Abstract
The loss of extracellular matrix in combination with the exposure of structures such as bone and tendon pose a major challenge; the development of granulation tissue and subsequent reepithelialization over these structures is extremely slow and often may not happen at all. Replacement of the matrix has been shown to significantly increase the chances of healing since, with revascularization of the matrix, a wound bed is created that may either heal by secondary intention or via the application of a skin graft.
A literature search on an esterified hyaluronic acid-based matrix (eHAM) returned five articles on the treatment of wounds with tendon and bone loss in which the eHAM was used. The etiologies of the wounds described varied among the articles, as did treatment modalities. However, all of them received proper debridement of necrosis with subsequent (although not always immediately) application of the eHAM. A very high percentage of all wounds reached the different primary endpoints in the studies, which were complete reepithelialization, complete coverage with granulation tissue and/or 10% coverage of the original wound size with epithelium, the latter being a strong indicator of the wound continuing to heal. The individual authors concluded that the esterified hyaluronic acid matrix (eHAM) is a valuable tool to assist in the complete healing of difficult to heal wounds.
Keywords: Hyaluronic acid, Extracellular matrix, Healing, Exposed bone, Exposed tendon
Introduction
Debridement, removal of devitalized tissue, is a crucial step in wound healing and an essential part of the initiation of the wound healing process,1, 2, 3 as reflected in acronyms such as DIMES.4
In lesions such as Wagner stage III and IV diabetic foot ulcers,5 debridement may extend into the subcutaneous tissues, often with exposure of tendon and/or bone. While grafting on other clean, subcutaneous tissues (i.e. fat or fascia) is possible and may lead to good results,6 direct coverage of tendon and bone is difficult without the development of granulation tissue over these structures.7 Because of the exposure of deep structures, typical wound management is often insufficient, and the lesion may not reach complete, or even partial, reepithelialization.8 It is estimated that these so-called “hard to heal wounds” represent a growing economic burden on Western society of approximately $25B annually.9
It has been shown that reconstruction or replacement of lost extracellular matrix (ECM) is beneficial, since it improves the development of granulation tissue, the speed of healing and overall quality of the tissues.10, 11 The first “replacement matrix” was made of a mixture of collagen and a glycosaminoglycan (chondroitin sulfate). It was developed in the 1980's for the treatment of large, full-thickness, excised burns12, 13 and was first tested in a clinical trial14 in the same decade, receiving wider acceptance and usage in the 1990's.15, 16 In the burn and trauma literature, it was shown that a matrix based on a glycosaminoglycan can also be used successfully in hand, foot, and ankle reconstruction and their associated tendon and joint exposures.17, 18, 19
Many matrices, including acellular dermal-epidermal matrices and bioengineered skin substitutes, are now available and all aim at replacing the lost ECM with a matrix that will allow and encourage the production of granulation tissue and a “neodermis”.20, 21, 22, 23 The matrices become vascularized from both the wound margins and the underlying tissues,24, 25, 26, 27 eventually covering the poorly vascularized wound bed with a vascularized scaffold, which can then support a skin graft or go on to closure via secondary intention.
Some of the newer matrices are based on hyaluronic acid (also called hyaluronan or HA), another glycosaminoglycan. Glycosaminoglycans, also called mucopolysaccharides, are long, unbranched polysaccharides consisting of repeating disaccharides. Among them, hyaluronic acid has a unique structure; it does not contain any sulfate groups and is not covalently attached to proteins. It is, however, a component of non-covalently formed complexes with proteoglycans in the ECM. The disaccharides in hyaluronic acid are d-glucuronic acid and D-N-acetylglucosamine units, and the molecule can reach a molecular mass of up to 107 daltons28 It has a unique mode of synthesis in which the molecule is extruded directly into the extracellular space upon formation.29
Hyaluronic acid is completely and consistently conserved throughout a large span of the evolutionary tree,30 indicating its fundamental biological importance. It is identified in all vertebrates and present in many tissues, but more than 50% of hyaluronan resides in the dermis where it is associated with versican.28
Case I.
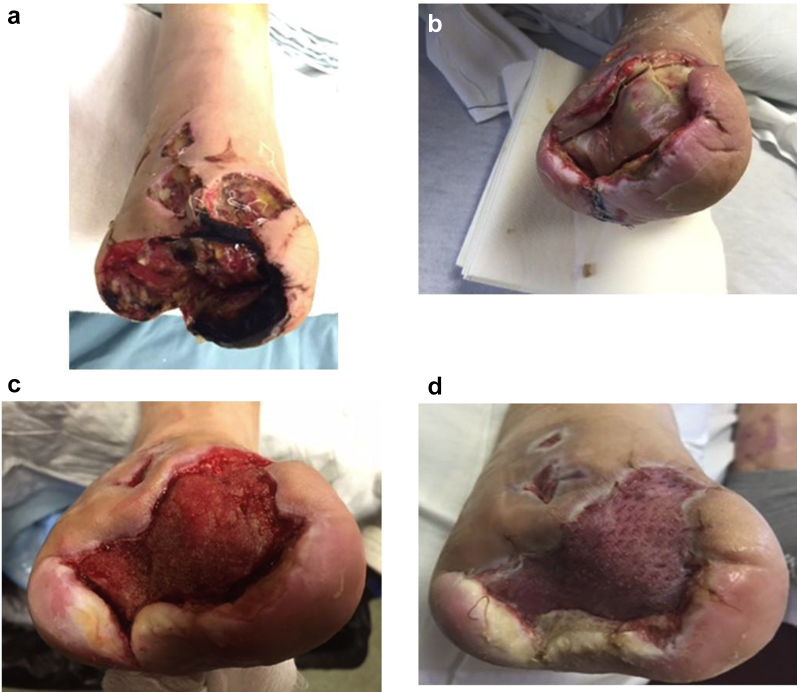
49-year-old male with type 2 diabetes mellitus and peripheral neuropathy. A right transmetatarsal amputation was performed because of a gangrenous forefoot with osteomyelitis. Conservative treatment and hyperbaric oxygen treatment had failed (Ia). Patient refused a below knee amputation and was admitted to a long-term acute care facility for wound care. Intravenous antibiotic therapy was combined with excision of necrotic soft tissue and osteomyelitic bone. The plantar defect was partially closed, and eHAM was applied in combination with NPWT (Ib). Two weeks later, the wound bed was covered with 100% granulation tissue (Ic): an STSG was applied, and NPWT was used for one week. The wound showed complete reepithelialization one week later (Id).
HA plays a multifaceted role through its complex biological and physicochemical interactions with matrix components and cells.31 This ranges from a purely structural function in the extracellular matrix to controlling cellular behavior via its influence on the tissue macro- and microenvironments, as well as through direct receptor-mediated effects on gene expression.32 Hyaluronic acid is a major component of synovial tissues and fluids, as well as other soft tissues, and endows their environments with remarkable rheological properties such as changing viscosity depending on shear stress within the joint.33, 34 Hyaluronan also takes part in the partitioning of plasma proteins between vascular and extravascular spaces. This way it affects solubility of macromolecules in the interstitium, changes chemical equilibria, and stabilizes the structure of collagen fibers.34
Case II.
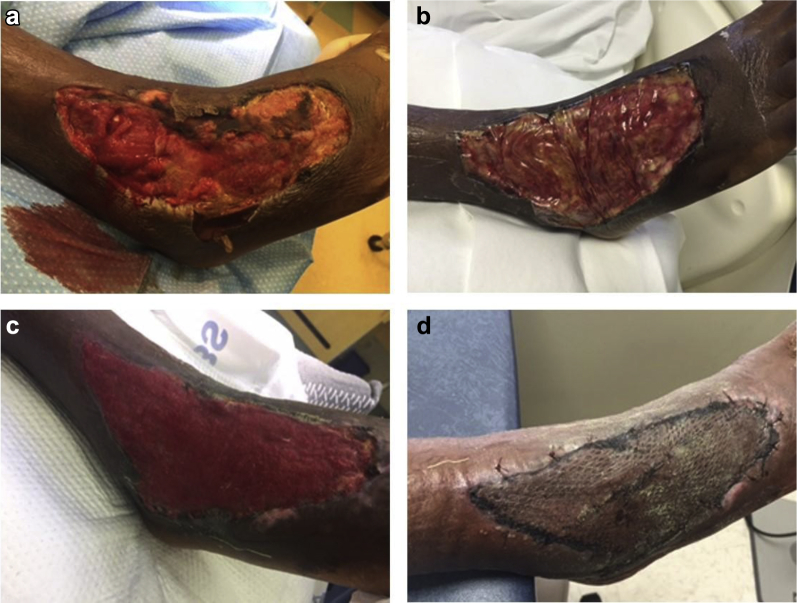
27-year-old male. After a lateral right ankle injury, presented with beta Streptococcus Group A necrotizing fasciitis. Surgical excision was performed (IIa). Patient was placed on wide spectrum antibiotics in combination with twice-daily dressings with Dakin's solution ¼ strength. At post-operative day 7, the wound was stable; a second debridement was performed and eHAM was applied over the exposed ankle capsule and extensor tendons (IIb) and combined with NPWT. Three weeks later, the wound bed showed 100% granulation tissue (IIc) and was grafted with an STSG. Two weeks later, reepithelialization was complete (IId).
Through complex signaling mechanisms, HA plays a major role in promoting angiogenesis.35 Indeed, hyaluronan content in skin is elevated transiently in granulation tissue during the wound healing process.32
Hyaluronic acid is metabolically very active; for example, its half-life in skin is less than one day.28 To avoid this rapid turnover when used in, for example, a matrix, hyaluronic acid can be esterified, and the level of esterification “controls” the half-life of the product. HYAFF®a is an esterified form of hyaluronic acid36 and Hyalomatrixa, b is an esterified hyaluronic acid matrix (eHAM) featuring HYAFF as the primary layer. eHAM acts as a 3-dimensional scaffold for cellular invasion and capillary growth, thus creating a vascularized wound bed. It has a protective outer silicone layer that can be removed upon incorporation of the matrix.
eHAM has been used in different indications for deep tissue loss, such as deep partial- and full-thickness burns37, 38, 39 and other full-thickness trauma,40, 41, 42, 43, 44, 45 as well as in the reconstruction of the scalp. Other indications include the reconstruction of contracted and hypertrophic scars,46, 47 the correction of syndactyly48 and as a matrix in the repair of wounds with exposed tendons and bone.45 In addition, its clinical efficacy has been evaluated in ulcers of various etiology, including large venous leg ulcers and diabetic foot ulcers with critical limb ischemia lesions.41, 42, 44
In lesions with exposed tendons and bone, the use of flaps for wound closure is generally considered the best option; fasciocutaneous flaps are the first choice for the repair of lesion with exposed tendon, since they provided the best functional repair with superior gliding properties for the underlying tendons.49, 50, 51 Myocutaneous flaps or muscle flaps in combination with a (split-thickness) skin graft (STSG) are a good choice for the coverage of exposed bones.52, 53, 54 However, due to several local and systemic conditions, not all patients and their wounds may be appropriate candidates for (extensive) surgery, in which case the use of an extracellular matrix may play an essential role in reconstructing the ECM, thus contributing to wound healing.
The aim of this article is to analyze and review clinical research on eHAM as an ECM in the repair of ulcers and other (surgical) lesions with exposed bone and/or tendon.
Method
An online search, using search engines such as PubMed, Google Scholar, Embase and Endnote, was initiated. (English) search terms included “hyaluronic acid,” “hyaluronan,” “ulcer,” “chronic wound,” “exposure,” “tendon,” “bone,” “matrix” and “Hyalomatrix.” Search results were limited to those articles that described ulcers with exposed bone and tendon, and for which eHAM was used as part of the treatment.
Results
Five articles, describing only lesions with exposed tendon and/or bone or with some exposed bone/tendon lesions among a more extensive series of deep ulcers, were found in the literature, with patient populations ranging from one (a case history) to 262. Evidence levels55 ranged from 2a42 (essentially a multicenter, non-comparative, prospective, observational study) to 456 (a single case study). Inclusion and exclusion criteria showed a significant diversity among the studies. At the same time, there was a high level of consistency with regard to wound care per se; all lesions were thoroughly (and, typically, surgically) debrided prior to application of eHAM with a neutral, non-adherent material as a cover dressing. In some cases, the matrix was fixated using sutures. Incorporation of eHAM into the wound base was among the consistent study objectives, as was (the level of) reepithelialization and, in some studies, the quality of healing.
The individual articles are described below. If wound bed preparation was significantly different from “standard”, including debridement, this was highlighted. In many cases the newly vascularized wound bed was used as a recipient site for an STSG, although “spontaneous” reepithelialization, without grafting, occurred in a number of cases. At least one of these clinical features was part of the endpoints of each study.
Vindigni et al.,56 in a case study, describe an 82 year male with a squamous cell carcinoma on the forehead which had been in existence for 3 years. The lesion underwent wide excision, including the underlying periosteum, resulting in a skin defect of 12 × 8 cm eHAM was applied immediately post-excision. The silicone top layer was removed on post-operative day 21, and an STSG was applied in the same session. Four weeks after the grafting procedure, the lesion was completely reepithelialized with good cosmetic results; the STSG resembled normal skin. A biopsy showed complete vascularization of eHAM, with a total integration of the graft into the wound bed. Long-term follow-up (2 years post-op) still showed proper cosmesis, and the patient was tumor-free.
Caravaggi et al.,41 in a study for which the type is not defined in the article, describe 23 diabetic patients with deep infections of the foot. All patients presented with critical limb ischemia (defined as TcPO2 < 30 mmHg); 18 of these underwent successful endoluminal revascularization. In every patient, some type of amputation had to be performed, with mid-foot amputation counting for eight of these (transmetatarsal: N = 3, Chopart: N = 5). Eight patients were subjected to open partial calcanectomy for a heel ulcer with osteomyelitis of the os calcis, and seven patients were subjected to open forefoot amputation (single or multiple rays). Post-operatively and prior to application of eHAM, topical antimicrobial and negative pressure wound therapy (NPWT) was used for 10 ± 3 days. If necessary, debridement of soft tissue and exposed cancellous bone was performed again; at that time, eHAM was applied, and wounds were grafted with an STSG upon ingrowth of the matrix or left to heal by secondary intention.
Complete coverage of the exposed cancellous bone was obtained in 21 out of 23 patients in a period of 28 ± 17 days of treatment. Further follow up lasted for a period of 176 ± 141 days. One patient was submitted to above-the-knee amputation due to recurrence of foot infection and severe ischemia and was excluded from further participation in the trial. One patient was lost at follow up. Two patients died during the follow up period from myocardial infarction.
Four patients were submitted to skin graft with complete reepithelialization, while six patients healed by secondary intention with initial reepithelialization from the wound edges. The remaining patients showed total coverage of the exposed bone with compact and well-organized new dermal tissue, which was the secondary objective of this study.
Dessy et al.46 describe a prospective study on 10 patients with deep carcinomatous lesions on the scalp. Seven of the described patients were male, and the average age of all patients was 74.4 years (range: 60–90). All patients suffered from infiltrating, giant, non-melanoma skin cancer, of which 2 were squamous cell- and eight were basal cell carcinomas. All tumors were treated with wide excision; when the lesion did not extend beyond galea, surgical excision was performed in subgaleal plane. In deeper lesions, the external calvarium was removed as well. eHAM was applied during the same surgical intervention, and after formation of a neodermis and granulation tissue, covered with an STSG. On average, skin grafts were applied on post-op day 18 (range: 14–21). Graft take was 97% on average (range: 80–100) with 8 patients (80%) reaching 100% take. One patient developed a post-operative infection, which was treated successfully; this was the patient with 80% take.
Average patient satisfaction, rated on a Visual Analogue Scale (VAS) (0: totally unsatisfactory, 10: completely satisfactory) was 8.5. The Vancouver scar57 scale highlighted good graft evolution, with normal vascularity in all cases, normopigmentation in nine cases, hypopigmentation in one case, and no hyperpigmentation. Pliability was normal, supple or yielding in all cases; the graft height was flat or less than 2 mm.
Valenti et al.44 published on a prospective, non-comparative, observational trial in patients with trauma, with exposed bone or tendon. 8 males and 7 females with a mean age of 36.6 years (range: 3–68) suffered from trauma to the lower limb (N = 8) or the upper limb (N = 7). The average size of the lesions was 103.5 cm2 (range 6–490 cm2). Twelve patients had exposed tendons, and two had exposed bone (data on one patient could not be confirmed from the article). Once, after debridement surgery, a clean wound bed was obtained, an eHAM was applied. After a minimum period of 15 days, assessment of the wound was performed by removing the silicone film; where necessary, a second application of eHAM was performed, which was the case for six patients (40%). On average, eHAM stayed in situ for 15.4 days (range: 14–21), and the average number of applications was 1.3 (range: 1–2).
10 patients (67%) experienced spontaneous reepithelialization, and in 5 cases (33%), the lesion was closed with an STSG. The mean time to complete healing was 26.8 days (range: 16–60).
The assessments of clinical epithelialization and the aesthetic quality of the newly formed skin (texture, color similarity and elasticity) were performed at least 6 weeks after product application.
The assessment of the quality of the new skin was optimal for 5 cases (33.3%), good in 7 (46.6%) and modest in 3 (20%). In one case (6.7%), reepithelialization was delayed by a wound infection that was treated with antibiotics. No other complications or adverse events occurred.
Caravaggi42 was the primary author of an article describing a multicenter trial.42 This was an IRB approved, prospective, observational study involving 70 Italian centers and 262 elderly patients. Patients with different types of ulcers and in whom conventional treatments for at least 2 months prior to enrollment had proven ineffective participated in the study. The main exclusion criterion was signs of local infection. Patients using medications known to interfere with healing (e.g., corticosteroids, immunosuppressive, or cytotoxic agents) were not excluded since concomitant therapy was “considered an essential part of wound treatment in ulcers with an immunological etiology”. Patients suffering from significant peripheral vascular disease underwent revascularization procedures in accordance with the criteria established by the Inter-Society Consensus for the Management of Peripheral Arterial Disease58, 59 (TASD II). Offloading was recommended for patients with neuropathic plantar foot ulcers. If patients were suffering from more than one ulcer of the same etiology, the largest ulcer was chosen to participate in the trial.
Ulcers were properly and extensively debrided. When a viable wound bed was obtained, treatment with eHAM was initiated. Study endpoints were defined as either wound coverage with dermal tissue suitable for a thin autograft or growth of new epithelium from the wound edges >10% of the wound surface. The 10% threshold has been shown to be a strong indicator towards reepithelialization60, 61 For patients with “normal” pain sensation, the level of pain during eHAM treatment was assessed at each visit using a Visual Analogue Scale (VAS), with 0 indicating no pain and 100 indicating excruciating pain. Quantitative variables were described as mean with range or, in instances of skewed distributions with median, first (Q1) and third (Q3) quartiles. For this analysis, only patients with a VAS score of >0 at baseline and at least one other pain evaluation within 1 month from study-start were enrolled in the data analysis.
A total of 262 patients with 262 ulcers participated in the study. The mean age was 70 years (range: 33–103), and 53% of the subjects were women. With regard to etiology, 121 (46%) of the ulcers were vascular in origin; 61 ulcers were venous, 60 had a mixed arterial/venous etiology, 66 lesions were diabetic foot ulcers, and 73 ulcers were of other origin. The leg was the most common location (111, 43%), followed by the foot (49, 19%) and the ankle (66, 25%). The 95 ulcers with exposed bone and tendons were not presented in the article as a separate cohort.
The median area was 21.5 cm2 and 34 ulcers (13%) were larger than 100 cm2. Thirty-six percent (n = 95) of the ulcers had exposure of tendon, joint, and/or bone. Of these, 46 (49%) were diabetic foot ulcers. Additional ulcer characteristics are presented in Table 1. The median number of times that eHAM was applied to an ulcer was 2, and median in situ residence was 8 days (range: 6–14) per application.
Table 1.
Ulcer characteristics, multicenter study (Caravaggi et al.42).
| Venous ulcer | Mixed, arterial venous ulcer | Diabetic foot ulcer | Other origin | Total | |
|---|---|---|---|---|---|
| Location | |||||
| Ankle | 18 (30) | 20 (33) | 6 (9) | 5 (7) | 49 (19) |
| Foot | 0 (0) | 3 (5) | 56 (85) | 7 (10) | 66 (25) |
| Leg | 39 (64) | 33 (55) | 0 (0) | 39 (53) | 111 (43) |
| Other | 4 (6) | 4 (7) | 4 (6) | 22 (30) | 34 (13) |
| Total | 61 (100) | 60 (100) | 66 (100) | 73 (100) | 260 (100) |
| Size | |||||
| <15 cm2 | 14 (23) | 20 (33) | 22 (33) | 26 (35) | 82 (310) |
| 15–50 cm2 | 30 (50) | 24 (40) | 34 (51) | 26 (35) | 114 (44) |
| >50 cm2 | 17 (28) | 16 (27) | 10 (15) | 22 (30) | 65 (25) |
| Total | 61 (100) | 60 (100) | 66 (100) | 74 (100) | 261 (100) |
| Duration | |||||
| <6 months | 11 (18) | 17 (28) | 45 (68) | 44 (59) | 117 (45) |
| 6–12 months | 12 (20) | 14 (23) | 11 (17) | 13 (17) | 50 (19) |
| >12 months | 38 (62) | 29 (49) | 10 (15) | 18 (24) | 95 (36) |
| Total | 61 (100) | 60 (100) | 66 (100) | 75 (100) | 262 (100) |
Re-epithelization of ten percent or higher was achieved in 83% of ulcers in a median time of 16 days. Twenty-six percent (26%) of wounds achieved 75% re-epithelization within the 60-day follow-up period using only eHAM treatment. A further follow-up showed that 84% of ulcers achieved complete re-epithelialization by secondary intention. Venous ulcers reached the study endpoint faster than the other types of ulcers. Smaller and more superficial ulcers also reached the 10% reepithelialization threshold faster, although these differences were not statistically significant (Kaplan Meier).
VAS distributions data at baseline and within 30 days from the initial treatment were available for the 229 patients; 16 patients reported no pain at baseline (VAS = 0), and 17 did not report any pain evaluation after baseline. The VAS median value at baseline was 50 (18–74), and the median VAS within 30 days was 15 (first quartile: 3, third quartile: 40). The median trend showed rapid pain relief after eHAM application. Pain intensity was reduced almost 3-fold within 30 days after the initial treatment with eHAM.
Of the 35 adverse events (AE's) (13.5% frequency), 4 infections and 5 increases in the pain level were possibly related to the treatment. Other possibly related AE's included micro-bleeding (N = 1), maceration and erythema of the perilesional skin (N = 2, N = 1, respectively), and “flush of the perilesional skin” (N = 1). All AE's resolved within a short time without further consequences, and removal of eHAM was unnecessary. One patient died due to a non-related disease.
General matrix properties, such as overall handling, ease of use, compatibility with secondary dressings, ease of silicone film removal, safety, tolerability, and overall performance were all rated “very good” or “optimal” by the treating clinician.
Limitations
A review article has inherent limitations since, as is the case here, it is highly unlikely that the different case series and trials described in articles found through (web) searches all have the same format and generate the some type of parameters. This implies that results cannot necessarily be combined or extrapolated, since study protocols, patient populations, indications and endpoints vary among the different studies. The level of evidence55 also usually varies among the different studies, as was the case in the articles reviewed here, ranging from 2a to 4.
All studies referenced in this article, however, included lesions that included exposed bone and/or tendon, albeit with different etiologies. In addition, the approach to the treatment of these lesions also was largely similar; all were extensively (surgically) debrided, which is one of the principles of good wound care61, 62, 63: Proper debridement eliminates a number of factors that are known to impede wound healing. To a large extent, the endpoints among the studies were also similar and included the rebuilding of a proper (neo) dermis, which either allows for the lesion to become a healthy recipient site for an STSG or allows for healing by secondary intention. Healing by secondary intention is a process that, in typical chronic wounds, usually is hampered by intrinsic factors in the lesion, such as a disbalance between metalloproteinases (MMP's) and their inhibitors (TIMP's), as well as the presence of biofilms.64, 65
Discussion and conclusion
144 lesions of different etiologies, but all with exposed bone and/or tendon, were treated with proper and extensive debridement (ulcers) or primary excision (malignancies). The wounds were subsequently covered with eHAM, comprised of a hyaluronan based material (HYAFF) with a protective silicone top-layer. The matrix, allows for cellular ingrowth, thus creating a viable wound bed. In a number of lesions, the “reconstructed” dermis was then covered with a split thickness skin graft. Alternatively, the lesions were left to heal by secondary intention.
The primary etiologies of the lesions and the endpoints of the studies were somewhat different (Table 2). A relatively small percentage of the lesions was surgically created, while the majority included different types of ulcers, particularly diabetic foot ulcers, venous leg ulcers, and mixed arterial/venous ulcers. Debriding the ulcers, however, created lesions that would favorably accept a dermal template, while the surgery, used to excise malignancies, resulted in the creation of a viable woundbed as well.
Table 2.
Overview endpoints & clinical results: patients with exposed bone/tendon, treatment with HMX.
| Type of study | # of subjects | Type of indication | Primary endpoint, % reached | Secondary Endpoint, % reached |
|---|---|---|---|---|
| Vindigni et al.56 | 1 | Excision squamous cell carcinoma | Full reepithelialization: 100% |
N/A? |
| Caravaggi et al.41 | 23 | Diabetic foot ulcer with exposed bone/tendon | Full reepithelialization: With STSG: 17% 2ND Intention: 26% |
“Rebuilding” dermis: 57% |
| Dessy et al.46 | 10 | Skin carcinoma | Graft take: 97% (80% of subjects: 100%) |
Average patient satisfaction (VAS, 1–10): 8.5 |
| Valenti et al.44 | 15 | Trauma with exposed bone/tendon | Full reepithelialization: 100%: With STSG: 33% 2ND Intention: 67% |
Optimal or good quality of skin: 80% |
| Caravaggi et al.42 | 262, 95 exposed tendon or bone | Ulcers of different etiology | Reepithelialization ≥ 10% from wound edges: 83% (16 days) | Reduction of pain: nearly 3-fold within 30 days of primary application. |
The endpoints for the different studies were challenging to analyze and included the creation of a proper wound bed through the application of eHAM, in some cases to be followed by closure of the lesion with an STSG. Wounds with exposed bone and/or tendon and which occur mostly in elderly patients are notoriously difficult to heal.7, 45 In addition, many patients, particularly those with ulcerations such as diabetic foot ulcers and venous leg ulcers, are known to suffer from concomitant diseases and/or use medications that negatively influence wound healing; this makes for an even more challenging patient population.
Since, in patients with serious and hard to heal wounds, the study endpoints were similar to a very high degree (Table 2), it can be concluded that eHAM is a valuable tool for the recreation of a healthy dermis in lesions with exposed bone and/or tendons. In addition, where analyzed, cosmesis and ease of use also received positive results.
Footnotes
Medline, Northfield, IL.
Hyalomatrix®. In the European Union, the matrix is called Hyalomatrix® PA.
References
- 1.Gfeller R.W., Crowe D.T. The emergency care of traumatic wounds: current recommendations. Vet Clin N Am Small Anim Pract. 1994;24(6):1249–1274. doi: 10.1016/s0195-5616(94)50137-2. [DOI] [PubMed] [Google Scholar]
- 2.Haury B., Rodeheaver G., Vensko J. Debridement: an essential component of traumatic wound care. Am J Surg. 1978;135(2):238–242. doi: 10.1016/0002-9610(78)90108-3. [DOI] [PubMed] [Google Scholar]
- 3.Quinby W.C., Jr., Burke J.F., Bondoc C.C. Primary excision and immediate wound closure. Intensive Care Med. 1981;7(2):71–76. doi: 10.1007/BF01687263. [DOI] [PubMed] [Google Scholar]
- 4.Snyder R.J., Fife C., Moore Z. Components and quality measures of DIME (devitalized tissue, infection/inflammation, moisture balance, and edge preparation) in wound care. Adv Skin Wound Care. 2016;29(5):205–215. doi: 10.1097/01.ASW.0000482354.01988.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner F.W., Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 6.Jones T., McDonald S., Deitch E.A. Effect of graft bed on long-term functional results of extremity skin grafts. J Burn Care Rehabil. 1988;9(1):72–74. doi: 10.1097/00004630-198801000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Shores J.T., Hiersche M., Gabriel A. Tendon coverage using an artificial skin substitute. J Plast Reconstr Aesthetic Surg. 2012;65(11):1544–1550. doi: 10.1016/j.bjps.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Janis J.E., Kwon R.K., Attinger C.E. The new reconstructive ladder: modifications to the traditional model. Plast Reconstr Surg. 2011;127(Suppl 1):205S–212S. doi: 10.1097/PRS.0b013e318201271c. [DOI] [PubMed] [Google Scholar]
- 9.Becker D.L., Thrasivoulou C., Phillips A.R. Connexins in wound healing; perspectives in diabetic patients. Biochim Biophys Acta. 2012;1818(8):2068–2075. doi: 10.1016/j.bbamem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Song C. Hypertrophic scars and keloids in surgery: current concepts. Ann Plast Surg. 2014;73(Suppl 1):S108–S118. doi: 10.1097/SAP.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 11.AbouIssa A., Mari W., Simman R. Clinical usage of an extracellular, collagen-rich matrix: a case series. Wounds. 2015;27(11):313–318. [PubMed] [Google Scholar]
- 12.Yannas I.V., Burke J.F. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14(1):65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 13.Yannas I.V., Burke J.F., Gordon P.L. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res. 1980;14(2):107–132. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- 14.Heimbach D., Luterman A., Burke J. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988;208(3):313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helvig E.I. Dermal replacement: an update. Semin Perioperat Nurs. 1997;6(4):233–235. [PubMed] [Google Scholar]
- 16.Lorenz C., Petracic A., Hohl H.P. Early wound closure and early reconstruction. Experience with a dermal substitute in a child with 60 per cent surface area burn. Burns. 1997;23(6):505–508. doi: 10.1016/s0305-4179(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Bhavsar D., Tenenhaus M. The use of acellular dermal matrix for coverage of exposed joint and extensor mechanism in thermally injured patients with few options. Eplasty. 2008;8(33) [PMC free article] [PubMed] [Google Scholar]
- 18.Jeng J.C., Fidler P.E., Sokolich J.C. Seven years' experience with Integra as a reconstructive tool. J Burn Care Res. 2007;28(1):120–126. doi: 10.1097/BCR.0b013E31802CB83F. [DOI] [PubMed] [Google Scholar]
- 19.Muangman P., Engrav L.H., Heimbach D.M. Complex wound management utilizing an artificial dermal matrix. Ann Plast Surg. 2006;57(2):199–202. doi: 10.1097/01.sap.0000218636.61803.d6. [DOI] [PubMed] [Google Scholar]
- 20.Cervelli V., Brinci L., Spallone D. The use of MatriDerm(R) and skin grafting in post-traumatic wounds. Int Wound J. 2011;8(4):400–405. doi: 10.1111/j.1742-481X.2011.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumenta D.B., Kamolz L.P., Frey M. Adult burn patients with more than 60% TBSA involved-Meek and other techniques to overcome restricted skin harvest availability–the Viennese Concept. J Burn Care Res. 2009;30(2):231–242. doi: 10.1097/BCR.0b013e318198a2d6. [DOI] [PubMed] [Google Scholar]
- 22.Philandrianos C., Andrac-Meyer L., Mordon S. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns. 2012;38(6):820–829. doi: 10.1016/j.burns.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Sheridan R., Hegarty M.T., Tompkins R. Artificial skin in massive burns-results to ten years. Eur J Plast Surg. 1994;17:91–93. [Google Scholar]
- 24.Campoccia D., Doherty P., Radice M. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;19(23):2101–2127. doi: 10.1016/s0142-9612(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 25.Reed R.K., Laurent U.B. Turnover of hyaluronan in the microcirculation. Am Rev Respir Dis. 1992;146(5 Pt 2):S37–S39. doi: 10.1164/ajrccm/146.5_Pt_2.S37. [DOI] [PubMed] [Google Scholar]
- 26.Rooney P., Kumar S., Ponting J. The role of hyaluronan in tumour neovascularization (review) Int J Canc. 1995;60(5):632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- 27.West D.C., Hampson I.N., Arnold F. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 28.Nusgens B.V. Hyaluronic acid and extracellular matrix: a primitive molecule? Ann Dermatol Venereol. 2010;137(Suppl 1):S3–S8. doi: 10.1016/S0151-9638(10)70002-8. [DOI] [PubMed] [Google Scholar]
- 29.Hagenfeld D., Borkenhagen B., Schulz T. Hyaluronan export through plasma membranes depends on concurrent K+ efflux by K(ir) channels. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Z., Yin-Min W., Jun Y. Hyaluronic acid: a versatile biomaterial in tissue engineering. Plast Aesthet Res. 2017;4(219-27) [Google Scholar]
- 31.Litwiniuk M., Krejner A., Speyrer M.S. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88. [PubMed] [Google Scholar]
- 32.Chen W.Y., Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 33.Fraser J.R., Laurent T.C., Laurent U.B. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 34.Laurent T.C., Laurent U.B., Fraser J.R. The structure and function of hyaluronan: an overview. Immunol Cell Biol. 1996;74(2):A1–A7. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 35.Park D., Kim Y., Kim H. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFbeta receptor interaction via CD44-PKCdelta. Mol Cell. 2012;33(6):563–574. doi: 10.1007/s10059-012-2294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aigner J., Tegeler J., Hutzler P. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42(2):172–181. doi: 10.1002/(sici)1097-4636(199811)42:2<172::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Tamisani A. The use of Hyalomatrix in deep paediatric burns. Ann Burns Fire Disast. 2004;17(4) [Google Scholar]
- 38.Gravante G., Sorge R., Merone A. Hyalomatrix PA in burn care practice: results from a national retrospective survey, 2005 to 2006. Ann Plast Surg. 2010;64(1):69–79. doi: 10.1097/SAP.0b013e31819b3d59. [DOI] [PubMed] [Google Scholar]
- 39.Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns and Trauma. 2014;2(4):162–168. doi: 10.4103/2321-3868.142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carapetis J.R., Jacoby P., Carville K. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59(3):358–365. doi: 10.1093/cid/ciu304. [DOI] [PubMed] [Google Scholar]
- 41.Caravaggi C., Barbara A., Sganzaroli A. Safety and efficacy of a dermal substitute in the coverage of cancellous bone after surgical debridement for severe diabetic foot ulceration. EWMA J. 2009;9(1):11–14. [Google Scholar]
- 42.Caravaggi C., Francesco Grigoletto M., Scuderi N. Wound bed preparation with a dermal substitute (Hyalomatrix® PA) facilitates re-epithelialization and healing: results of a multicenter, prospective, observational study on complex chronic ulcers the FAST study. Wounds. 2011;8(23):228–235. [PubMed] [Google Scholar]
- 43.Motolese A., Vignati F., Brambilla R. Interaction between a regenerative matrix and wound bed in nonhealing ulcers: results with 16 cases. BioMed Res Int. 2013;2013:849321. doi: 10.1155/2013/849321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaienti L., Marchesi A., Palitta G. Limb trauma: the use of an advanced wound care device in the treatment of full-thickness wounds. Strategies Trauma Limb Reconstr. 2013;8(2):111–115. doi: 10.1007/s11751-013-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simman R., Mari W., Younes S. Use of hyaluronic acid–based biological bilaminar matrix in wound bed preparation: a case series. e-Plasty. 2018;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 46.Dessy L.A., Mazzocchi M., Rizzo M.I. Scalp reconstruction using dermal induction template: state of the art and personal experience. In Vivo. 2013;27(1):153–158. [PubMed] [Google Scholar]
- 47.Faga A., Nicoletti G., Brenta F. Hyaluronic acid three-dimensional scaffold for surgical revision of retracting scars: a human experimental study. Int Wound J. 2012;10:1–8. doi: 10.1111/j.1742-481X.2012.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landi A., Garagnani L., Leti Acciaro A. Hyaluronic acid scaffold for skin defects in congenital syndactyly release surgery: a novel technique based on the regenerative model. J Hand Surg Eur Vol. 2014;39(9):994–1000. doi: 10.1177/1753193414529046. [DOI] [PubMed] [Google Scholar]
- 49.Lin C.T., Chen S.G., Chen T.M. Bipedicled flap for the reconstruction of soft tissue defects of the Achilles tendon. Ann Plast Surg. 2015;74(4):484–487. doi: 10.1097/SAP.0b013e3182a1e508. [DOI] [PubMed] [Google Scholar]
- 50.Rainer C., Schwabegger A.H., Bauer T. Free flap reconstruction of the foot. Ann Plast Surg. 1999;42(6):595–606. doi: 10.1097/00000637-199906000-00003. discussion 06–7. [DOI] [PubMed] [Google Scholar]
- 51.Sobanko J.F., Fischer J., Etzkorn J.R. Local fasciocutaneous sliding flaps for soft-tissue defects of the dorsum of the hand. JAMA Dermatol. 2014;150(11):1187–1191. doi: 10.1001/jamadermatol.2014.954. [DOI] [PubMed] [Google Scholar]
- 52.Delgove A., Leclere F.M., Villani F. Medial triceps brachii free flap in reconstructive surgery: a prospective study in eight patients. Arch Orthop Trauma Surg. 2015;135(2):275–282. doi: 10.1007/s00402-014-2102-9. [DOI] [PubMed] [Google Scholar]
- 53.Kim S.W., Jeon S.B., Hwang K.T. Coverage of amputation stumps using a latissimus dorsi flap with a serratus anterior muscle flap: a comparative study. Ann Plast Surg. 2016;76(1):88–93. doi: 10.1097/SAP.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzetti F., Lazzeri D., Bonini L. Distally based peroneus brevis muscle flap in reconstructive surgery of the lower leg: postoperative ankle function and stability evaluation. J Plast Reconstr Aesthetic Surg. 2010;63(9):1523–1533. doi: 10.1016/j.bjps.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Burns P.B., Rohrich R.J., Chung K.C. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vindigni V., Bassetto F., Abatangelo S. Temporary coverage of a forehead defect following tumor resection with a hyaluronic acid biological dressing: a case report. Ostomy/Wound Manag. 2011;4(57):56–60. [PubMed] [Google Scholar]
- 57.Sullivan T., Smith J., Kermode J. Rating the burn scar. J Burn Care Rehabil. 1990;11(3):256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Dormandy J.A., Rutherford R.B., Management of Peripheral Arterial Disease (PAD). TASC Working Group TransAtlantic inter-society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- 59.Norgren L., Hiatt W.R., Dormandy J.A. Inter-society Consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Donohoe K., Falanga V. Healing rate as a prognostic indicator of complete healing. Wounds. 2003;15(3):71–76. [Google Scholar]
- 61.Margolis D.J., Kantor J., Santanna J. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol. 2000;136(12):1531–1535. doi: 10.1001/archderm.136.12.1531. [DOI] [PubMed] [Google Scholar]
- 62.Ayello E.A., Dowsett C., Schultz G.S. TIME heals all wounds. Nursing. 2004;34(4):36–41. doi: 10.1097/00152193-200404000-00040. quiz, 41-2. [DOI] [PubMed] [Google Scholar]
- 63.Uccioli L., Giurato L., Ruotolo V. Two-step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: results of a multicenter, randomized controlled clinical trial with long-term follow-up. Int J Low Extrem Wounds. 2011;10(2):80–85. doi: 10.1177/1534734611409371. [DOI] [PubMed] [Google Scholar]
- 64.Trengove N.J., Stacey M.C., MacAuley S. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–452. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 65.Wolcott R. Disrupting the biofilm matrix improves wound healing outcomes. J Wound Care. 2015;24(8):366–371. doi: 10.12968/jowc.2015.24.8.366. [DOI] [PubMed] [Google Scholar]