Abstract
Background
Clostridium difficile is a leading cause of morbidity and mortality in several countries. However, there are limited evidence characterizing its role as a global public health problem. We conducted a systematic review to provide a comprehensive overview of C. difficile infections (CDI) rates.
Methods
Seven databases were searched (January 2016) to identify studies and surveillance reports published between 2005 and 2015 reporting CDI incidence rates. CDI incidence rates for health care facility-associated (HCF), hospital onset-health care facility-associated, medical or general intensive care unit (ICU), internal medicine (IM), long-term care facility (LTCF), and community-associated (CA) were extracted and standardized. Meta-analysis was conducted using a random effects model.
Results
229 publications, with data from 41 countries, were included. The overall rate of HCF-CDI was 2.24 (95% confidence interval CI = 1.66-3.03) per 1000 admissions/y and 3.54 (95%CI = 3.19-3.92) per 10 000 patient-days/y. Estimated rates for CDI with onset in ICU or IM wards were 11.08 (95%CI = 7.19-17.08) and 10.80 (95%CI = 3.15-37.06) per 1000 admission/y, respectively. Rates for CA-CDI were lower: 0.55 (95%CI = 0.13-2.37) per 1000 admissions/y. CDI rates were generally higher in North America and among the elderly but similar rates were identified in other regions and age groups.
Conclusions
Our review highlights the widespread burden of disease of C. difficile, evidence gaps, and the need for sustainable surveillance of CDI in the health care setting and the community.
Clostridium difficile is a leading cause of health care-associated infections (HAIs) and an important public health threat. C. difficile has been associated with substantial morbidity and mortality worldwide and among individuals of all ages beyond the traditionally recognized at-risk groups (eg, elderly, hospitalized patients, or those under antimicrobial therapy) [1]. In the United States, C. difficile caused an estimated half a million infections and 29 000 deaths in 2012 [2]. Almost two thirds of these were associated with inpatient care, and more than 80% of these deaths occurred in those 65 years and over [2]. Challenges remain to control incidence of C. difficile infections (CDI) rates in other world regions such as Europe [3-5] and Asia [6]. It is estimated that approximately 40 000 cases among inpatients are potentially underdiagnosed each year in Europe [5]. Furthermore, recurrence of CDI is estimated to occur among a considerable percentage of cases (approximately 20%-30%) [7]. Though the global health care costs associated with CDI are not known, these are likely to be substantial with estimates suggesting attributable costs of US$ 5.4-6.3 billion per year in the United States [8].
Considering the evolving epidemiology of C. difficile morbidity and mortality, global challenges regarding antibiotic stewardship and limited alternative preventative options for CDI, it is important to assess its burden to inform public health action. The emergence of hyper virulent strain PCR ribotype 027/NAP1, significant increases in incidence of hospitalizations associated with C. difficile by the mid-2000s, and outbreaks of CDI in hospitals globally are examples of the major impact C. difficile can have on health care systems [1,9-12]. Through the implementation of standardized surveillance case definitions, a considerable proportion of cases of CDI occurring outside hospitals has also been identified [1,13-15]. Increased awareness of the role of C. difficile as a global health problem is required to reduce morbidity and control rates of CDI [16,17]. However, there are limited evidence characterizing its role as a global public health problem. We aimed to conduct a comprehensive examination of globally reported rates of CDI incidence, in order to develop baseline epidemiological estimates of incidence and identify characteristics and gaps in the available evidence base.
METHODS
Identification of eligible publications
We searched seven electronic databases (Medline, Embase, Global Health (through Ovid), CINAHL, LILACS, WHO Library, and Web of Science) in January 2016 to identify publications reporting incidence rates of CDI. Search strategies were developed with the assistance of a medical librarian and included a combination of MeSH and keywords relevant for CDI and incidence or burden of disease. An internet search to identify the most recent data from national surveillance reports was also conducted to supplement evidence from published literature (Table S1 in Online Supplementary Document). Details on eligibility criteria for the review are described in Table 1. Two reviewers (EB, TS) screened the records obtained through electronic medical databases and identified articles that met the inclusion criteria. We included studies and surveillance reports with a publication year between 2005 and 2015 inclusive and reporting CDI cases identified through positive laboratory assay or endoscopic findings using surveillance case definitions [18] or administratively coded CDI hospitalizations (eg, International Classification of Diseases (ICD) ICD-9-ICM = 00845.0 or ICD-10 = A04.7). Studies focusing on recurrence, asymptomatic colonization or mortality, or those published in languages other than English or Spanish were excluded. We also excluded data from periods of outbreaks or from intervention arms of infection control studies.
Table 1.
Eligibility criteria
| Inclusion criteria |
|---|
| Observational (during non-outbreak periods) or interventional studies (pre-intervention period) and national surveillance reports published between 2005 and 2015. |
| English and Spanish language (for peer-reviewed publications). |
| Publication reports rates of CDI incidence for individuals of all ages in any of the following settings: hospitals, medical intensive care units, internal medicine wards, long term care facilities or nursing homes and in the community. |
| Case ascertainment methods and case definition compatible with current CDI surveillance (laboratory or histological-diagnosis) or administrative coded hospitalizations (ICD9-9-CM:008.45; ICD-10:A04.7) are clearly reported. |
| Data on incidence of CDI rate can be extracted as an independent outcome and at least two of the following are available: number of cases during study period, study population (denominator) or rate. |
| Exclusion criteria: |
| Articles published before 2005 or reporting data for a study population already considered in the review. |
| Methods for case ascertainment are not clearly reported. |
| Interventional before-and-after studies without clear background rate or studies reporting rates outbreak periods exclusively. |
| C. difficile assessed as a co-infection, composite outcome (eg, set of health care-associated infections), or defined as an adverse drug reaction or antibiotic associated diarrhoea without C. difficile laboratory/code confirmation. |
| Study population at risk from which cases were identified narrowed by a prior selection process (eg, only diarrheal patients, those on antibiotics, co-morbidity-specific groups). |
| Case-reports, systematic reviews, narrative reviews, letters to editors, brief communications, commentary pieces. |
Data extraction
Data from the included studies were extracted by at least two reviewers (EB, CL, JB, IL) using an Excel form which was piloted before its final use. Uncertainties or discrepancies on data extraction were resolved by discussion between extractors. We extracted data on the study setting, duration, and design, case ascertainment methods, and case definition details. Data were extracted in accordance to the following settings of CDI acquisition: health care facility-associated (HCF), hospital onset health care facility-associated (HO-HCF), community-associated (CA), any setting /unspecified CDI and for three selected high-risk settings: medical or general intensive care units (ICU), internal medicine wards (IM), and long-term care facilities (LTCF), including nursing homes. For ICD-coded hospitalizations, we included publications reporting the number of hospitalizations with CDI code as a primary and secondary diagnosis. or data extraction, incidence rates were collected based on number of CDI cases that adhered to recommended surveillance case definitions, but also included studies with modified case definitions if this was clearly reported. Authors of publications eligible for inclusion were contacted to obtain the number of cases if additional eligible categories were reported in order to enable inclusion in the meta-analysis. To minimize potential bias towards large studies in meta-analyses, data from multicentre publications were extracted per health care facility and counted as an individual data point. Studies with data from facilities also included in national surveillance or other multicentre publications were excluded to avoid potential duplication of the same study population. Depending on the case definition, incidence for the following metrics were extracted and standardized: number of CDI cases per admissions (per 1000 admissions per year), incidence density (per 10 000 patient-days), and cumulative incidence of CDI cases over the total population at risk (per 100 000 population per year).
Statistical analysis
The total number of CDI cases by admissions, total patient-days, or population for each category, rates and 95% confidence intervals (CI) were extracted from publications as reported by authors or calculated based on available data. The distribution of CDI incidence rates identified was summarized in terms of interquartile ranges and median values as follows: for HCF and HO-HCF (including high-risk settings: ICU, IM, and LTCF) in terms of incidence density and cases per admissions, and for CA, any or unspecified setting of acquisition, or ICD-coded hospitalization in terms of cases per admissions and cumulative incidence.
Rates of CDI incidence were pooled using the metan command in Stata version 14 (StataCorp, College Station, Texas, USA). When a publication reported different rates for the same study population using different case ascertainment methods (eg, based on review of patients or laboratory charts vs administrative records) we used the number of CDI cases where patient’s records had been reviewed. A small value (0.05) was used as continuity correction to include rates with zero CDI cases in the meta-analysis. Estimates were developed using a random effects model, acknowledging the heterogeneity in observational studies conducted in diverse settings.
Where possible, we performed and reported subgroup meta-analyses by location (WHO regions; the Americas region was divided into North America and Latin America) and age group (children [≤15 years or pediatric hospitals], adults [≥15 years], elderly [≥65 years], and all or unspecified age [≥0-2 years and studies without information on age or where children were not explicitly excluded]). Subgroup analyses were performed for categories with at least three different data points.
RESULTS
Overview of included studies
A total of 229 publications [2,11,12,19-244], including 14 national surveillance reports, were included from over 12 000 publications assessed for eligibility. The literature review process is summarized in the PRISMA flowchart in Figure 1. Data extracted from each of the included studies are available in (Tables S2-S8 in Online Supplementary Document).
Figure 1.
PRISMA flowchart.
We included data on rates of CDI incidence from 41 countries. The majority of reports (195/229) were from Europe and North America. Incidence data eligible for inclusion from other regions: Western Pacific, Latin America, Eastern Mediterranean, and Africa were identified less frequently, and no reports from the South-East Asia region was included. Sub-analyses on CDI rates by world region are reported for Europe, North America and the Western Pacific regions for categories with suitable number of data points to conduct a meta-analysis.
Of the 229 publications included, 89 publications reported rates of HCF-CDI (incidence density and/or cases per admissions) and 57 publications reported data for HO-HCF CDI (incidence density and/or cases per admissions). Rates of CA-CDI were reported in 39 publications with almost all data from countries in North America, Europe, and the Western Pacific. CDI in high-risk settings (ICU, IM, and LTCF; 56 publications) were also for the most part identified from countries in North America, Europe, Latin America and the Western Pacific. CDI rates for children and the elderly were reported in 37 and 33 publications, respectively. The number of individual data on rates for each of the CDI categories and age groups are presented in Table 2.
Table 2.
Number of data-points on CDI rates included in the review by category, age group, and world region
| CDI | Hospital onset-health care facility associated |
Intensive care unit |
Internal Medicine |
Healthcare facility-associated |
Community-associated |
Any setting |
ICD-coded hospitalization |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children |
Adults |
Elderly |
All ages |
Any |
Ward* |
Any |
Ward* |
Children |
Adults |
Elderly |
All ages |
Children |
Adults |
Elderly |
All ages |
Children |
Adult |
Elderly |
All ages |
Children |
Adults |
Elderly |
All ages |
|||||||
| CDI per 1000 admissions: | ||||||||||||||||||||||||||||||
| Total (%)† |
2 (5.5) |
20 (54.1) |
3 (8.2) |
12 (32.5) |
5 (31.3) |
11 (68.8) |
7 (63.7) |
4 (36.4) |
5 (10.7) |
24 (51.1) |
5 (10.7) |
13 (27.7) |
3 (11.6) |
12 (46.2) |
4 (15.4) |
7 (27) |
5 (4.7) |
24 (22.5) |
2 (1.9) |
76 (71.1) |
9 (27.3) |
7 (21.3) |
4 (12.2) |
13 (39.4) |
||||||
| NA |
1 |
14 |
2 |
7 |
5 |
1 |
2 |
15 |
3 |
3 |
1 |
10 |
3 |
2 |
3 |
16 |
2 |
5 |
9 |
7 |
4 |
10 |
||||||||
| EU |
2 |
3 |
3 |
4 |
5 |
3 |
3 |
4 |
1 |
9 |
2 |
1 |
1 |
4 |
2 |
4 |
64 |
2 |
||||||||||||
| WP |
1 |
3 |
1 |
1 |
1 |
2 |
1 |
4 |
1 |
1 |
1 |
3 |
3 |
1 |
||||||||||||||||
| LA |
1 |
1 |
1 |
1 |
1 |
3 |
||||||||||||||||||||||||
| AF |
1 |
1 |
||||||||||||||||||||||||||||
| EM |
1 |
|||||||||||||||||||||||||||||
|
CDI incidence density (per 10 000 patient-days) |
CDI cumulative incidence (per 100 000 population per year) |
|||||||||||||||||||||||||||||
| Total (%)† |
2 (4.0) |
20 (40.0) |
2 (4.0) |
26 (52.0) |
9 (39.2) |
14 (60.9) |
5 (38.5) |
8 (61.6) |
10 (6.9) |
39 (26.8) |
6 (4.2) |
91 (62.4) |
6 (16.7) |
10 (27.8) |
6 (16.7) |
14 (38.9) |
9 (23.1) |
7 (18.0) |
8 (20.6) |
15 (38.5) |
4 (15.4) |
6 (23.1) |
8 (30.8) |
8 (30.8) |
||||||
| NA |
1 |
15 |
2 |
16 |
1 |
7 |
1 |
2 |
6 |
21 |
2 |
12 |
5 |
6 |
3 |
6 |
4 |
3 |
3 |
4 |
4 |
6 |
7 |
6 |
||||||
| EU |
4 |
4 |
7 |
3 |
6 |
3 |
12 |
4 |
72 |
1 |
4 |
3 |
7 |
4 |
3 |
3 |
8 |
1 |
2 |
|||||||||||
| WP |
3 |
6 |
3 |
1 |
4 |
5 |
1 |
1 |
1 |
1 |
3 |
|||||||||||||||||||
| LA |
1 |
1 |
1 |
1 |
||||||||||||||||||||||||||
| AF |
||||||||||||||||||||||||||||||
| EM | 2 | 1 |
1 | 1 | ||||||||||||||||||||||||||
CDI – Clostridium difficile infection, LTCF – long-term care facility, NA – North America, EU – Europe, WP – Western Pacific, LA – Latin America, AF – Africa, EM – Eastern Mediterranean, Any – health care facility and community-associated-CDI combined, also includes data from studies without distinction of case acquisition setting, ICD-coded – CDI hospitalization based on discharge code (ICD-CM 9 or ICM-10) for C. difficile, ICD – International Classification of Diseases
* Ward-onset.
†Percentage of data available by age group within category.
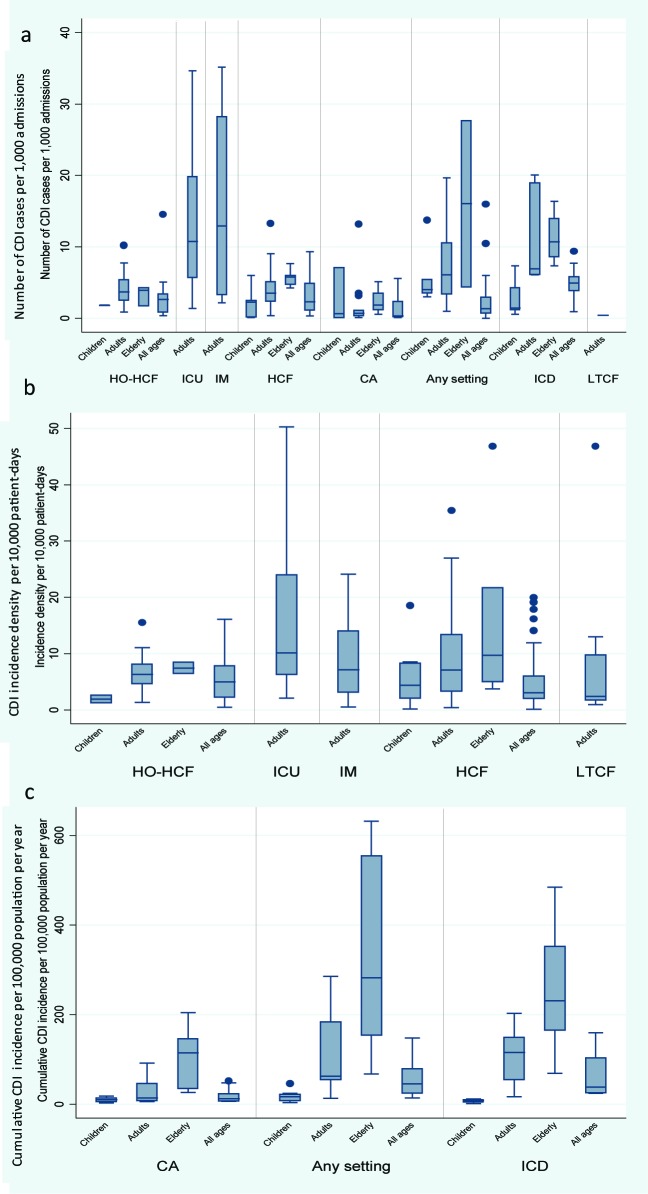
Regardless of metric, there were large variations in rates of CDI incidence across categories and age groups. Figure 2 depicts the median and range of all rates of CDI incidence reported in included studies in the review.
Figure 2.
Distribution of CDI incidence rates by category and age groupI a) number of CDI cases per 1000 admissions per year, b) incidence density per 10 000 patient-days, c) cumulative incidence per 100 000 population per year. Abbreviations: CDI, Clostridium difficile infection; HO-HCF, hospital-onset health care facility-associated; ICU, intensive care unit; IM, internal medicine; HCF, health care facility-associated; CA, community-associated; ICD, international classification of diseases; LTCF, long term care facility.
Meta-analysis
Table 3 shows the results from the meta-analyses, by CDI categories, regions and age groups.
Table 3.
CDI incidence rates meta-estimates by category, age group, and region
| CDI category |
Age group |
Overall |
North America |
Europe |
Western Pacific |
Other | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
N |
IR (95% CI) |
N |
IR (95% CI) |
N |
IR (95% CI) |
N |
IR (95% CI) |
N |
||
|
Number of CDI cases per 1000 admissions | ||||||||||
|
HO-HCF |
Children |
2 |
1.78-1.80 |
1 |
1.78 |
1 |
1.80 |
|||
| Adults |
20 |
3.58 (2.48-5.18) |
14 |
4.27 (2.75-6.62) |
2 |
1.50-4.22 |
3 |
3.05 (2.66-3.50) |
1 |
|
| Elderly |
3 |
3.05 (1.48-6.26) |
2 |
1.69-4.29 |
1 |
3.93 |
||||
| All ages |
12 |
2.09 (1.16-3.74) |
7 |
2.60 (0.96-7.05) |
3 |
0.98 (0.45-2.14) |
1 |
2.68 |
1 |
|
|
ICU |
Hospital |
5 |
7.82 (3.81-16.05) |
1 |
6.62 |
3 |
7.98 (1.72-36.98) |
1 |
||
| Ward-onset |
11 |
11.08 (7.19-17.08) |
4 |
15.82 (8.75-28.59) |
4 |
11.32 (5.24-24.46) |
1 |
2 |
||
|
IM |
Hospital |
7 |
9.41 (3.95-22.40) |
5 |
10.81 (3.88-30.14) |
1 |
3.25 |
1 |
||
| Ward-onset |
4 |
10.80 (3.15-37.06) |
1 |
35.15 |
3 |
7.26 (1.57-33.74) |
||||
|
HCF |
Children |
5 |
0.90 (0.17-4.80) |
2 |
2.27-2.50 |
3 |
0.45 (0.3-8.01) |
|||
| Adults |
24 |
3.26 (2.37-4.50) |
15 |
4.36 (3.01-6.31) |
4 |
1.72 (0.89-3.32) |
4 |
3.42 (2.74-4.27) |
1 |
|
| Elderly |
5 |
5.54 (4.81-6.38) |
3 |
5.72 (4.29-7.63) |
1 |
5.75 |
1 |
4.7 |
||
| All ages |
13 |
2.24 (1.66-3.03) |
3 |
7.14 (4.26-11.98) |
9 |
1.37 (1.06-1.79) |
1 |
6.49 |
||
|
CA |
Children |
3 |
0.52 (0.02-16.06) |
1 |
0.66 |
2 |
0.03-7.09 |
|||
| Adults |
12 |
0.60 (0.25-1.42) |
10 |
0.77 (0.51-1.17) |
1 |
0.26 |
1 |
0.12 |
||
| Elderly |
4 |
1.77 (0.52-6.04) |
3 |
2.58 (1.21-5.51) |
1 |
|||||
| All ages |
7 |
0.55 (0.13-2.37) |
2 |
2.36-5.59 |
4 |
0.23 (0.18-0.29) |
1 |
|||
|
Any |
Children |
5 |
5.05 (3.12-8.17) |
3 |
4.19 (3.28-5.34) |
2 |
3.00-13.75 |
|||
| Adult |
24 |
5.89 (4.64-7.50) |
16 |
7.44 (5.62-9.85) |
4 |
4.12 (0.84-20.40) |
3 |
4.31 (3.02-6.15) |
1 |
|
| Elderly |
2 |
4.4-27.67 |
2 |
4.4-27.67 |
||||||
| All ages |
76 |
1.57 (1.34-1.85) |
5 |
7.52 (4.00-14.13) |
64 |
1.21 (0.98-1.49) |
3 |
2.44 (0.80-7.42) |
4 |
|
|
ICD |
Children |
9 |
2.01 (1.36-2.96) |
9 |
2.00 (1.40-2.85) |
|||||
| Adults |
7 |
9.64 (7.04-13.2) |
7 |
9.69 (7.11-13.19) |
||||||
| Elderly |
4 |
10.77 (7.98-14.54) |
4 |
10.77 (7.98-14.54) |
||||||
| All ages |
13 |
4.26 (3.61-5.05) |
10 |
5.54 (4.69-6.54) |
2 |
0.90-2.39 |
1 |
2.5 |
||
|
Incidence density per 10 000 patient-days | ||||||||||
|
HO-HCF |
Children |
2 |
1.26-2.6 |
1 |
2.6 |
1 |
1.26 |
|||
| Adults |
20 |
5.68 (4.91-6.56) |
15 |
7.07 (6.09-8.20) |
3 |
3.44 (2.49-4.76) |
2 |
|||
| Elderly |
2 |
6.39-8.46 |
2 |
6.39-8.46 |
||||||
| All ages |
26 |
4.14 (3.10-5.53) |
16 |
6.36 (5.53-7.19) |
4 |
1.67 (0.58-4.84) |
6 |
2.69 (1.32-5.49) |
||
|
ICU |
Hosp |
9 |
7.05 (4.42-11.24) |
1 |
2.68 |
4 |
7.72 (3.72-16.00) |
3 |
10.50 (7.57-14.56) |
1 |
| Ward-onset |
16 |
13.74 (9.46-19.93) |
7 |
18.66 (13.04-26.69) |
7 |
11.17 (5.44-22.93) |
1 |
1 |
||
|
IM |
Hosp |
4 |
7.24 (3.57-14.71) |
1 |
2.16 |
3 |
10.64 (5.02-21.78) |
|||
| Ward-onset |
8 |
9.21 (5.74-14.77) |
2 |
16.00-24.13 |
6 |
7.13 (4.03-12.63) |
||||
|
HCF |
Children |
10 |
3.53 (1.48-8.42) |
6 |
7.43 (4.68-11.78) |
3 |
0.88 (0.18-4.27) |
1 |
2.00 |
|
| Adults |
39 |
5.78 (4.63-7.24) |
21 |
10.25 (7.77-13.53) |
12 |
2.30 (1.95-2.70) |
4 |
5.30 (3.44-8.17) |
2 |
|
| Elderly |
6 |
10.95 (5.08-23.59) |
2 |
11.76-46.83 |
4 |
7.48 (1.8-19.32) |
||||
| All ages |
91 |
3.54 (3.19-3.92) |
12 |
7.03 (5.23-9.44) |
72 |
3.14 (2.80-3.53) |
5 |
3.45 (2.41-4.95) |
2 |
|
|
LTCF |
Ward-onset |
8 |
4.41 (2.36-8.23) |
7 |
5.52 (2.78-10.97) |
1 |
0.94 |
0 |
||
|
Cumulative incidence of CDI per 100 000 population | ||||||||||
|
CA |
Children |
5 |
8.878 (5.67-13.88) |
4 |
9.58 (5.96-15.41) |
1 |
5.42 |
0 |
||
| Adults |
9 |
19.95 (11.57-34.38) |
6 |
25.45 (12.13-45.33) |
3 |
14.36 (6.58-31.35) |
0 |
|||
| Elderly |
6 |
74.41 (37.74-146.70) |
3 |
83.67 (33.23-210.65) |
3 |
65.69 (18.73-230.33) |
0 |
|||
| All ages |
14 |
14.37 (9.92-20.82) |
6 |
13.75 (7.9-23.95) |
7 |
16.57 (8.80-31.22) |
1 |
6.98 |
0 |
|
|
Any |
Children |
8 |
15.27 (7.72-30.192) |
4 |
23.35 (15.08-36.14) |
3 |
10.63 (3.04-37.17) |
1 |
7.49 |
0 |
| Adults |
7 |
78.95 (47.70-130.67) |
3 |
89.57 (51.51-155.73) |
3 |
77.25 (41.07-145.32) |
1 |
58.93 |
0 |
|
| Elderly |
7 |
323.32 (217.82-479.91) |
3 |
502.04 (331.51-760.31) |
3 |
272.44 (159.16-466.35) |
1 |
142.76 |
0 |
|
| All ages |
15 |
41.94 (30.30-58.05) |
4 |
53.52 (24.08-118.98) |
8 |
42.5 (26.94-66.92) |
3 |
29.05 (21.61-37.33) |
0 |
|
| ICD | Children |
4 |
5.45 (2.65-11.23) |
4 |
5.45 (2.65-11.23) |
0 |
||||
| Adults |
6 |
83.62 (64.72-108.04) |
6 |
83.62 (64.71-108.04) |
0 |
|||||
| Elderly |
8 |
219.29 (187.33-256.69) |
7 |
242.64 (207.01-284.39) |
1 |
108 |
0 |
|||
| All ages | 8 | 49.36 (34.01-71.75) | 6 | 59.91 (43.66-82.19) | 2 | 32.70-23.60 | 0 | |||
CDI – Clostridium difficile infection, HO-HCF – hospital onset, health care facility-associated, ICU – intensive care unit, IM – internal medicine, HCF – health care facility-associated, CA – community-associated, ICD – International classification of diseases, LTCF – long term care facility, Other – Latin America, Africa, Eastern Mediterranean, N – Number of data entries in meta-analysis, IR – incidence rate point estimate; 95% CI – 95% confidence intervals
CDI cases per admission
Incidence of CDI reported in individual studies ranged from 0 to 35.15 cases per 1000 admissions per year (Figure 2, panel a). The highest median number of CDI cases per admissions were reported from ICU and IM wards; with the highest meta-estimate for incidence rate (IR) of 11.08 (95% CI = 7.19-17.08) per 1000 admissions per year for ICU-onset CDI. Among all ages, meta-estimates rates of HO-HCF and HCF-CDI were similar (IR = 2.09 (95% CI = 1.16-3.74) per 1000 admissions per year and 2.24 (95% CI = 1.66-3.03) per 1000 admissions per year, respectively), and rates for CA-CDI were lower (IR = 0.55 (95% CI = 0.13-2.37) per 1000 admissions per year) (Table 3).
Estimates in the elderly were similar to those than for adults or higher compared to the other age groups (with children having the lowest incidence rates). Although regional level estimates were not strictly comparable, North America generally reported higher rates across CDI categories and age groups (Table 3).
CDI incidence density
CDI incidence density reported in individual studies ranged from 0.11 to 50.3 per 10 000 patient-days (Figure 2, panel b). The highest estimated incidence density by meta-analysis was in the ICU and IM wards, the highest incidence rate 13.74 (95% CI = 9.46-19.93) per 10 000 patient-days was for ICU-onset CDI. The meta-analysis estimates for HO-HCF and HCF were similar (IR = 4.14 (95% CI = 3.10-5.53) per 10 000 patient-days and 3.54 (95% CI = 3.19-3.92) per 10 000 patient-days, respectively), as was the incidence density in LTCF (IR = 4.41 (95% CI = 2.36-8.23) per 10 000 patient-days) (Table 3).
Meta-estimates for the four age categories were calculated for HCF-CDI incidence density, where the highest density was observed among the elderly (IR = 10.95 (95% CI = 5.08-23.59) per 10 000 patient-days). Incidence density of HO-HCF CDI and HCF-CDI were also higher for adult populations compared to that in patients of all ages. Based on the limited data at regional level, we observed that North America had the highest CDI incidence density.
Cumulative incidence of CDI
Cumulative incidence of CDI reported in individual studies ranged from 1.12 to 631.80 per 100 000 population per year (Figure 2, panel c). The majority of data for cumulative incidence of CDI hospitalizations were available from large databases in the United States. Based on data from ICD- coded hospitalizations, the estimate of cumulative incidence rate for all ages is 49.36 (95% CI = 34.01-71.75) per 100 000 population per year. This estimate is similar to the rate based on CDI cases identified through positive laboratory assay and clinical criteria (IR = 41.94 (95% CI = 30.30-58.05) per 100 000 population per year) (Table 3). For the latter, estimates from individual studies ranged from 13.42 per 100 000 population per year in a study conducted in Spain in 2003 where national laboratories were surveyed to 147.20 per 100 000 population per year in a study with sentinel surveillance in the United States where rates were adjusted for use of molecular laboratory assays. At the national level, increases in CDI hospitalization rates per 100 000 population were reported, for instance, in Finland (from 16.0 in 1996 to 34.0 in 2004) [12], in Belgium (from 16.5 in 1999 to 44.3 in 2008), and in the United States (from 48.8 in 1999 to 114.6 in 2008) [11].
Where meta-analysis by age groups was possible, the results for the cumulative incidence for the elderly was higher compared with the other age groups; for any CDI (regardless of setting of acquisition), the overall estimate from meta-analysis was 323.32 (95% CI = 217.82-479.91) per 100 000 population per year. Europe had the highest estimates of cumulative incidence rate CA-CDI in all age groups, though confidence intervals overlap with the estimate for North America.
DISCUSSION
The aim of this study was to examine reports of CDI incidence rates in order to develop an overall estimate and to identify gaps in the current evidence base globally. We found variations of CDI occurrence in terms of rates, within categories as well as between world regions. From our meta-analyses, the estimated overall incidence rate of HCF-CDI for patients of all ages was 2.24 per 1000 admissions per year and 3.54 per 10 000 patient-days.
The implementation of robust surveillance systems is important to ascertain the burden of CDI across and to help identify any future changes in rates [2,14]. Through our review of the literature, we found that the highest rates of CDI for the majority of categories assessed were reported in North America. It is likely that this finding is due to improvements in case detection and influenced by the use of high-sensitivity testing methods such as nucleic acid amplification testing which can lead to overestimation of CDI rates [2,149,245]. In Europe, our meta-estimate for health care-associated CDI density rates (3.14 [95%CI = 2.80-3.53] per 10 000 patient-days) is similar to estimates from regional surveys in 2005, 2008 and 2012-13 [3-5] (for which the overall estimate is 4.08 (95%CI = 3.52-4.74) per 10 000 patient days when data are analyzed by meta-analysis, (Appendix S9 in Online Supplementary Document). Our meta-estimate is also similar to the median rate based on aggregate data from 37 European hospitals in 14 countries in 2013 (median 3.7 (range: 0.6-18.5) per 10 000 patients days) [246]. Variations in CDI rates may also be due to other differences in case ascertainment, both regionally and globally, such as practices and criteria for specimen collection, testing policies and methodologies, under-ascertainment of cases, and reporting requirements [5,246,247]. Efforts to harmonize CDI surveillance protocols within and across countries, such as in Europe, will facilitate the monitoring of epidemiological changes, implementation of infection control protocols [246,248]. Our review shows there is a paucity of data on the incidence of CDI from regions other than North America, Europe and the Western Pacific. This gap in the evidence base is noteworthy as there is evidence of high burden of CDI in other world regions [249]. Limited country-specific data may be due to a combination of lower prevalence and C. difficile not being tested commonly in these regions [250,251]. Identifying the global transmission of C. difficile by molecular characterization is also an important component of surveillance [252] that will further advance our understanding of its associated burden.
Our assessment of rates by age groups shows the importance of age-specific rates to monitor and address the burden of CDI effectively. Our meta-estimates of CDI rates provide evidence for the magnitude of the burden among across age groups and consistently show that elderly populations are disproportionally affected by CDI; as incidence rates increase with increasing age [11] and over 80% of CDI deaths occur in those over 65 years [2]. The high burden of C. difficile on older adults may be due to a range of factors including: more frequent interactions with health care systems, higher use of antimicrobials, and physiologic changes such as decreases in immune responses and multi-morbidity [2]. Some risk factors for CDI in the elderly are modifiable, through actions such as antimicrobial stewardship [253] and the targeting of severe and recurrent cases of CDI, which may impact morbidity and outcomes [254]. These approaches are also relevant to cases of CDI in other age groups. Though we found low rates of CDI among the paediatric population, we also identified discrepancies in the inclusion criteria for classifying pediatric CDI cases. There is a high carriage rate of C. difficile in neonates. Consequently, C. difficile is often thought to be non-pathogenic in infants and assessing the burden of CDI in this population is more difficult [255]. Further, episodes of disease are often of shorter duration and fewer complications as compared to adults [256]. Considering that increases in the rate of CDI hospitalizations in children have been reported, in North America for instance [244,257,258], and the limited information regarding the burden of CDI, careful assessment of surveillance data among the pediatric population is warranted. It is important that studies adhere to standardized surveillance recommendations (eg, infants should be excluded both from the number of cases and the study population in incidence rate calculations) and that CDI rates are analyzed for key age groups to identify monitor subgroups at risk of disease.
While there is evidence from individual studies that the incidence of CDI in ICU patients is decreasing [37,112], our study showed CDI rates were consistently high in the ICU or IM setting. These high rates may be related to the multiple major risk factors that are often found in critically ill or older hospitalized patients [259]. CDI in the ICU has been associated with an increase in mortality, and increased length of stay compared to other settings [259,260] highlighting the importance for developing strategies and protocols for reducing infection in these settings. Our review also indicates the need for assessments of CDI in nursing homes and other LTCF, where limited data are available currently and the burden is estimated to be high [222,261,262].
The rates of CA-CDI identified through this analysis were generally low, yet, comparable across world regions. Under-ascertainment or under-reporting of CA-CDI may have contributed to the lower rates identified, as well as the lower density of individuals in the community who are at high-risk for CDI. In countries where research has been conducted, such as in the US and the UK, a substantial proportion of CDI cases that are acquired and have symptom onset in the community (20%-45% of all CDI are CA-CDI [1,2,75,263]). Understanding the burden of CA-CDI is important as these cases may be characterized by different risk factors, with a younger age group, presenting with less severe disease, and with less prior exposure to antimicrobials [121]. Further knowledge of these factors and on the links with health care setting is important to develop strategies for lowering the rate of CDI in these distinct populations.
To our knowledge, this is the largest meta-analysis of CDI incidence data to date obtained data using publications from a 10-year period to identify a substantial amount of data. This comprehensive review allowed us to estimate CDI rates for a range of metrics across settings. Through the exclusion of studies that reported rates from outbreaks, our estimates provide a baseline epidemiological rate of the burden associated with CDI. However, there are limitations to our review and meta-analysis. One limitation is the low number of reports from several countries, preventing a truly global picture of CDI rates. Though this could partially be influenced by our inclusion criteria (peer-reviewed articles in English and Spanish), we added to the global reach of our search by including data from publically available surveillance reports. Other sources of data, beyond the scope of our study, including short bulletins and publications in other languages need to be systematically assessed. We also identified a limited number of reports of CDI for some clinical settings and case definitions (such as LTCF and community), which limits the estimation of the impact of C. difficile on these diverse populations. We used data in studies published between 2005 and 2015 to include a wide range of relevant reports. However, rates in the early years of these publications (ranging from 1993 until 2015) may be less representative of the current epidemiology of CDI due to changes in rates over time. In addition, some recent data on CDI incidence from 2015 may have not been included in our estimates because our search criteria might not have captured studies during this year. Finally, results from meta-analyses are impacted by the characteristics of the primary studies, which may include variations in case definitions, testing methods, and case identification. We aimed to limit these variations in this study through our inclusion criteria and analysis, yet, heterogeneity remains.
CONCLUSIONS
This study provides baseline epidemiological CDI rates and uncertainty ranges for a variety of settings highlighting the high global burden of disease associated with C. difficile. Our results also emphasize the need for the application of standardized surveillance recommendations, the reporting of age-specific incidence rates, and research in countries were sustainable surveillance by national or regional authorities is not possible to obtain a fuller picture of the burden of CDI. Improving surveillance and case ascertainment rates globally is important to characterize, to understand the burden of disease associated with C. difficile, and to implement effective prevention and infection control measures.
Additional material
Acknowledgements
We are thankful to University of Edinburgh, Medical Librarian, Ms. Marshall Dozier for her assistance in developing search strategies and to investigators who kindly provided data for meta-analyses. Editorial assistance with the preparation of the manuscript was provided by a professional medical writer, Nicola Truss PhD of inScience Communications, Springer Healthcare, UK.
Footnotes
Funding: This work was supported financially by Sanofi Pasteur.
Authorship contributions: EB, HN, MHK, and HC conceived and designed the study. EB, TS, CL, JB, and IL conducted the study. EB analysed the data. EB, CL, JB, IL, CW, HC, MHK, and HN interpreted the data. EB and MHK wrote the first draft and are accountable for the accuracy and integrity of the contents in the manuscript. All authors provided critical revisions of manuscript for the intellectual content. All authors provided approval of the final draft of manuscript.
Competing interests: MHK is an employee of Sanofi Pasteur. EB, TS, CL, IL, JB, CW have no relevant conflict of interest to declare. HC and HN received grants from Sanofi Pasteur. HC is the Editor-in-Chief of the Journal of Global Health. All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no other conflicts of interest.
REFERENCES
- 1.Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. (Special Issue: Fidaxomicin and the evolving approach to the treatment of Clostridium difficile infection.). Clin Infect Dis. 2012;55:S65–70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbut F, Mastrantonio P, Delmee M, Brazier J, Kuijper E, Poxton I, et al. Prospective study of Clostridium difficile infections in Europe with phenotypic and genotypic characterisation of the isolates. Clin Microbiol Infect. 2007;13:1048–57. doi: 10.1111/j.1469-0691.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 5.Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis. 2014;14:1208–19. doi: 10.1016/S1473-3099(14)70991-0. [DOI] [PubMed] [Google Scholar]
- 6.Borren NZ, Ghadermarzi S, Hutfless S, Ananthakrishnan AN. The emergence of Clostridium difficile infection in Asia: A systematic review and meta-analysis of incidence and impact. PLoS One. 2017;12:e0176797. doi: 10.1371/journal.pone.0176797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakken JS, Polgreen PM, Beekmann SE, Riedo FX, Streit JA. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–4. doi: 10.1016/j.anaerobe.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Palazuelos-Munoz S, Balsells EM, Harish N, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447. doi: 10.1186/s12879-016-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducomble T, et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016;13:e1002150. doi: 10.1371/journal.pmed.1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81:1–14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Lucado J, Gould C, Elixhauser A; Healthcare Cost and Utilization Project. Clostridium difficile Infection (CDI) in hospital stays, 2009. 2012. Available: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. Accessed: [01 March 2017]. [PubMed]
- 12.Lyytikainen O, Turunen H, Sund R, Rasinpera M, Kononen E, Ruutu P, et al. Hospitalizations and deaths associated with Clostridium difficile infection, Finland, 1996-2004. Emerg Infect Dis. 2009;15:761–5. doi: 10.3201/eid1505.081154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359–67. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control. European surveillance of Clostridium difficile infections. Stockholm: ECDC, 2015. [Google Scholar]
- 15.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:987–94. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. The national and state healthcare-associated infections progress report. 2016.
- 17.Services; TDoHaH. National action plan to prevent health care-associated infections: road map to elimination (HAI Action Plan). 2013.
- 18.McDonald LC, Coignard B, Dubberke E, Song XY, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140–5. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 19.Ahmetagic S, Salkic N, Ahmetagic A, Custovic A, Tihic N, Smajlovic J, et al. Clostridium difficile infection in hospitalized patients at University Clinical Center Tuzla, Bosnia and Herzegovina: a 4 year experience. Mater Sociomed. 2013;25:153–7. doi: 10.5455/msm.2013.25.153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcala L, Marin M, Martin A, Sanchez-Somolinos M, Catalan P, Pelaez MT, et al. Laboratory diagnosis of Clostridium difficile infection in Spain: a population-based survey. J Hosp Infect. 2011;79:13–7. doi: 10.1016/j.jhin.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Aldeyab MA, Harbarth S, Vernaz N, Kearney MP, Scott MG, Funston C, et al. Quasiexperimental study of the effects of antibiotic use, gastric acid-suppressive agents, and infection control practices on the incidence of Clostridium difficile-associated diarrhea in hospitalized patients. Antimicrob Agents Chemother. 2009;53:2082–8. doi: 10.1128/AAC.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Eidan FA. Proton pump inhibitors and the increased risk of Clostridium difficile infections: A case-control study. Int J Pharma Bio Sci. 2013;4:B735–41. [Google Scholar]
- 23.Alfa MJ, Lo E, Olson N, MacRae M, Buelow-Smith L. Use of a daily disinfectant cleaner instead of a daily cleaner reduced hospital-acquired infection rates. American Journal of Infection Control. 2015;43:141-6 6p. [DOI] [PubMed] [Google Scholar]
- 24.Al-Tawfiq JA, Abed MS. Clostridium difficile-associated disease among patients in Dhahran, Saudi Arabia. Travel Med Infect Dis. 2010;8:373–6. doi: 10.1016/j.tmaid.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Lerma F, Palomar M, Villasboa A, Amador J, Almirall J, Posada MP, et al. Epidemiological study of Clostridium difficile infection in critical patients admitted to the Intensive Care Unit. Med Intensiva. 2014;38:558–66. doi: 10.1016/j.medin.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Ang CW, Heyes G, Morrison P, Carr B. The acquisition and outcome of ICU-acquired Clostridium difficile infection in a single centre in the UK. J Infect. 2008;57:435–40. doi: 10.1016/j.jinf.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Argamany JR, Aitken SL, Lee GC, Boyd NK, Reveles KR. Regional and seasonal variation in Clostridium difficile infections among hospitalized patients in the United States, 2001-2010. Am J Infect Control. 2015;43:435–40. doi: 10.1016/j.ajic.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armbruster S, Goldkind L. A 5-year retrospective review of experience with Clostridium difficile-associated diarrhea. Mil Med. 2012;177:456–9. doi: 10.7205/MILMED-D-11-00389. [DOI] [PubMed] [Google Scholar]
- 29.Babey K, Kelton S, Milne WK, Muileboom J, Voth B, Kelly L, et al. Clostridium difficile infection in rural Ontario: a retrospective multisite population-based study. Can J Rural Med. 2015;20:117–20. [PubMed] [Google Scholar]
- 30.Balihar K, Kozak F, Kozeluhova J, Hejda V, Fremundova L, Krcma M, et al. Clostridium difficile infection in hospitalized patients at a Czech tertiary center: analysis of epidemiology, clinical features, and risk factors of fulminant course. Eur J Gastroenterol Hepatol. 2014;26:880–7. doi: 10.1097/MEG.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 31.Barbut F, Gariazzo B, Bonne L, Lalande V, Burghoffer B, Luiuz R, et al. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect Control Hosp Epidemiol. 2007;28:131–9. doi: 10.1086/511794. [DOI] [PubMed] [Google Scholar]
- 32.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18:714. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bengualid V, Umesh KC, Alapati J, Berger J. Clostridium difficile at a community hospital in the Bronx, New York: incidence prevalence and risk factors from 2006 to 2008. Am J Infect Control. 2011;39:183–7. doi: 10.1016/j.ajic.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Benoit SR, McDonald LC, English R, Tokars JI. Automated surveillance of Clostridium difficile infections using BioSense. Infect Control Hosp Epidemiol. 2011;32:26–33. doi: 10.1086/657633. [DOI] [PubMed] [Google Scholar]
- 35.Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol. 2007;28:1233–5. doi: 10.1086/520732. [DOI] [PubMed] [Google Scholar]
- 36.Bishara J, Farah R, Mograbi J, Khalaila W, Abu-Elheja O, Mahamid M, et al. Obesity as a risk factor for Clostridium difficile infection. Clin Infect Dis. 2013;57:489–93. doi: 10.1093/cid/cit280. [DOI] [PubMed] [Google Scholar]
- 37.Bouza E, Rodríguez-Créixems M, Alcalá L, Marín M, De Egea V, Braojos F, et al. Is Clostridium difficile infection an increasingly common severe disease in adult intensive care units? A 10-year experience. Journal of Critical Care. 2015;30:543-9 7p. [DOI] [PubMed] [Google Scholar]
- 38.Brakovich B, Bonham E, VanBrackle L. War on the spore: Clostridium difficile disease among patients in a long-term acute care hospital. J Healthc Qual. 2013;35:15–21. doi: 10.1111/j.1945-1474.2011.00182.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown K, Valenta K, Fisman D, Simor A, Daneman N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med. 2015;175:626–33. doi: 10.1001/jamainternmed.2014.8273. [DOI] [PubMed] [Google Scholar]
- 40.Buendgens L, Bruensing J, Matthes M, Duckers H, Luedde T, Trautwein C, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. J Crit Care. 2014;29:696.e11. doi: 10.1016/j.jcrc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Camacho-Ortiz A, Galindo-Fraga A, Rancel-Cordero A, Macias AE, Lamothe-Molina P, Ponce de Leon-Garduno A, et al. Factors associated with Clostridium difficile disease in a tertiary-care medical institution in Mexico: a case-control study. Rev Invest Clin. 2009;61:371–7. [PubMed] [Google Scholar]
- 42.Campbell RJ, Giljahn L, Machesky K, Cibulskas-White K, Lane LM, Porter K, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30:526–33. doi: 10.1086/597507. [DOI] [PubMed] [Google Scholar]
- 43.Catanzaro M, Cirone J. Real-time polymerase chain reaction testing for Clostridium difficile reduces isolation time and improves patient management in a small community hospital. Am J Infect Control. 2012;40:663–6. doi: 10.1016/j.ajic.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease C Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk–four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1201–5. [PubMed] [Google Scholar]
- 45.Centers for Disease C Prevention. Surveillance for community-associated Clostridium difficile–Connecticut, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:340–3. [PubMed] [Google Scholar]
- 46.Chan M, Lim P, Chow A, Win M, Barkham TM. Surveillance for Clostridium difficile infection: ICD-9 coding has poor sensitivity compared to laboratory diagnosis in hospital patients, Singapore. PLoS One. 2011;6:e15603. doi: 10.1371/journal.pone.0015603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandler RE, Hedberg K, Cieslak PR. Clostridium difficile-associated disease in Oregon: increasing incidence and hospital-level risk factors. Infect Control Hosp Epidemiol. 2007;28:116–22. doi: 10.1086/511795. [DOI] [PubMed] [Google Scholar]
- 48.Chen KT, Stephens DJ, Anderson E, Acton R, Saltzman D, Hess DJ. Clostridium difficile infection in the pediatric surgery population. J Pediatr Surg. 2012;47:1385–9. doi: 10.1016/j.jpedsurg.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Cheng VCC, Yam WC, Lam OTC, Tsang JLY, Tse EYF, Siu GKH, et al. Clostridium difficile isolates with increased sporulation: emergence of PCR ribotype 002 in Hong Kong. Eur J Clin Microbiol Infect Dis. 2011;30:1371–81. doi: 10.1007/s10096-011-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chopra T, Neelakanta A, Dombecki C, Awali RA, Sharma S, Kaye KS, et al. Burden of Clostridium difficile infection on hospital readmissions and its potential impact under the Hospital Readmission Reduction Program. Am J Infect Control. 2015;43:314–7. doi: 10.1016/j.ajic.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Chung CH, Wu CJ, Lee HC, Yan JJ, Chang CM, Lee NY, et al. Clostridium difficile infection at a medical center in southern Taiwan: incidence, clinical features and prognosis. J Microbiol Immunol Infect. 2010;43:119–25. doi: 10.1016/S1684-1182(10)60019-9. [DOI] [PubMed] [Google Scholar]
- 52.Cloud J, Noddin L, Pressman A, Hu M, Kelly C. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin Gastroenterol Hepatol. 2009;7:868–73.e2. doi: 10.1016/j.cgh.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Collins CE, Ayturk MD, Flahive JM, Emhoff TA, Anderson FA, Jr, Santry HP. Epidemiology and outcomes of community-acquired Clostridium difficile infections in Medicare beneficiaries. J Am Coll Surg. 2014;218:1141–7.e1. doi: 10.1016/j.jamcollsurg.2014.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper PB, Heuer AJ, Warren CA. Electronic screening of patients for predisposition to Clostridium difficile infection in a community hospital. Am J Infect Control. 2013;41:232–5. doi: 10.1016/j.ajic.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Czepiel J, Kedzierska J, Biesiada G, Birczynska M, Perucki W, Nowak P, et al. Epidemiology of Clostridium difficile infection: results of a hospital-based study in Krakow, Poland. Epidemiol Infect. 2015;143:3235–43. doi: 10.1017/S0950268815000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daneman N, Stukel TA, Ma X, Vermeulen M, Guttmann A. Reduction in Clostridium difficile infection rates after mandatory hospital public reporting: findings from a longitudinal cohort study in Canada. PLoS Medicine. 2012;9:e1001268. doi: 10.1371/journal.pmed.1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Bella S, Musso M, Cataldo MA, Meledandri M, Bordi E, Capozzi D, et al. Clostridium difficile infection in Italian urban hospitals: data from 2006 through 2011. BMC Infect Dis. 2013;13:146. doi: 10.1186/1471-2334-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 59.Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179:767–72. doi: 10.1503/cmaj.071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiDiodato G. Has improved hand hygiene compliance reduced the risk of hospital-acquired infections among hospitalized patients in Ontario? Analysis of publicly reported patient safety data from 2008 to 2011. Infect Control Hosp Epidemiol. 2013;34:605–10. doi: 10.1086/670637. [DOI] [PubMed] [Google Scholar]
- 61.Dodek PM, Norena M, Ayas NT, Romney M, Wong H. Length of stay and mortality due to Clostridium difficile infection acquired in the intensive care unit. J Crit Care. 2013;28:335–40. doi: 10.1016/j.jcrc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Drudy D, Harnedy N, Fanning S, O’Mahony R, Kyne L. Isolation and characterisation of toxin A-negative, toxin B-positive Clostridium difficile in Dublin, Ireland. Clin Microbiol Infect. 2007;13:298–304. doi: 10.1111/j.1469-0691.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 63.Dubberke ER, Butler AM, Yokoe DS, Mayer J, Hota B, Mangino JE, et al. Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31:1030–7. doi: 10.1086/656245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubberke ER, McMullen KM, Mayfield JL, Reske KA, Georgantopoulos P, Warren DK, et al. Hospital-associated Clostridium difficile infection: is it necessary to track community-onset disease? Infect Control Hosp Epidemiol. 2009;30:332–7. doi: 10.1086/596604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubberke ER, Reske KA, McDonald LC, Fraser VJ. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis. 2006;12:1576–9. doi: 10.3201/eid1210.060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duleba K, Pawlowska M, Wietlicka-Piszcz M. Clostridium difficile infection in children hospitalized due to diarrhea. Eur J Clin Microbiol Infect Dis. 2014;33:201–9. doi: 10.1007/s10096-013-1946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durkin MJ, Baker AW, Dicks KV, Lewis SS, Chen LF, Anderson DJ, et al. A comparison between National Healthcare Safety Network laboratory-identified event reporting versus traditional surveillance for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015;36:125–31. doi: 10.1017/ice.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eckert C, Coignard B, Hebert M, Tarnaud C, Tessier C, Lemire A, et al. Clinical and microbiological features of Clostridium difficile infections in France: the ICD-RAISIN 2009 national survey. Med Mal Infect. 2013;43:67–74. doi: 10.1016/j.medmal.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Elixhauser A, Jhung MA, Healthcare Cost and Utilization Project Clostridium difficile-associated disease in U.S. hospitals, 1993. Statistical Brief. 2005;50:2008. [Google Scholar]
- 70.Elligsen M, Walker SA, Pinto R, Simor A, Mubareka S, Rachlis A, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33:354–61. doi: 10.1086/664757. [DOI] [PubMed] [Google Scholar]
- 71.Ergen EK, Akalin H, Yilmaz E, Sinirtas M, Alver O, Heper Y, et al. Nosocomial diarrhea and Clostridium Difficile associated diarrhea in a Turkish University Hospital. Med Mal Infect. 2009;39:382–7. doi: 10.1016/j.medmal.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Evans ME, Simbartl LA, Kralovic SM, Jain R, Roselle GA. Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35:1037–42. doi: 10.1086/677151. [DOI] [PubMed] [Google Scholar]
- 73.Faires MC, Pearl DL, Ciccotelli WA, Berke O, Reid-Smith RJ, Weese JS. Detection of Clostridium difficile infection clusters, using the temporal scan statistic, in a community hospital in southern Ontario, Canada, 2006-2011. BMC Infect Dis. 2014;14:254. doi: 10.1186/1471-2334-14-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fashner J, Ribble L, Garcia M. Clostridium difficile-associated diarrhea at a community hospital: Ten-year analysis of infection rates and the relationship with proton pump inhibitor use. Hosp Pharm. 2012;47:446–50. doi: 10.1310/hpj4706-446. [DOI] [Google Scholar]
- 75.Fellmeth G, Yarlagadda S, Iyer S. Epidemiology of community-onset Clostridium difficile infection in a community in the South of England. J Infect Public Health. 2010;3:118–23. doi: 10.1016/j.jiph.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Fenner L, Frei R, Gregory M, Dangel M, Stranden A, Widmer AF. Epidemiology of Clostridium difficile-associated disease at University Hospital Basel including molecular characterisation of the isolates 2006-2007. Eur J Clin Microbiol Infect Dis. 2008;27:1201–7. doi: 10.1007/s10096-008-0564-9. [DOI] [PubMed] [Google Scholar]
- 77.Ferguson JK, Cheng AC, Gilbert GL, Gottlieb T, Korman T, McGregor A, et al. Clostridium difficile laboratory testing in Australia and New Zealand: national survey results and Australasian Society for Infectious Diseases recommendations for best practice. Pathology. 2011;43:482–7. doi: 10.1097/PAT.0b013e328348c9b4. [DOI] [PubMed] [Google Scholar]
- 78.Folkhälsomyndigheten. Clostridium difficile ĺrsrapport 2014. 2015. Available: https://www.folkhalsomyndigheten.se/pagefiles/20544/Clostridium-difficile-arsrapport-2014-15028.pdf. Accessed: [March 2017].
- 79.Foster NF, Collins DA, Ditchburn SL, Duncan CN, van Schalkwyk JW, Golledge CL, et al. Epidemiology of Clostridium difficile infection in two tertiary-care hospitals in Perth, Western Australia: A cross-sectional study. New Microbes New Infect. 2014;2:64–71. doi: 10.1002/nmi2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia C, Samalvides F, Vidal M, Gotuzzo E, Dupont HL. Epidemiology of Clostridium difficile-associated diarrhea in a Peruvian tertiary care hospital. Am J Trop Med Hyg. 2007;77:802–5. doi: 10.4269/ajtmh.2007.77.802. [DOI] [PubMed] [Google Scholar]
- 81.Gase KA, Haley VB, Xiong K, Van Antwerpen C, Stricof RL. Comparison of 2 Clostridium difficile surveillance methods: National Healthcare Safety Network’s laboratory-identified event reporting module versus clinical infection surveillance. Infect Control Hosp Epidemiol. 2013;34:284–90. doi: 10.1086/669509. [DOI] [PubMed] [Google Scholar]
- 82.Gastmeier P, Weitzel-Kage D, Behnke M, Eckmanns T. Surveillance of Clostridium difficile-associated diarrhoea with the German nosocomial infection surveillance system KISS (CDAD-KISS). Int J Antimicrob Agents. 2009;33(Suppl 1):S19–23. doi: 10.1016/S0924-8579(09)70011-1. [DOI] [PubMed] [Google Scholar]
- 83.Gilca R, Hubert B, Fortin E, Gaulin C, Dionne M. Epidemiological patterns and hospital characteristics associated with increased incidence of Clostridium difficile infection in Quebec, Canada, 1998-2006. Infect Control Hosp Epidemiol. 2010;31:939–47. doi: 10.1086/655463. [DOI] [PubMed] [Google Scholar]
- 84.Goorhuis A, Legaria MC, van den Berg RJ, Harmanus C, Klaassen CH, Brazier JS, et al. Application of multiple-locus variable-number tandem-repeat analysis to determine clonal spread of toxin A-negative Clostridium difficile in a general hospital in Buenos Aires, Argentina. Clin Microbiol Infect. 2009;15:1080–6. doi: 10.1111/j.1469-0691.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- 85.Gordin FM, Schultz ME, Huber RA, Gill JA. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infection Control & Hospital Epidemiology. 2005;26:650-3 4p. [DOI] [PubMed] [Google Scholar]
- 86.Gravel D, Miller M, Simor A, Taylor G, Gardam M, McGeer A, et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48:568–76. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 87.Gutierrez RL, Riddle MS, Porter CK. Epidemiology of Clostridium difficile infection among active duty United States military personnel (1998-2010). BMC Infect Dis. 2013;13:609. doi: 10.1186/1471-2334-13-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gweon TG, Choi M, Baeg M, Lim C, Park J, Lee I, et al. Hematologic diseases: high risk of Clostridium difficile associated diarrhea. World J Gastroenterol. 2014;20:6602–7. doi: 10.3748/wjg.v20.i21.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haas JP, Menz J, Dusza S, Montecalvo MA. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am J Infect Control. 2014;42:586–90. doi: 10.1016/j.ajic.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 90.Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, Mills S. Clostridium difficile colitis in the United States: a decade of trends, outcomes, risk factors for colectomy, and mortality after colectomy. J Am Coll Surg. 2013;217:802–12. doi: 10.1016/j.jamcollsurg.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 91.Haley VB, DiRienzo AG, Lutterloh EC, Stricof RL. Quantifying sources of bias in National Healthcare Safety Network laboratory-identified Clostridium difficile infection rates. Infect Control Hosp Epidemiol. 2014;35:1–7. doi: 10.1086/674389. [DOI] [PubMed] [Google Scholar]
- 92.Hamel M, Zoutman D, O’Callaghan C. Exposure to hospital roommates as a risk factor for health care-associated infection. Am J Infect Control. 2010;38:173–81. doi: 10.1016/j.ajic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 93.Han SH, Kim H, Lee K, Jeong SJ, Park KH, Song JY, et al. Epidemiology and clinical features of toxigenic culture-confirmed hospital-onset Clostridium difficile infection: a multicentre prospective study in tertiary hospitals of South Korea. J Med Microbiol. 2014;63:1542–51. doi: 10.1099/jmm.0.070672-0. [DOI] [PubMed] [Google Scholar]
- 94.Han Z, McMullen KM, Russo AJ, Copper SM, Warren DK, Dubberke ER. A Clostridium difficile infection “intervention”: change in toxin assay results in fewer C. difficile infection cases without changes in patient outcomes. Am J Infect Control. 2012;40:349–53. doi: 10.1016/j.ajic.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harbrecht BG, Franklin GA, Shirley RM, Smith JW, Miller FB, Richardson JD. Statewide experience with Clostridium difficile colitis in academic and non-academic medical centers. Surg Infect (Larchmt) 2012;13:88–92. doi: 10.1089/sur.2009.033. [DOI] [PubMed] [Google Scholar]
- 96.Hensgens MP, Goorhuis A, Dekkers OM, van Benthem BH, Kuijper EJ. All-cause and disease-specific mortality in hospitalized patients with Clostridium difficile infection: a multicenter cohort study. Clin Infect Dis. 2013;56:1108–16. doi: 10.1093/cid/cis1209. [DOI] [PubMed] [Google Scholar]
- 97.Hensgens MPM, Dekkers OM, Demeulemeester A, Buiting AGM, Bloembergen P, Benthem BHB, et al. Diarrhoea in general practice: when should a Clostridium difficile infection be considered? Results of a nested case-control study. Clin Microbiol Infect. 2014;20:O1067–74. doi: 10.1111/1469-0691.12758. [DOI] [PubMed] [Google Scholar]
- 98.Hikone M, Ainoda Y, Tago S, Fujita T, Hirai Y, Takeuchi K, et al. Risk factors for recurrent hospital-acquired Clostridium difficile infection in a Japanese university hospital. Clin Exp Gastroenterol. 2015;8:191–6. doi: 10.2147/CEG.S85007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Honda H, Yamazaki A, Sato Y, Dubberke ER. Incidence and mortality associated with Clostridium difficile infection at a Japanese tertiary care center. Anaerobe. 2014;25:5–10. doi: 10.1016/j.anaerobe.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Hooker EA, Bochan M, Reiff TT, Blackwell C, Webb KW, Hart KW. Decreasing Clostridium difficile health care-associated infections through use of a launderable mattress cover. Am J Infect Control. 2015;43:1326–30. doi: 10.1016/j.ajic.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–90. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 102.Health Protection Scotland. Commentary on quarterly epidemiological data on Clostridium difficile infection (CDI) and Staphylococcus aureus bacteraemias (SAB) in Scotland. April to June (Q2) 2015. 2015. Available: http://www.hps.scot.nhs.uk/haiic/sshaip/resourcedetail.aspx?id=1690. Accessed: [01 March 2017].
- 103.Health Protection Surveillance Centre. Annual Epidemiological Reports. 2012-15. Available: http://www.hpsc.ie/az/gastroenteric/clostridiumdifficile/surveillance/notifiablesurveillance/annualreports/. Accessed: [03 March 2017].
- 104.Health & Social Care Public Health Agency Northern Ireland. Clostridium difficile surveillance. 2010-15. Available: http://www.publichealth.hscni.net. Accessed: [01 March 2017].
- 105.Hsu MS, Wang JT, Huang WK, Liu YC, Chang SC. Prevalence and clinical features of Clostridium difficile-associated diarrhea in a tertiary hospital in northern Taiwan. J Microbiol Immunol Infect. 2006;39:242–8. [PubMed] [Google Scholar]
- 106.Institute Scientifique de Sante Publique Belgium. Neely F, Catry B, Lambert M-L. Epidemiology of Clostridium difficile infection in Belgium 2015. Available: http://www.nsih.be/download/CDIF/CDIF-AR-2015-EN.pdf. Accessed: [03 March 2017].
- 107.Ivanova K, Petrov P, Asseva G, Dobreva E, Ivanov I, Vatcheva-Dobrevska R, et al. Prevalence of Clostridium difficile PCR ribotypes in Bulgaria 2008-2010. Dokl Bulg Akad Nauk. 2011;64:1051–8. [Google Scholar]
- 108.Jayatilaka S, Shakov R, Eddi R, Bakaj G, Baddoura WJ, DeBari VA. Clostridium difficile infection in an urban medical center: five-year analysis of infection rates among adult admissions and association with the use of proton pump inhibitors. Ann Clin Lab Sci. 2007;37:241–7. [PubMed] [Google Scholar]
- 109.Jen MH, Saxena S, Bottle A, Pollok R, Holmes A, Aylin P. Assessment of administrative data for evaluating the shifting acquisition of Clostridium difficile infection in England. Journal of Hospital Infection. 2012;80:229-37 9p. [DOI] [PubMed] [Google Scholar]
- 110.Jiang Y, Viner-Brown S, Baier R. Burden of hospital-onset Clostridium difficile infection in patients discharged from Rhode Island hospitals, 2010-2011: application of present on admission indicators. Infect Control Hosp Epidemiol. 2013;34:700–8. doi: 10.1086/670993. [DOI] [PubMed] [Google Scholar]
- 111.Jones G, Taright N, Boelle PY, Marty J, Lalande V, Eckert C, et al. Accuracy of ICD-10 codes for surveillance of Clostridium difficile infections, France. Emerg Infect Dis. 2012;18:979–81. doi: 10.3201/eid1806.111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kanamori H, Weber DJ, DiBiase LM, Sickbert-Bennett EE, Brooks R, Teal L, et al. Longitudinal trends in all healthcare-associated infections through comprehensive hospital-wide surveillance and infection control measures over the past 12 years: substantial burden of healthcare-associated infections outside of intensive care units and “other” types of infection. Infect Control Hosp Epidemiol. 2015;36:1139–47. doi: 10.1017/ice.2015.142. [DOI] [PubMed] [Google Scholar]
- 113.Kanerva M, Mentula S, Virolainen-Julkunen A, Karki T, Mottonen T, Lyytikainen O, et al. Reduction in Clostridium difficile infections in Finland, 2008-2010. J Hosp Infect. 2013;83:127–31. doi: 10.1016/j.jhin.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 114.Kang J, Sickbert-Bennett EE, Brown VM, Weber DJ, Rutala WA. Changes in the incidence of health care-associated pathogens at a university hospital from 2005 to 2011. Am J Infect Control. 2014;42:770–5. doi: 10.1016/j.ajic.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 115.Kassavin DS, Pham D, Pascarella L, Yen-Hong K, Goldfarb MA. The combined use of proton pump inhibitors and antibiotics as risk factors for Clostridium difficile infection. Healthc Infect. 2013;18:76–9. doi: 10.1071/HI12039. [DOI] [Google Scholar]
- 116.Kazakova SV, Ware K, Baughman B, Bilukha O, Paradis A, Sears S, et al. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med. 2006;166:2518–24. doi: 10.1001/archinte.166.22.2518. [DOI] [PubMed] [Google Scholar]
- 117.Khan FY, Abu-Khattab M, Anand D, Baager K, Alaini A, Siddique MA, et al. Epidemiological features of Clostridium difficile infection among inpatients at Hamad General Hospital in the state of Qatar, 2006-2009. Travel Med Infect Dis. 2012;10:179–85. doi: 10.1016/j.tmaid.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 118.Khanafer N, Blais L, Barbut F, Hirschel B, Vanhems P. Treatment of Clostridium difficile infection in a French university hospital. Scand J Gastroenterol. 2015;50:1253–60. doi: 10.3109/00365521.2015.1033746. [DOI] [PubMed] [Google Scholar]
- 119.Khanafer N, Toure A, Chambrier C, Cour M, Reverdy ME, Argaud L, et al. Predictors of Clostridium difficile infection severity in patients hospitalised in medical intensive care. World J Gastroenterol. 2013;19:8034–41. doi: 10.3748/wjg.v19.i44.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khanna S, Baddour LM, Huskins WC, Kammer PP, Faubion WA, Zinsmeister AR, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis. 2013;56:1401–6. doi: 10.1093/cid/cit075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2013;107:89–95. doi: 10.1038/ajg.2011.398. [Erratum appears in Am J Gastroenterol. 2012 Jan;107(1):150] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim J, Kang JO, Kim H, Seo MR, Choi TY, Pai H, et al. Epidemiology of Clostridium difficile infections in a tertiary-care hospital in Korea. Clin Microbiol Infect. 2012;19:521–7. doi: 10.1111/j.1469-0691.2012.03910.x. [DOI] [PubMed] [Google Scholar]
- 123.Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001-2006. Pediatrics. 2008;122:1266–70. doi: 10.1542/peds.2008-0469. [DOI] [PubMed] [Google Scholar]
- 124.Kim JH, Toy D, Muder RR. Clostridium difficile infection in a long-term care facility: hospital-associated illness compared with long-term care-associated illness. Infect Control Hosp Epidemiol. 2011;32:656–60. doi: 10.1086/660767. [DOI] [PubMed] [Google Scholar]
- 125.Kim YS, Han DS, Kim YH, Kim WH, Kim JS, Kim HS, et al. Incidence and clinical features of Clostridium difficile infection in Korea: a nationwide study. Epidemiol Infect. 2013;141:189–94. doi: 10.1017/S0950268812000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.King RN, Lager SL. Incidence of Clostridium difficile infections in patients receiving antimicrobial and acid-suppression therapy. Pharmacotherapy. 2011;31:642–8. doi: 10.1592/phco.31.7.642. [DOI] [PubMed] [Google Scholar]
- 127.KISS Hospital Infection Surveillance System. Module CDAD-KISS Reference Data. 2015. Available: http://www.nrz-hygiene.de/en/surveillance/hospital-infection-surveillance-system/cdad-kiss/. Accessed: [01 March 2017].
- 128.Knight N, Strait T, Anthony N, Lovell R, Norton HJ, Sautter R, et al. Clostridium difficile colitis: a retrospective study of incidence and severity before and after institution of an alcohol-based hand rub policy. Am J Infect Control. 2010;38:523–8. doi: 10.1016/j.ajic.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 129.Koh TH, Tan AL, Tan ML, Wang G, Song KP. Epidemiology of Clostridium difficile infection in a large teaching hospital in Singapore. Pathology. 2007;39:438–42. doi: 10.1080/00313020701444507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohler P, Bregenzer-Witteck A, Rafeiner P, Schlegel M. Presumably hospital-transmitted Clostridium difficile infections based on epidemiological linkage. Swiss Med Wkly. 2013;143:w13824. doi: 10.4414/smw.2013.13824. [DOI] [PubMed] [Google Scholar]
- 131.Koo HL, Van JN, Zhao M, Ye X, Revell PA, Jiang ZD, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol. 2014;35:667–73. doi: 10.1086/676433. [DOI] [PubMed] [Google Scholar]
- 132.van der Kooi TI, Koningstein M, Lindemans A, Notermans DW, Kuijper E, van den Berg R, et al. Antibiotic use and other risk factors at hospital level for outbreaks with Clostridium difficile PCR ribotype 027. J Med Microbiol. 2008;57:709–16. doi: 10.1099/jmm.0.47711-0. [DOI] [PubMed] [Google Scholar]
- 133.Kotila SM, Virolainen A, Snellman M, Ibrahem S, Jalava J, Lyytikainen O. Incidence, case fatality and genotypes causing Clostridium difficile infections, Finland, 2008. Clin Microbiol Infect. 2011;17:888–93. doi: 10.1111/j.1469-0691.2010.03384.x. [DOI] [PubMed] [Google Scholar]
- 134.Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011;11:194. doi: 10.1186/1471-2334-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuntz JL, Johnson ES, Raebel MA, Petrik AF, Yang X, Thorp ML, et al. Clostridium difficile infection, Colorado and the northwestern United States, 2007. Emerg Infect Dis. 2012;18:960–2. doi: 10.3201/eid1806.111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuntz JL, Polgreen PM. The importance of considering different healthcare settings when estimating the burden of Clostridium difficile. Clin Infect Dis. 2015;60:831–6. doi: 10.1093/cid/ciu955. [DOI] [PubMed] [Google Scholar]
- 137.Kurti Z, Lovasz BD, Mandel MD, Csima Z, Golovics PA, Csako BD, et al. Burden of Clostridium difficile infection between 2010 and 2013: trends and outcomes from an academic center in Eastern Europe. World J Gastroenterol. 2015;21:6728–35. doi: 10.3748/wjg.v21.i21.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kutty PK, Benoit SR, Woods CW, Sena AC, Naggie S, Frederick J, et al. Assessment of Clostridium difficile-associated disease surveillance definitions, North Carolina, 2005. Infect Control Hosp Epidemiol. 2008;29:197–202. doi: 10.1086/528813. [DOI] [PubMed] [Google Scholar]
- 139.Kutty PK, Woods CW, Sena AC, Benoit SR, Naggie S, Frederick J, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197–204. doi: 10.3201/eid1602.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laffan AM, Bellantoni MF, Greenough WB, III, Zenilman JM. Burden of Clostridium difficile-associated diarrhea in a long-term care facility. J Am Geriatr Soc. 2006;54:1068–73. doi: 10.1111/j.1532-5415.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 141.Lambert PJ, Dyck M, Thompson LH, Hammond GW. Population-based surveillance of Clostridium difficile infection in Manitoba, Canada, by using interim surveillance definitions. Infect Control Hosp Epidemiol. 2009;30:945–51. doi: 10.1086/605719. [DOI] [PubMed] [Google Scholar]
- 142.Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28:123–30. doi: 10.1086/511793. [DOI] [PubMed] [Google Scholar]
- 143.Laza R, Jurac R, Crisan A, Lazureanu V, Licker M, Popovici ED, et al. Clostridium difficile in western Romania: unfavourable outcome predictors in a hospital for infectious diseases. BMC Infect Dis. 2015;15:141. doi: 10.1186/s12879-015-0895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee J, Tashjian DB, Moriarty KP. Is partial colectomy the operation of choice in pediatric Clostridium difficile colitis? Pediatr Surg Int. 2012;28:603–7. doi: 10.1007/s00383-012-3097-3. [DOI] [PubMed] [Google Scholar]
- 145.Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self-stewardship: trainee-led structured antibiotic time-outs to improve antimicrobial use. Ann Intern Med. 2014;161:S53–8. doi: 10.7326/M13-3016. [DOI] [PubMed] [Google Scholar]
- 146.Lee YC, Wang JT, Chen AC, Sheng WH, Chang SC, Chen YC. Changing incidence and clinical manifestations of Clostridium difficile-associated diarrhea detected by combination of glutamate dehydrogenase and toxin assay in Northern Taiwan. J Microbiol Immunol Infect. 2012;45:287–95. doi: 10.1016/j.jmii.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 147.Lessa FC, Mu Y, Winston LG, Dumyati GK, Farley MM, Beldavs ZG, et al. Determinants of Clostridium difficile Infection Incidence Across Diverse United States Geographic Locations. Open Forum Infect Dis. 2014;1:ofu048. doi: 10.1093/ofid/ofu048. . -ofu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lipp MJ, Nero DC, Callahan MA. Impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol. 2012;27:1733–7. doi: 10.1111/j.1440-1746.2012.07242.x. [DOI] [PubMed] [Google Scholar]
- 149.Longtin Y, Trottier S, Brochu G, Paquet-Bolduc B, Garenc C, Loungnarath V, et al. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin Infect Dis. 2013;56:67–73. doi: 10.1093/cid/cis840. [DOI] [PubMed] [Google Scholar]
- 150.Mah ND, Ahern JW, Terhune CJ, Alston WK. Interaction of age and levofloxacin exposure on the incidence of clostridium difficile infection. Infect Dis Clin Pract. 2011;19:262–4. doi: 10.1097/IPC.0b013e31820994a2. [DOI] [Google Scholar]
- 151.Manian FA, Griesnauer S, Bryant A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am J Infect Control. 2013;41:537–41. doi: 10.1016/j.ajic.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 152.Marco-Martinez J, Barba-Martin R, Plaza-Canteli S, Canora-Lebrato J, Mendez-Baillon M, Miguel-Yanes JMd, et al. Clostridium difficile infections in Spanish Internal Medicine departments during the period 2005-2010: the burden of the disease. Enferm Infecc Microbiol Clin. 2015;33:16–21. doi: 10.1016/j.eimc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 153.Marwick CA, Yu N, Lockhart MC, McGuigan CC, Wiuff C, Davey PG, et al. Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: cohort study with nested case-control. J Antimicrob Chemother. 2013;68:2927–33. doi: 10.1093/jac/dkt257. [DOI] [PubMed] [Google Scholar]
- 154.Mattner F, Winterfeld I, Knobloch J, Solbach W. Successful bundle of prevention measures against a high CDAD incidence at a university hospital. Hyg Med. 2008;33:346–52. [Google Scholar]
- 155.Centers for Disease Control and Prevention (CDC) Vital signs: preventing Clostridium difficile infections. Morbidity and Mortality Weekly Report. 2012;61:157–62. [PubMed] [Google Scholar]
- 156.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12:409–15. doi: 10.3201/eid1205.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis. 2007;45:1141–51. doi: 10.1086/522187. [DOI] [PubMed] [Google Scholar]
- 158.Mellace L, Consonni D, Jacchetti G, Del Medico M, Colombo R, Velati M, et al. Epidemiology of Clostridium difficile-associated disease in internal medicine wards in northern Italy. Intern Emerg Med. 2013;8:717–23. doi: 10.1007/s11739-012-0752-6. [DOI] [PubMed] [Google Scholar]
- 159.Mertz D, Frei R, Plagge H, Battegay M, Widmer AF. Stronger correlation between antibiotic use and the incidence of Clostridium difficile determined by culture results instead of faecal toxin detection only. Eur J Clin Microbiol Infect Dis. 2010;29:1575–8. doi: 10.1007/s10096-010-1022-z. [DOI] [PubMed] [Google Scholar]
- 160.Meyer E, Gastmeier P, Weizel-Kage D, Schwab F. Associations between nosocomial meticillin-resistant Staphylococcus aureus and nosocomial Clostridium difficile-associated diarrhoea in 89 German hospitals. J Hosp Infect. 2012;82:181–6. doi: 10.1016/j.jhin.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 161.Mitchell B, Ware C, McGregor A, Brown S, Wells A. Clostridium difficile infection in Tasmanian public hospitals 2006 - 2010. Healthc Infect. 2011;16:101–6. doi: 10.1071/HI11009. [DOI] [Google Scholar]
- 162.Mitchell BG. Clostridium difficile infection: Incidence in an Australian setting. Asian Nurs Res. 2014;8:213–8. doi: 10.1016/j.anr.2014.07.003. [DOI] [Google Scholar]
- 163.Mitchell BG, Wilson F, McGregor A. An increase in community onset Clostridium difficile infection: A population-based study, Tasmania, Australia. Healthc Infect. 2012;17:127–32. doi: 10.1071/HI12029. [DOI] [Google Scholar]
- 164.MoH Uruguay MdSPU. Infecciones por Clostridium difficile en hospitales centinela. 2012. Available: http://www.msp.gub.uy/sites/default/files/Informe_ICD_2012.pdf. Accessed: [01 March 2017].
- 165.Montoya M, Detorres O. Antimicrobial selection and its impact on the incidence of Clostridium difficile-associated diarrhea. J Pharm Pract. 2013;26:483–7. doi: 10.1177/0897190013499524. [DOI] [PubMed] [Google Scholar]
- 166.Mori N, Yoshizawa S, Saga T, Ishii Y, Murakami H, Iwata M, et al. Incorrect diagnosis of Clostridium difficile infection in a university hospital in Japan. J Infect Chemother. 2015;21:718–22. doi: 10.1016/j.jiac.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 167.Murphy CR, Avery TR, Dubberke ER, Huang SS. Frequent hospital readmissions for Clostridium difficile infection and the impact on estimates of hospital-associated C. difficile burden. Infect Control Hosp Epidemiol. 2012;33:20–8. doi: 10.1086/663209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muto CA, Blank MK, Marsh JW, Vergis EN, O’Leary MM, Shutt KA, et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis. 2007;45:1266–73. doi: 10.1086/522654. [DOI] [PubMed] [Google Scholar]
- 169.Mylotte JM, Russell S, Sackett B, Vallone M, Antalek M. Surveillance for Clostridium difficile infection in nursing homes. J Am Geriatr Soc. 2013;61:122–5. doi: 10.1111/jgs.12041. [DOI] [PubMed] [Google Scholar]
- 170.Nagaraja A, Visintainer P, Haas JP, Menz J, Wormser GP, Montecalvo MA. Clostridium difficile infections before and during use of ultraviolet disinfection. American Journal of Infection Control. 2015;43:940-5 6p. [DOI] [PubMed] [Google Scholar]
- 171.National Institute for Health and Welfare. Infectious diseases in Finland 2013. Helsinki: Department of Infectious Disease Surveillance and Control, 2013. [Google Scholar]
- 172.Novak A, Spigaglia P, Barbanti F, Goic-Barisic I, Tonkic M. First clinical and microbiological characterization of Clostridium difficile infection in a Croatian University Hospital. Anaerobe. 2014;30:18–23. doi: 10.1016/j.anaerobe.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 173.Nowak MA, Nelson RE, Breidenbach JL, Thompson PA, Carson PJ. Clinical and economic outcomes of a prospective antimicrobial stewardship program. Am J Health Syst Pharm. 2012;69:1500–8. doi: 10.2146/ajhp110603. [DOI] [PubMed] [Google Scholar]
- 174.Nseir W, Bishara J, Mograbi J, Mahamid M, Khalaila W, Taha M, et al. Do statins protect against the development of Clostridium difficile-associated diarrhoea? Journal of Antimicrobial Chemotherapy (JAC). 2013;68:1889-93 5p. [DOI] [PubMed] [Google Scholar]
- 175.Oake N, Taljaard M, van Walraven C, Wilson K, Roth V, Forster AJ. The effect of hospital-acquired Clostridium difficile infection on in-hospital mortality. Arch Intern Med. 2010;170:1804–10. doi: 10.1001/archinternmed.2010.405. [DOI] [PubMed] [Google Scholar]
- 176.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–27. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 177.Paltansing S, van den Berg RJ, Guseinova RA, Visser CE, van der Vorm ER, Kuijper EJ. Characteristics and incidence of Clostridium difficile-associated disease in The Netherlands, 2005. Clin Microbiol Infect. 2007;13:1058–64. doi: 10.1111/j.1469-0691.2007.01793.x. [DOI] [PubMed] [Google Scholar]
- 178.Passaretti CL, Otter JA, Reich NG, Myers J, Shepard J, Ross T, et al. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis. 2013;56:27–35. doi: 10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- 179.Pawar D, Tsay R, Nelson DS, Elumalai MK, Lessa FC, Clifford McDonald L, et al. Burden of Clostridium difficile infection in long-term care facilities in Monroe County, New York. Infect Control Hosp Epidemiol. 2012;33:1107–12. doi: 10.1086/668031. [DOI] [PubMed] [Google Scholar]
- 180.Public Health Agency of Canada. Healthcare-associated Clostridium difficile infection in Canadian acute-care hospitals. 2012. Available: http://www.phac-aspc.gc.ca/id-mi/c-difficile-sum-res-eng.php. Accessed: [01 March 2017].
- 181.Public Health England. Clostridium difficile: annual data. 2004-15. Available: http://webarchive.nationalarchives.gov.uk. Accessed: [01 March 2017].
- 182.Pituch H, Obuch-Woszczatynski P, Lachowicz D, Wultanska D, Karpinski P, Mlynarczyk G, et al. Hospital-based Clostridium difficile infection surveillance reveals high proportions of PCR ribotypes 027 and 176 in different areas of Poland, 2011 to 2013. Euro Surveill. 2015;20:30025. doi: 10.2807/1560-7917.ES.2015.20.38.30025. [DOI] [PubMed] [Google Scholar]
- 183.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30:959–63. doi: 10.1086/605925. [DOI] [PubMed] [Google Scholar]
- 184.Price MF, Dao-Tran T, Garey KW, Graham G, Gentry LO, Dhungana L, et al. Epidemiology and incidence of Clostridium difficile-associated diarrhoea diagnosed upon admission to a university hospital. J Hosp Infect. 2007;65:42–6. doi: 10.1016/j.jhin.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 185.Rajabally NM, Pentecost M, Pretorius G, Whitelaw A, Mendelson M, Watermeyer G. The Clostridium difficile problem: a South African tertiary institution’s prospective perspective. South African Medical Journal Suid-Afrikaanse Tydskrif Vir Geneeskunde. 2013;103:168–72. doi: 10.7196/samj.6012. [DOI] [PubMed] [Google Scholar]
- 186.Reddy S, Taori S, Poxton IR. Changes in laboratory and clinical workload for Clostridium difficile infection from 2003 to 2007 in hospitals in Edinburgh. Clin Microbiol Infect. 2010;16:340–6. doi: 10.1111/j.1469-0691.2010.03141.x. [DOI] [PubMed] [Google Scholar]
- 187.Reigadas E, Alcalá L, Marín M, Burillo A, Muńoz P, Bouza E. Missed diagnosis of Clostridium difficile infection; a prospective evaluation of unselected stool samples. J Infect. 2015;70:264–72. doi: 10.1016/j.jinf.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 188.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028–32. doi: 10.1016/j.ajic.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 189.Rhee SM, Tsay R, Nelson DS, Van Wijngaarden E, Dumyati G. Clostridium difficile in the pediatric population of Monroe county, New York. J Pediatric Infect Dis Soc. 2014;3:183–8. doi: 10.1093/jpids/pit091. [DOI] [PubMed] [Google Scholar]
- 190.Ricciardi R, Harriman K, Baxter NN, Hartman LK, Town RJ, Virnig BA. Predictors of Clostridium dificile colitis infections in hospitals. Epidemiol Infect. 2008;136:913–21. doi: 10.1017/S0950268807009387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624–31, discussion 31. doi: 10.1001/archsurg.142.7.624. [DOI] [PubMed] [Google Scholar]
- 192.National Reference Laboratory Netherlands RIVM. Annual Report of the National Reference Laboratory for Clostridium difficile and results of the sentinel surveillance. 2012-14. Available: http://www.rivm.nl/. Accessed: [01 March 2017].
- 193.Rodriguez-Pardo D, Almirante B, Bartolome RM, Pomar V, Mirelis B, Navarro F, et al. Epidemiology of Clostridium difficile infection and risk factors for unfavorable clinical outcomes: results of a hospital-based study in Barcelona, Spain. J Clin Microbiol. 2013;51:1465–73. doi: 10.1128/JCM.03352-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rogers MA, Greene MT, Saint S, Chenoweth CE, Malani PN, Trivedi I, et al. Higher rates of Clostridium difficile infection among smokers. PLoS One. 2012;7:e42091. doi: 10.1371/journal.pone.0042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sabbah MA, Schorr C, Czosnowski QA, Hunter K, Torjman MC, Fraimow HS, et al. Risk of Clostridium difficile infection in intensive care unit patients with sepsis exposed to metronidazole. Infect Dis (Lond) 2015;47:197–202. doi: 10.3109/00365548.2014.978890. [DOI] [PubMed] [Google Scholar]
- 196.Salazar M, Garey KW, Jiang ZD, Dao-Tran T, Dupont H. Changing Clostridium difficile infection testing and treatment trends at a large tertiary care teaching hospital. Pharm World Sci. 2009;31:565–71. doi: 10.1007/s11096-009-9316-x. [DOI] [PubMed] [Google Scholar]
- 197.Salgado CD, Mauldin PD, Fogle PJ, Bosso JA. Analysis of an outbreak of Clostridium difficile infection controlled with enhanced infection control measures. Am J Infect Control. 2009;37:458–64. doi: 10.1016/j.ajic.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 198.Salva S, Duran N, Rodriguez V, Nieto L, Serra J, Rello J, et al. Clostridium difficile in the ICU: study of the incidence, recurrence, clinical characteristics and complications in a university hospital. Med Intensiva. 2014;38:140–5. doi: 10.1016/j.medin.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 199.Sammons JS, Localio R, Xiao R, Coffin SE, Zaoutis T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin Infect Dis. 2013;57:1–8. doi: 10.1093/cid/cit155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sansone S, Aschbacher R, Staffler M, Bombonato M, Girardi F, Larcher C, et al. Nosocomial diarrhoea in adult medical patients: the role of Clostridium difficile in a North Italian acute care teaching hospital. J Prev Med Hyg. 2009;50:117–20. [PubMed] [Google Scholar]
- 201.Santiago B, Guerra L, Garcia-Morin M, Gonzalez E, Gonzalvez A, Izquierdo G, et al. Clostridium difficile isolation in children hospitalized with diarrhea. An Pediatr (Barc) 2015;82:417–25. doi: 10.1016/j.anpedi.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 202.Sathyendran V, McAuliffe GN, Swager T, Freeman JT, Taylor SL, Roberts SA. Clostridium difficile as a cause of healthcare-associated diarrhoea among children in Auckland, New Zealand: clinical and molecular epidemiology. Eur J Clin Microbiol Infect Dis. 2014;33:1741–7. doi: 10.1007/s10096-014-2139-2. [DOI] [PubMed] [Google Scholar]
- 203.Schmiedeskamp M, Harpe S, Polk R, Oinonen M, Pakyz A. Use of International Classification of Diseases, Ninth Revision, Clinical Modification codes and medication use data to identify nosocomial Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:1070–6. doi: 10.1086/606164. [DOI] [PubMed] [Google Scholar]
- 204.Schwartz KL, Darwish I, Richardson SE, Mulvey MR, Thampi N. Severe clinical outcome is uncommon in Clostridium difficile infection in children: a retrospective cohort study. BMC Pediatr. 2014;14:28. doi: 10.1186/1471-2431-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shaklee J, Zerr DM, Elward A, Newland J, Leckerman K, Asti L, et al. Improving surveillance for pediatric Clostridium difficile infection: derivation and validation of an accurate case-finding tool. Pediatr Infect Dis J. 2011;30:e38–40. doi: 10.1097/INF.0b013e3182027c22. [DOI] [PubMed] [Google Scholar]
- 206.Shears P, Prtak L, Duckworth R. Hospital-based epidemiology: a strategy for ‘dealing with Clostridium difficile’. J Hosp Infect. 2010;74:319–25. doi: 10.1016/j.jhin.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 207.Siller-Ruiz M, Calvo-Garcia N, Hernandez-Egido S, Maria-Blazquez A, de Frutos-Serna M, Garcia-Sanchez JE. Epidemiology of Clostridium difficile-associated disease (CDAD) in Salamanca. Rev Esp Quimioter. 2014;27:122–6. [PubMed] [Google Scholar]
- 208.Silva M, Jr, Marra AR, Camargo TZ, Almeida SM, Siqueira I, Correa L, et al. Secular trends in the epidemiology of Clostridium difficile infection (CDI): relationship with alcohol gel and antimicrobial usage in a hospital. Int J Infect Dis. 2013;17:e418–21. doi: 10.1016/j.ijid.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 209.Skyum F, Karim Abed O, Backer Mogensen C. Clinical information on admission is insufficient to determine the appropriate isolation regimen for acute gastroenteritis. Dan Med J. 2014;61:A4850. [PubMed] [Google Scholar]
- 210.Slimings C, Armstrong P, Beckingham WD, Bull AL, Hall L, Kennedy KJ, et al. Increasing incidence of Clostridium difficile infection, Australia, 2011-2012. Med J Aust. 2014;200:272–6. doi: 10.5694/mja13.11153. [DOI] [PubMed] [Google Scholar]
- 211.Smith LC, Ratard R. Clostridium difficile hospitalizations in Louisiana: a 10 year review. J La State Med Soc. 2011;163:192–5. [PubMed] [Google Scholar]
- 212.Soes LM, Holt HM, Bottiger B, Nielsen HV, Torpdahl M, Nielsen EM, et al. The incidence and clinical symptomatology of Clostridium difficile infections in a community setting in a cohort of Danish patients attending general practice. Eur J Clin Microbiol Infect Dis. 2014;33:957–67. doi: 10.1007/s10096-013-2033-3. [DOI] [PubMed] [Google Scholar]
- 213.Sohn S, Climo M, Diekema D, Fraser V, Herwaldt L, Marino S, et al. Varying rates of Clostridium difficile-associated diarrhea at prevention epicenter hospitals. Infect Control Hosp Epidemiol. 2005;26:676–9. doi: 10.1086/502601. [DOI] [PubMed] [Google Scholar]
- 214.Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29:823–8. doi: 10.1086/588756. [DOI] [PubMed] [Google Scholar]
- 215.Souza Dias MB, Yamashiro J, Borrasca VL, Stempliuk VA, Araújo MRE, Costa SF, et al. Pseudo-outbreak of Clostridium difficile associated diarrhea (CDAD) in a tertiary-care hospital. Rev Inst Med Trop Săo Paulo. 2010;52:133–7. doi: 10.1590/S0036-46652010000300004. [DOI] [PubMed] [Google Scholar]
- 216.Statens Serum Institut SSI. Surveillance of hospital-acquired Clostridium difficile infection through HAIBA. 2015. Available: http://www.ssi.dk/English/News/EPI-NEWS/2015/No%2010%20-%202015.aspx. Accessed: [01 March 2017].
- 217.Starzengruber P, Segagni Lusignani L, Wrba T, Mitteregger D, Indra A, Graninger W, et al. Severe Clostridium difficile infection: incidence and risk factors at a tertiary care university hospital in Vienna, Austria. Wien Klin Wochenschr. 2014;126:427–30. doi: 10.1007/s00508-014-0549-x. [DOI] [PubMed] [Google Scholar]
- 218.Stausberg J, Hasford J. Drug-related admissions and hospital-acquired adverse drug events in Germany: a longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. BMC Health Serv Res. 2011;11:134. doi: 10.1186/1472-6963-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Suzuki H, Senda J, Yamashita K, Tokuda Y, Kanesaka Y, Kotaki N, et al. Impact of intensive infection control team activities on the acquisition of methicillin-resistant Staphylococcus aureus, drug-resistant Pseudomonas aeruginosa and the incidence of Clostridium difficile-associated disease. J Infect Chemother. 2013;19:1047–52. doi: 10.1007/s10156-013-0621-x. [DOI] [PubMed] [Google Scholar]
- 220.Tan ET, Robertson CA, Brynildsen S, Bresnitz E, Tan C, McDonald LC. Clostridium difficile-associated disease in New Jersey hospitals, 2000-2004. Emerg Infect Dis. 2007;13:498–500. doi: 10.3201/eid1303.060294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tan XQ, Verrall AJ, Jureen R, Riley TV, Collins DA, Lin RT, et al. The emergence of community-onset Clostridium difficile infection in a tertiary hospital in Singapore: a cause for concern. Int J Antimicrob Agents. 2014;43:47–51. doi: 10.1016/j.ijantimicag.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 222.Taori SK, Wroe A, Hardie A, Gibb AP, Poxton IR. A prospective study of community-associated Clostridium difficile infections: the role of antibiotics and co-infections. J Infect. 2014;69:134–44. doi: 10.1016/j.jinf.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 223.Tartof SY, Rieg GK, Wei R, Tseng H, Jacobsen SJ, Yu KC. A comprehensive assessment across the healthcare continuum: risk of hospital-associated Clostridium difficile infection due to outpatient and inpatient antibiotic exposure. Infect Control Hosp Epidemiol. 2015;36:1409–16. doi: 10.1017/ice.2015.220. [DOI] [PubMed] [Google Scholar]
- 224.Tartof SY, Yu KC, Wei R, Tseng HF, Jacobsen SJ, Rieg GK. Incidence of polymerase chain reaction-diagnosed Clostridium difficile in a large high-risk cohort, 2011-2012. Mayo Clin Proc. 2014;89:1229–38. doi: 10.1016/j.mayocp.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 225.Teltsch DY, Hanley J, Loo V, Goldberg P, Gursahaney A, Buckeridge DL. Infection acquisition following intensive care unit room privatization. Arch Intern Med. 2011;171:32–8. doi: 10.1001/archinternmed.2010.469. [DOI] [PubMed] [Google Scholar]
- 226.Thibault R, Graf S, Clerc A, Delieuvin N, Heidegger CP, Pichard C. Diarrhoea in the ICU: respective contribution of feeding and antibiotics. Crit Care. 2013;17:R153. doi: 10.1186/cc12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tschudin-Sutter S, Tamma PD, Naegeli AN, Speck KA, Milstone AM, Perl TM. Distinguishing community-associated from hospital-associated Clostridium difficile infections in children: implications for public health surveillance. Clin Infect Dis. 2013;57:1665–72. doi: 10.1093/cid/cit581. [DOI] [PubMed] [Google Scholar]
- 228.Van Gessel H. Measuring the incidence of Clostridium difficile-associated diarrhoea in a group of Western Australian hospitals. Healthcare Infection. 2008;13:56-62 7p. [Google Scholar]
- 229.VerLee KE, Finks JL, Wilkins MJ, Wells EV. Michigan Clostridium difficile hospital discharges: frequency, mortality, and charges, 2002-2008. Public Health Rep. 2012;127:62–71. doi: 10.1177/003335491212700107. [Erratum appears in Public Health Rep. 2015 Jul-Aug;130(4):301; PMID: 26345610] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vernaz N, Hill K, Leggeat S, Nathwani D, Philips G, Bonnabry P, et al. Temporal effects of antibiotic use and Clostridium difficile infections. J Antimicrob Chemother. 2009;63:1272–5. doi: 10.1093/jac/dkp128. [DOI] [PubMed] [Google Scholar]
- 231.Vesteinsdottir I, Gudlaugsdottir S, Einarsdottir R, Kalaitzakis E, Sigurdardottir O, Bjornsson ES. Risk factors for Clostridium difficile toxin-positive diarrhea: a population-based prospective case-control study. Eur J Clin Microbiol Infect Dis. 2012;31:2601–10. doi: 10.1007/s10096-012-1603-0. [DOI] [PubMed] [Google Scholar]
- 232.Wang X, Cai L, Yu R, Huang W, Zong Z. ICU-onset Clostridium difficile infection in a university hospital in China: a prospective cohort study. PLoS One. 2014;9:e111735. doi: 10.1371/journal.pone.0111735. . Electronic Resource. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Weitzel-Kage D, Behnke M, Eckmanns T, Gastmeier P. Incidence of Clostridium-difficile-associated disease: first results of CDAD-KISS as component of the German nosocomial infection surveillance system. Hyg Med. 2008;33:353–6. doi: 10.1016/S0924-8579(09)70011-1. [DOI] [PubMed] [Google Scholar]
- 234.Welker JA, Bertumen JB. Toxin assay is more reliable than ICD-9 data and less time-consuming than chart review for public reporting of Clostridium difficile hospital case rates. J Hosp Med. 2012;7:170–5. doi: 10.1002/jhm.990. [DOI] [PubMed] [Google Scholar]
- 235.Wenisch JM, Schmid D, Kuo HW, Simons E, Allerberger F, Michl V, et al. Hospital-acquired Clostridium difficile infection: determinants for severe disease. Eur J Clin Microbiol Infect Dis. 2012;31:1923–30. doi: 10.1007/s10096-011-1522-5. [DOI] [PubMed] [Google Scholar]
- 236.Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62:388–96. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 237.Wilson AP, Smyth D, Moore G, Singleton J, Jackson R, Gant V, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: A randomized crossover study in critical care units in two hospitals. Critical Care Medicine. 2011;39:651-8 8p. [DOI] [PubMed] [Google Scholar]
- 238.Yin J, Schweizer ML, Herwaldt LA, Pottinger JM, Perencevich EN. Benefits of universal gloving on hospital-acquired infections in acute care pediatric units. Pediatrics. 2013;131:e1515–20. doi: 10.1542/peds.2012-3389. [DOI] [PubMed] [Google Scholar]
- 239.Young-Xu Y, Kuntz JL, Gerding DN, Neily J, Mills P, Dubberke ER, et al. Clostridium difficile infection among Veterans Health Administration patients. Infect Control Hosp Epidemiol. 2015;36:1038–45. doi: 10.1017/ice.2015.138. [DOI] [PubMed] [Google Scholar]
- 240.Zahar JR, Schwebel C, Adrie C, Garrouste-Orgeas M, Francais A, Vesin A, et al. Outcome of ICU patients with Clostridium difficile infection. Crit Care. 2012;16:R215. doi: 10.1186/cc11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zarowitz BJ, Allen C, O’Shea T, Strauss ME. Risk factors, clinical characteristics, and treatment differences between residents with and without nursing home- and non-nursing home-acquired Clostridium difficile Infection. J Manag Care Spec Pharm. 2015;21:585–95. doi: 10.18553/jmcp.2015.21.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008;14:929–31. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zilberberg MD, Tabak YP, Sievert DM, Derby KG, Johannes RS, Sun X, et al. Using electronic health information to risk-stratify rates of Clostridium difficile infection in US hospitals. Infect Control Hosp Epidemiol. 2011;32:649–55. doi: 10.1086/660360. [DOI] [PubMed] [Google Scholar]
- 244.Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997-2006. Emerg Infect Dis. 2010;16:604–9. doi: 10.3201/eid1604.090680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gould CV, Edwards JR, Cohen J, Bamberg WM, Clark LA, Farley MM, et al. Effect of nucleic acid amplification testing on population-based incidence rates of Clostridium difficile infection. Clin Infect Dis. 2013;57:1304–7. doi: 10.1093/cid/cit492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.van Dorp SM, Kinross P, Gastmeier P, Behnke M, Kola A, Delmee M, et al. Standardised surveillance of Clostridium Difficile Infection in European acute care hospitals: A pilot study, 2013. Euro Surveill. 2016;21:30293. doi: 10.2807/1560-7917.ES.2016.21.29.30293. [DOI] [PubMed] [Google Scholar]
- 247.Davies K, Davis G, Barbut F, Eckert C, Petrosillo N, Wilcox MH. Variability in testing policies and impact on reported Clostridium difficile infection rates: results from the pilot Longitudinal European Clostridium difficile Infection Diagnosis surveillance study (LuCID). Eur J Clin Microbiol Infect Dis. 2016;35:1949–56. doi: 10.1007/s10096-016-2746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kola A, Wiuff C, Akerlund T, van Benthem BH, Coignard B, Lyytikainen O, et al. Survey of Clostridium difficile infection surveillance systems in Europe, 2011. Euro Surveill. 2016;21:30291. doi: 10.2807/1560-7917.ES.2016.21.29.30291. [DOI] [PubMed] [Google Scholar]
- 249.Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;8:1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Balassiano IT, Yates EA, Domingues RM, Ferreira EO. Clostridium difficile: a problem of concern in developed countries and still a mystery in Latin America. J Med Microbiol. 2012;61:169–79. doi: 10.1099/jmm.0.037077-0. [DOI] [PubMed] [Google Scholar]
- 251.Hawkey PM, Marriott C, Liu W, Jian Z, Gao Q, Ling K, et al. Molecular epidemiology of Clostridium difficile infection in a major Chinese hospital: an underrecognized problem in Asia? J Clin Microbiol. 2013;51:3308–13. doi: 10.1128/JCM.00587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45:109–13. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lawes T, Lopez-Lozano JM, Nebot CA, Macartney G, Subbarao-Sharma R, Wares KD, et al. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: a non-linear time-series analysis. Lancet Infect Dis. 2017;17:194–206. doi: 10.1016/S1473-3099(16)30397-8. [DOI] [PubMed] [Google Scholar]
- 254.Zilberberg MD, Shorr AF, Jesdale WM, Tjia J, Lapane K. Recurrent Clostridium difficile infection among Medicare patients in nursing homes: A population-based cohort study. Medicine (Baltimore) 2017;96:e6231. doi: 10.1097/MD.0000000000006231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lees EA, Miyajima F, Pirmohamed M, Carrol ED. The role of Clostridium difficile in the paediatric and neonatal gut - a narrative review. Eur J Clin Microbiol Infect Dis. 2016;35:1047–57. doi: 10.1007/s10096-016-2639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.McFarland LV, Ozen M, Dinleyici EC, Goh S. Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J Gastroenterol. 2016;22:3078–104. doi: 10.3748/wjg.v22.i11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nylund CM, Eide M, Gorman GH. Association of Clostridium difficile Infections with Acid Suppression Medications in Children. Journal of Pediatrics. 2014;165:979-84.e1 1p. [DOI] [PubMed] [Google Scholar]
- 258.Le Saux N, Gravel D, Mulvey MR, Dennis J, Yasseen AS, III, Barrowman N, et al. Pediatric Clostridium difficile infection: 6-year active surveillance in a defined patient population. Infect Control Hosp Epidemiol. 2014;35:904–6. doi: 10.1086/676875. [DOI] [PubMed] [Google Scholar]
- 259.Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and Clinical Outcomes of Clostridium difficile Infection in the Intensive Care Unit: A Systematic Review and Meta-Analysis. Open Forum Infect Dis. 2015;3:ofv186. doi: 10.1093/ofid/ofv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lofgren ET, Cole SR, Weber DJ, Anderson DJ, Moehring RW. Hospital-acquired Clostridium difficile infections: estimating all-cause mortality and length of stay. Epidemiology. 2014;25:570–5. doi: 10.1097/EDE.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hunter JC, Mu Y, Dumyati GK, Farley MM, Winston LG, Johnston HL, et al. Burden of Nursing Home-Onset Clostridium difficile Infection in the United States: Estimates of Incidence and Patient Outcomes. Open Forum Infect Dis. 2016;3:ofv196. doi: 10.1093/ofid/ofv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Daneman N, Bronskill SE, Gruneir A, Newman AM, Fischer HD, Rochon PA, et al. Variability in Antibiotic Use Across Nursing Homes and the Risk of Antibiotic-Related Adverse Outcomes for Individual Residents. JAMA Intern Med. 2015;175:1331–9. doi: 10.1001/jamainternmed.2015.2770. [DOI] [PubMed] [Google Scholar]
- 263.Banks A, Brown DJ, Mather H, Coia JE, Wiuff C. Sentinel community Clostridium difficile infection (CDI) surveillance in Scotland, April 2013 to March 2014. Anaerobe. 2016;37:49–53. doi: 10.1016/j.anaerobe.2015.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.