triggered cameras for surveying phasianids in Sichuan Province, China. Ibis 152, 299–309. ( 10.1111/j.1474-919X.2009.00989.x) [DOI] [Google Scholar]
- 62.Garrote G. eet al. 2011. Estimation of the Iberian lynx (Lynx pardinus) population in the Doñana area, SW Spain, using capture–recapture analysis of camera-trapping data. Eur. J. Wildl. Res. 57, 355–362. ( 10.1007/s10344-010-0440-7) [DOI] [Google Scholar]
- 63.Flatters I, Culmer P, Holt RJ, Wilkie RM, Mon-Williams M. 2014. A new tool for assessing head movements and postural sway in children. Behav. Res. 46, 950–959. ( 10.3758/s13428-013-0419-x) [DOI] [PubMed] [Google Scholar]
- 64.Kaizu Y, Imou K. 2008. A dual-spectral camera system for paddy rice seedling row detection. Comput. Electron. Agric. 63, 49–56. ( 10.1016/j.compag.2008.01.012) [DOI] [Google Scholar]
- 65.White JL, Scurr JC, Smith NA. 2009. The effect of breast support on kinetics during overground running performance. Ergonomics 52, 492–498. ( 10.1080/00140130802707907) [DOI] [PubMed] [Google Scholar]
- 66.Sakamoto T, Gitelson AA, Wardlow BD, Arkebauer TJ, Verma SB, Suyker AE, Shibayama M. 2012. Application of day and night digital photographs for estimating maize biophysical characteristics. Precis. Agric. 13, 285–301. ( 10.1007/s11119-011-9246-1) [DOI] [Google Scholar]
- 67.Nagy A, Tamás J. 2013. Noninvasive water stress assessment methods in orchards. Commun. Soil Sci. Plant 44, 366–376. ( 10.1080/00103624.2013.742308) [DOI] [Google Scholar]
- 68.Pierce AJ, Pobprasert K. 2007. A portable system for continuous monitoring of bird nests using digital video recorders. J. Field Ornithol. 78, 322–328. ( 10.1111/j.1557-9263.2007.00119.x) [DOI] [Google Scholar]
- 69.White PA, Frank LG, Barber PH. 2007. A remotely operated motorized burrow probe to investigate carnivore neonates. J. Wildl. Manage. 71, 1708–1711. ( 10.2193/2006-425) [DOI] [Google Scholar]
- 70.Huckschlag D. 2008. Development of a digital infrared video camera system for recording and remote capturing. Eur. J. Wildl. Res. 54, 651–655. ( 10.1007/s10344-008-0191-x) [DOI] [Google Scholar]
- 71.Wellman AE, Downs CT. 2009. A behavioural study of sleep patterns in the malachite sunbird, Cape white-eye and fan-tailed widowbird. Anim. Behav. 77, 61–66. ( 10.1016/j.anbehav.2008.09.010) [DOI] [Google Scholar]
- 72.Graham EA, Lam Y, Yuen EM. 2010. Forest understory soil temperatures and heat flux calculated using a Fourier model and scaled using a digital camera. Agric. Forest Meteorol. 150, 640–649. ( 10.1016/j.agrformet.2010.02.005) [DOI] [Google Scholar]
- 73.Schmidt M, Ammon C, Christian Schön P, Manteuffel C, Hoffman G. 2014. The suitability of infrared temperature measurements for continuous temperature monitoring in gilts. Arch. Anim. Breed. 57, 1–12. ( 10.7482/0003-9438-57-021) [DOI] [Google Scholar]
- 74.Bowman AS, Nolting JM, Workman JD, Cooper M, Fisher AE, Marsh B, Forshey T. 2016. The inability to screen exhibition swine for influenza a virus using body temperature. Zoonoses Public Health 63, 34–39. ( 10.1111/zph.12201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amri A, Saidane A, Pulko S. 2011. Thermal analysis of a three-dimensional breast model with embedded tumour using the transmission line matrix (TLM) method. Comput. Biol. Med. 41, 76–86. ( 10.1016/j.compbiomed.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 76.Luna JM, Romero-Mendez R, Hernandez-Guerrero A, Elizalde-Blancas F. 2012. Procedure to estimate thermophysical and geometrical parameters of embedded cancerous lesions using thermography. J. Biomech. Eng. 134, 031008 ( 10.1115/1.4006197) [DOI] [PubMed] [Google Scholar]
- 77.Absalan H, SalmanOgli A, Rostami R, Maghoul A. 2012. Simulation and investigation of quantum dot effects as internal heat-generator source in breast tumor site. J. Therm. Biol. 37, 490–495. ( 10.1016/j.jtherbio.2012.05.001) [DOI] [Google Scholar]
- 78.Padra C, Salva NN. 2013. Locating multiple tumors by moving shape analysis. Math. Biosci. 245, 103–110. ( 10.1016/j.mbs.2013.07.002) [DOI] [PubMed] [Google Scholar]
- 79.Deng Y, Song X, Li Y. 2011. Impact of pressure reduction rate on the quality of steamed stuffed bun. J. Agr. Sci. Tech. 13, 377–386. [Google Scholar]
- 80.Aoyagi M, Hiraguri T, Ueno T. 2014. Nondestructive detection of cracks near the surface of wooden boards by dynamic heat dissipation. Wood Sci. Technol. 48, 773–786. ( 10.1007/s00226-014-0639-y) [DOI] [Google Scholar]
- 81.Mauranen A, Ovaska M, Koivisto J, Salminen LI, Alava M. 2015. Thermal conductivity of wood: effect of fatigue treatment. Wood Sci. Technol. 49, 359–370. ( 10.1007/s00226-015-0705-0) [DOI] [Google Scholar]
- 82.Antikainen T, Rohumaa A, Bulota M, Kotilahti T, Hughes M. 2015. Estimating birch veneer (Betula pendula Roth) moisture content using infrared technology. Eur. J. Wood Prod. 73, 617–625. ( 10.1007/s00107-015-0944-7) [DOI] [Google Scholar]
- 83.Gasiorwski JC, Richardson DW, Boston RC, Schaer TP. 2011. Influence of a resilient, hard-carbon thin film on drilling efficiency and thermogenesis. Vet. Surg. 40, 875–880. [DOI] [PubMed] [Google Scholar]
- 84.Fiorelli J, Schmidt R, Kawabata CY, Lins de Oliveira CE, Savastano H Jr, Rossignolo JA. 2012. Thermal efficiency of fiber cement corrugated sheets applied to individual housing for calves exposed to sun and shade. Ciência Rural, Santa Maria 42, 64–67. ( 10.1590/S0103-84782012000100011) [DOI] [Google Scholar]
- 85.Huang Y, Fipps G, Maas SJ, Fletcher RS. 2009. Airborne remote sensing for detection of irrigation canal leakage. Irrig. Drain. 59, 524–534. ( 10.1002/ird.511) [DOI] [Google Scholar]
- 86.Bougherara H, Rahim E, Shah S, Dubov A, Schemitsch EH, Zdero R. 2011. A preliminary biomechanical assessment of a polymer composite hip implant using an infrared thermography technique validated by strain gage measurements. J. Biomech. Eng. 133, 074503 ( 10.1115/1.4004414) [DOI] [PubMed] [Google Scholar]
- 87.Paul A, Narasimhan A, Kahlen FJ, Das SD. 2014. Temperature evolution in tissues embedded with large blood vessels during photo-thermal heating. J. Therm. Biol. 41, 77–87. ( 10.1016/j.jtherbio.2014.02.010) [DOI] [PubMed] [Google Scholar]
- 88.Heussner N, Vagos M, Spitzer MS, Stork W. 2015. A prediction model for ocular damage – experimental validation. J. Therm. Biol. 52, 38–44. ( 10.1016/j.jtherbio.2015.05.005) [DOI] [PubMed] [Google Scholar]
- 89.Betke M, et al. 2008. Thermal imaging reveals significantly smaller Brazilian free-tailed bat colonies than previously estimated. J. Mamm. 89, 18–24. ( 10.1644/07-MAMM-A-011.1) [DOI] [Google Scholar]
- 90.Udevitz MS, Burn DM, Webber MA. 2008. Estimation of walrus populations on sea ice with infrared imagery and aerial photography. Mar. Mamm. Sci. 24, 57–70. ( 10.1111/j.1748-7692.2007.00169.x) [DOI] [Google Scholar]
- 91.Horn JW, Arnett EB, Kunz TH. 2008. Behavioral responses of bats to operating wind turbines. J. Wildl. Manage. 72, 123–132. ( 10.2193/2006-465) [DOI] [Google Scholar]
- 92.R Development Core Team. 2017. R version 3.4.1. Vienna, Austria: The R Foundation for Statistical Computing Platform. [Google Scholar]
- 93.Šumbera R, Zelová J, Kunc P, Knížková I, Burda H. 2007. Patterns of surface temperatures in two mole-rats (Bathyergidae) with different social systems as revealed by IR-thermography. Physiol. Behav. 92, 526–532. ( 10.1016/j.physbeh.2007.04.029) [DOI] [PubMed] [Google Scholar]
- 94.Deeming DC, Pike TW. 2015. Nest surface temperature predicts fledging success of blue tits Cyanistes caeruleus but not great tits Parus major. Acta Ornithol. 50, 247–251. ( 10.3161/00016454AO2015.50.2.012) [DOI] [Google Scholar]
- 95.Kleinhenz MD, Van Engen NK, Gordem PJ, Ji J, Walsh P, Coetzee JF. 2017. Effects of transdermal flunixin meglumine on pain biomarkers at dehorning in calves. J. Animal Sci. 95, 1993–2000. [DOI] [PubMed] [Google Scholar]
- 96.Paterson W, Pomeroy PP, Sparling CE, Moss S, Thompson D, Currie JI, McCafferty DJ. 2010. Assessment of flipper tag site healing in gray seal pups using thermography. Mar. Mamm. Sci. 27, 295–305. ( 10.1111/j.1748-7692.2010.00400.x) [DOI] [Google Scholar]
- 97.Samara EM, Ayadi M, Aljumaah RS. 2014. Feasibility of utilising an infrared-thermographic technique for early detection of subclinical mastitis in dairy camels (Camelus dromedarius). J. Dairy Res. 81, 38–45. ( 10.1017/S0022029913000605) [DOI] [PubMed] [Google Scholar]
- 98.Goff RP, Quallich SG, Buechler RO, Bischof JC, Iaizzo PA. 2017. Determination of cryothermal injury thresholds in tissues impacted by cardiac cryoablation. Cryobiology 75, 125–133. ( 10.1016/j.cryobiol.2017.01.002) [DOI] [PubMed] [Google Scholar]
- 99.Wright CI, et al. 2008. Arterial stiffness, endothelial function and microcirculatory reactivity in healthy young males. Clin. Physiol. Funct. Imaging 28, 299–306. ( 10.1111/j.1475-097X.2008.00807.x) [DOI] [PubMed] [Google Scholar]
- 100.Holzer K, Rijkenhuizen BM, Simhofer H. 2010. Thermographic imaging in the diagnosis of equine sinonasal disease. Pferdeheilkunde 26, 168–172. ( 10.21836/PEM20100208) [DOI] [Google Scholar]
- 101.Liang Y-K, Xie X, Lindsay SE, Wang YB, Masle J, Williamson L, Leyser O, Hetherington AM. 2010. Cell wall composition contributes to the control of transpiration efficiency in Arabidopsis thaliana. Plant J. 64, 679–686. ( 10.1111/j.1365-313X.2010.04362.x) [DOI] [PubMed] [Google Scholar]
- 102.Zhang K, Jiao L, Zhao X, Dong D. 2016. An instantaneous approach for determining the infrared emissivity of swine surface and the influencing factors. J. Therm. Biol. 57, 78–83. ( 10.1016/j.jtherbio.2016.03.003) [DOI] [PubMed] [Google Scholar]
- 103.Steward M, Stafford KJ, Dowling SK, Schaefer AL, Webster JR. 2008. Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol. Behav. 93, 789–797. ( 10.1016/j.physbeh.2007.11.044) [DOI] [PubMed] [Google Scholar]
- 104.Stokes JE, Leach KA, Main DCJ, Whay HR. 2012. An investigation into the use of infrared thermography (IRT) as a rapid diagnostic tool for foot lesions in dairy cattle. Vet. J. 193, 674–678. ( 10.1016/j.tvjl.2012.06.052) [DOI] [PubMed] [Google Scholar]
- 105.Zadeh HG, Haddadnia J, Seryasat OR, Mohammad S, Isfahani M. 2016. Segmenting breast cancerous regions in thermal images using fuzzy active contours. EXCLI J. 15, 532–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the electronic supplementary material.