Abstract
Background
Influenza is a major cause of respiratory illness in young children. Assessing the impact of infection on children and the community is required to guide immunisation policies.
Objectives
To describe the impact of laboratory‐proven influenza in young children and to compare its impact with that of other respiratory viruses on the child, their family and the health care system.
Methods
Preschool children presenting for care or admission to a tertiary paediatric hospital during the 2008‐2014 influenza seasons were tested for respiratory virus by polymerase chain reaction and culture. Parental surveys were used to determine the impact of infection on illness duration, medication use, absenteeism and health service utilisation. Multivariate regression analyses were used to assess the impact of influenza and to evaluate the association between influenza status and outcomes.
Results
Among 1191 children assessed, 238 had influenza. Among children with influenza, 87.8% were administered antipyretics and 40.9% antibiotics. 28.6% had secondary complications. 65.4% of children missed school/day care, and 53.4% of parents missed work. When influenza and other viruses were compared, significant differences were noted including duration of illness (influenza: 9.54 days, other viruses: 8.50 days; P = 0.005) and duration of absenteeism for both the child (23.1 vs 17.3 hours; P = 0.015) and their parents (28.5 vs 22.7 hours; P = 0.012).
Conclusions
Influenza infection in young children has a significant impact on medication use, absenteeism and the use of health care service. Significant differences are identified when compared with other ILI. These data demonstrate that influenza prevention strategies including immunisation are likely to have wide and significant impacts.
Keywords: children, impact, influenza, influenza‐like illness
1. INTRODUCTION
Influenza is a major cause of respiratory illness in young children,1 with those <5 years at greatest risk of disease. The rate of influenza‐associated hospitalisations in this age group exceeds that seen in the elderly.2 Assessing the impact of influenza infection in young children is challenging as it co‐circulates with other respiratory viruses, from which it is clinically indistinguishable without laboratory testing.3, 4 It is well recognised that estimates of influenza morbidity and mortality are likely to be underestimated.5
The majority of influenza‐related disease in children is seen in outpatient settings.1, 6 Despite lower severity, it is speculated that non‐hospitalised influenza represents a greater burden overall.6, 7 Influenza in children is associated with the frequent use of antipyretics, antibiotics,6, 8, 9 and parental and child absenteeism.6, 10, 11, 12, 13, 14 Health care visits due to influenza illness have been previously estimated to cost the Australian health care system AU$115 million annually (2008).15 Indirect costs from parental absenteeism are thought to be the largest contributor to the economic impact of influenza in young children.10, 14, 16
The impact of influenza in children has been well described in the Northern Hemisphere,6, 10, 14, 17 yet impact in Australia remains unknown. To date, data on the impact of influenza have been limited to hospitalised children,18, 19 single influenza seasons16, 18, 19, 20 or studies with small numbers of influenza‐positive children.16, 20
In Australia, influenza vaccine is not included on the National Immunisation Program (NIP), except for Aboriginal and Torres Strait Islander children for whom it was funded in 2015.21 Western Australia (WA) introduced a free influenza programme for children aged 6‐59 months in 2008, although uptake rates have been low since safety concerns were identified with one brand of vaccine in 2010.22, 23 In 2018, following the largest influenza season since 2009, several other Australian jurisdictions introduced influenza vaccine programmes for young children. Prior to inclusion on the NIP, cost‐effectiveness of preschool influenza vaccination needs to be demonstrated. Understanding the impact of paediatric influenza on families and the community is essential to inform such analyses.
Commencing in 2008, the WA Influenza Vaccine Effectiveness (WAIVE) study was established to assess vaccine effectiveness in young children22, 24, 25, 26 and to assess the impact of influenza on young children and their families. We describe the impact of laboratory‐proven influenza on the child, their family and health care services, and compare the impact of influenza with other respiratory viruses presenting as influenza‐like illness (ILI).
2. METHODS
2.1. Study population
As previously described,24, 25 young children eligible for vaccination (aged 6‐59 months at the time of vaccination) presenting to the emergency department (ED) or admitted with ILI to Princess Margaret Hospital, were eligible for recruitment. Princess Margaret Hospital was the only tertiary paediatric hospital in Perth WA (population 2.5 million people 27), since replaced by Perth Children’s Hospital. ILI was defined as a history of fever ≥37.5°C (by parental report or documented on presentation), plus at least one acute respiratory symptom or sign in the previous 96 hours. Enrolment occurred over seven influenza seasons (2008‐2014), with the season being defined by an increase in statewide influenza detections above baseline. Due to the cohort being part of the wider influenza vaccine effectiveness study, children with a contraindication to influenza vaccination, history of immunosuppression or recent receipt of an immunomodulatory medication were ineligible.
2.2. Study procedures
Following written consent, bilateral flocked nasopharyngeal swabs were obtained (Copan Diagnostics Inc. Murrieta, CA). Children from whom a nasopharyngeal aspirate (NPA) was already taken at presentation did not require additional testing. Using previously published methods, samples were tested for respiratory viruses including influenza, respiratory syncytial virus (RSV), human metapneumoviruses, parainfluenza types 1‐4, picornaviruses (including rhinovirus A‐C), human adenoviruses, coronaviruses HKU1/OC43/229E/NL63 and bocaviruses using a polymerase chain reaction (PCR) assay and viral culture.28, 29, 30
Two parent‐completed questionnaires were collected: one at enrolment collecting demographics, comorbidities, school/day care attendance, household composition, passive smoke exposure and influenza vaccination status, and a second questionnaire administered 7‐10 days after enrolment collecting information on the impact of the illness.
2.3. Exclusions
Enrolled children for whom a respiratory sample was not successfully collected (n = 16), a second questionnaire was not completed (n = 1946) or a second questionnaire was completed either too early or too late to accurately capture impact data (<6 days or >40 days after enrolment; n = 360) were excluded from the analysis. In addition, to minimise parents reporting the impact from consecutive and/or concurrent viruses not detected by the respiratory sample, children who reportedly had a continuous fever commencing >7 days prior to enrolment were also excluded (n = 102).
2.4. Exposure ascertainment and outcomes
Primary exposure was defined as laboratory‐proven influenza A or B by either PCR or culture. Two comparison groups were used: (a) influenza‐negative children and (b) influenza‐negative children testing positive for other respiratory viruses. The second comparison group was used to reduce the probability of including false‐negative influenza infection due to inadequate specimen collection, storage or transport and to exclude children whose symptoms were due to non‐infectious causes.24
The outcomes examined were parental reported duration of symptoms (resolution being defined as the majority of symptoms resolving and the child being back to usual activities); prevalence and duration of medication use (over the counter [OTC] and prescription); diagnosis of secondary infections or complications, child absenteeism from playgroup, day care or school; parental absenteeism from work; and health care service use including hospitalisation, visits to general practitioners (GP) or other health care professionals and the performance of diagnostic tests or procedures.
2.5. Statistical analysis
Statistical analysis was performed using SPSS® (IBM Corp., Version 22, Armonk, NY) with a level of significance set at P < 0.05. Descriptive statistics were performed for baseline characteristics and for all study outcomes. Crude comparisons between children with influenza and other respiratory virus were initially performed using chi‐squared test for categorical variables and independent t tests for continuous variables. Separate multivariate regression models were used to evaluate the association between influenza status and study outcomes. Logistic regression was used for binary outcomes and linear regression for continuous variables. All models were controlled for a range of covariates including age, sex, race, comorbidities, prematurity, day care or school attendance >4 hours per week and influenza vaccination status.
The Princess Margaret Hospital for Children Ethics Committee (1673/EP), the South Metropolitan Area Health Service, the Western Australian Aboriginal Health Information and Ethics Committee and the University of Western Australia Ethics Committee (RA/4/1/6456) provided approval for the study.
3. RESULTS
A total of 1191 children were included in the analysis: 938 ED presentations and 233 hospitalisations. The highest enrolment occurred in 2011 (n = 243) and the lowest in 2010 (n = 101), peaking in August to September each year (Figure 1). Baseline sociodemographic and clinical characteristics of the study sample are shown in Table 1.
Figure 1.
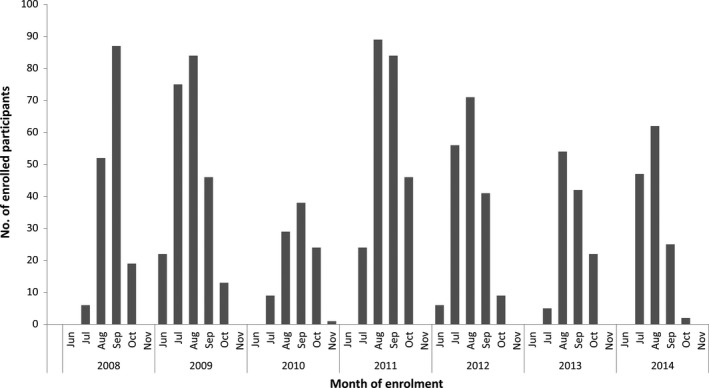
Participant enrolments by month and year, 2008‐14
Table 1.
Comparison of demographic and baseline characteristics between influenza‐positive, influenza‐negative and other respiratory virus‐positive children
| All participants (n = 1191) | Influenza‐positive (n = 238) | Influenza‐negative (n = 953) | Other respiratory virus‐positive (n = 670) | Comparison of influenza‐positive and other virus‐positive (P value) | |
|---|---|---|---|---|---|
| Age (y)a | 2.22 ± 1.27 | 2.85 ± 1.35 | 2.06 ± 1.20 | 1.99 ± 1.15 | <0.001 |
| Male gender | 690 (57.9) | 126 (52.9) | 564 (59.2) | 397 (59.3) | 0.091 |
| Aboriginal/Torres Strait Islander | 40 (3.4) | 11 (4.6) | 29 (3.1) | 17 (2.6) | 0.114 |
| Preterm (<37 wk) | 172 (14.5) | 29 (12.4) | 143 (15.1) | 98 (14.7) | 0.380 |
| Any comorbidityb | 174 (15.0) | 31 (13.7) | 143 (15.4) | 31 (13.7) | 0.929 |
| ≥4 h childcare/school/wk | 741 (62.6) | 169 (72.2) | 572 (60.3) | 402 (60.3) | 0.001 |
| No. of adults in householda | 2.09 ± 0.63 | 2.12 ± 0.68 | 2.08 ± 0.62 | 2.05 ± 0.62 | 0.253 |
| No. of children in householda | 1.93 ± 0.95 | 1.96 ± 0.96 | 1.92 ± 0.94 | 1.91 ± 0.93 | 0.515 |
| Smoker in household | 205 (17.5) | 38 (16.5) | 167 (17.7) | 125 (18.8) | 0.431 |
| Influenza vaccination status | |||||
| Unvaccinated | 853 (71.7) | 204 (86.1) | 649 (68.1) | 453 (67.6) | <0.001 |
| Partially vaccinatedc | 100 (8.4) | 10 (4.2) | 90 (9.4) | 68 (10.1) | |
| Fully vaccinatedd | 237 (19.9) | 23 (9.7) | 214 (22.5) | 149 (22.2) | |
Continuous variables expressed as mean ± SD.
Including asthma, congenital heart disease, chronic neurological conditions, chronic renal disease, chronic liver disease and inborn errors of metabolism.
Receipt of current years TIV and at least 2 doses in the current year or in preceding years.
Receipt of only one dose of TIV in the current year without meeting the definition of fully vaccinated.
Influenza was detected via PCR in 238 (20.0%), while 953 (80.0%) tested negative for influenza, of which 670 (70.3%) tested positive for another respiratory virus. Influenza detection varied significantly between years (P < 0.001). The lowest proportion of influenza positivity was seen in 2011 (13.2%) and highest in 2014 (30.9%; Figure 2).
Figure 2.
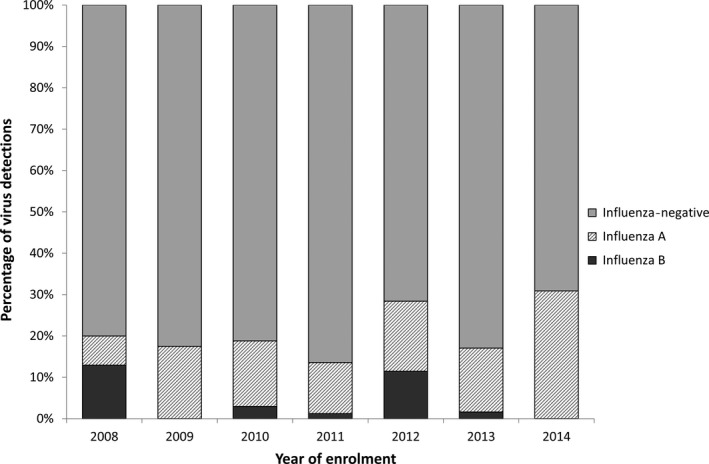
Influenza detections by year (proportion of respiratory samples), 2008‐14
Influenza A/H1, of which the majority was pandemic influenza A/H1N1‐09, was the most commonly detected (n = 117; 49.2% of influenza‐positive samples), followed by A/H3 (n = 72; 30.3%) and influenza B (n = 49 children; 20.6%, Figure 2). Only one child had more than one subtype of influenza isolated from the same sample (A/H1N1‐ 09 and A/H3). The most commonly detected other respiratory viruses were picornaviruses (inclusive of both human rhinoviruses and enteroviruses; n = 409, 34.3%) followed by RSV (n = 204, 17.1%; Figure 3). Co‐infection was common, with 240 children (20.2%) having two viruses detected and 54 (4.6%) having three or more viruses detected. Of children with influenza, co‐infection was observed in 79/238 (33.2%).
Figure 3.
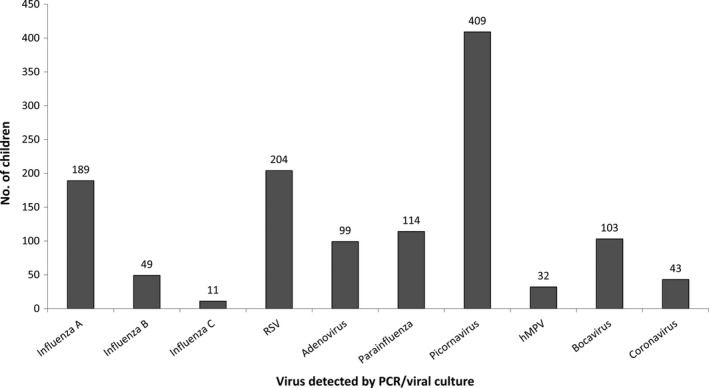
Influenza and other virus detections, 2008‐14
Influenza‐infected children were older (mean age 2.85 vs 1.99 years, P < 0.001), more likely to be unvaccinated against influenza (86.1% vs 67.6%, P < 0.001) and more likely to attend day care or school ≥4 hours a week (72.2% vs 60.3%, P = 0.001) compared with children with other respiratory viruses.
3.1. Impact of influenza and other respiratory viruses
Impact study outcomes are shown in Tables 2 and 3. As similar results were found, only the comparison between the influenza‐positive and other respiratory virus‐positive groups is shown. The mean duration of influenza illness was 9.54 days, compared to 8.50 days in other respiratory viruses (P = 0.005 [Table 3]).
Table 2.
Categorical variables: number and percentage of children in influenza‐positive, influenza‐negative and other respiratory virus‐positive groups and odds ratio comparing influenza‐positive and other respiratory virus‐positive groups
| Influenza‐positive (n = 238) n (%) | Influenza‐negative (n = 953) n (%) | Other respiratory virus‐positive (n = 670) n (%) | Unadjusted OR (95% CI) (influenza‐positive vs other virus‐positive) | Adjusted ORc (95% CI) (influenza‐positive vs other virus‐positive) | |
|---|---|---|---|---|---|
| Medication use | |||||
| Antipyreticsa | 209 (87.8) | 788 (82.7) | 551 (82.2) | 1.56 (1.01‐2.41) | 2.05 (1.24‐3.38) |
| Decongestant/cough medicine | 55 (22.3) | 117 (12.4) | 80 (12.1) | 2.21 (1.51‐3.23) | 1.82 (1.19‐2.80) |
| Antibiotics | 97 (40.9) | 394 (41.6) | 270 (40.6) | 1.01 (0.75‐1.37) | 0.94 (0.67‐1.31) |
| Secondary infections | |||||
| Pneumonia/chest infection | 17 (7.1) | 108 (11.3) | 70 (10.5) | 0.66 (0.38‐1.14) | 0.59 (0.33‐1.09) |
| Febrile convulsion | 5 (2.2) | 16 (1.7) | 9 (1.4) | 1.58 (0.52‐4.76) | 2.22 (0.61‐8.07) |
| Ear infection/otitis media | 25 (10.8) | 94 (10.1) | 75 (11.5) | 0.93 (0.58‐1.51) | 0.90 (0.53‐1.53) |
| Croup | 12 (5.2) | 26 (2.8) | 13 (2.0) | 2.69 (1.21‐5.98) | 3.53 (1.43‐8.70) |
| Any secondary complication | 68 (28.6) | 273 (28.6) | 191 (28.5) | 1.00 (0.72‐1.38) | 1.04 (0.72‐1.49) |
| Absenteeism | |||||
| Child (school/day care) | 155 (65.4) | 573 (60.6) | 414 (62.3) | 0.98 (0.66‐1.45) | 0.87 (0.56‐1.35) |
| Parent (work) | 109 (53.4) | 449 (55.2) | 329 (57.6) | 0.84 (0.61‐1.16) | 1.02 (0.70‐1.47) |
| Health service use | |||||
| Hospitalisation | 38 (16.0) | 247 (26.0) | 183 (27.5) | 0.50 (0.34‐0.74) | 0.61 (0.40‐0.93) |
| ≥2 ED visits | 45 (18.9) | 177 (18.7) | 120 (18.0) | 1.07 (0.73‐1.56) | 1.24 (0.80‐1.90) |
| GP visit | 155 (65.1) | 639 (67.2) | 455 (68.0) | 0.88 (0.64‐1.20) | 0.90 (0.64‐1.28) |
| ≥2 GP visits | 79 (33.1) | 296 (31.1) | 221 (33.0) | 1.11 (0.77‐1.60) | 1.13 (0.76‐1.70) |
| Paediatrician/other specialist visit | 7 (3.0) | 60 (6.3) | 41 (6.1) | 0.47 (0.21‐1.60) | 0.58 (0.24‐1.40) |
| ≥2 paediatrician visits | 0 (0.0) | 23 (2.4) | 14 (2.1) | ‐ | ‐ |
| Medical helpline callb | 56 (23.5) | 184 (19.3) | 126 (18.8) | 1.33 (0.93‐1.89) | 1.46 (0.99‐2.16) |
| ≥2 medical helpline calls | 16 (6.7) | 47 (4.9) | 37 (5.5) | 0.96 (0.48‐1.93) | 1.05 (0.50‐2.22) |
| Diagnostic tests/procedures | |||||
| Blood test | 27 (11.4) | 105 (11.1) | 70 (10.5) | 1.10 (0.69‐1.77) | 1.18 (0.69‐2.02) |
| NPA | 19 (8.1) | 102 (10.2) | 77 (11.5) | 0.67 (0.40‐1.14) | 0.98 (0.55‐1.75) |
| Chest X‐ray | 24 (10.2) | 142 (15.0) | 100 (15.0) | 0.64 (0.40‐1.03) | 1.72 (0.43‐1.20) |
| Lumbar puncture | 2 (0.8) | 6 (0.6) | 2 (0.3) | 2.85 (0.40‐20.3) | 1.59 (0.12‐20.99) |
Paracetamol and/or ibuprofen.
HealthDirect or Swine Flu Hotline (2009).
Adjusted for age, sex, Aboriginal and/or Torres Strait Islander status, any comorbidity, prematurity, attendance at day care/school >4 h/wk and TIV vaccination status.
Table 3.
Continuous variables: number and percentage of children in influenza‐positive, influenza‐negative and other respiratory virus‐positive groups and linear regression comparing influenza‐positive and other respiratory virus‐positive groups
| Influenza‐positive (n = 238) | Influenza‐negative (n = 953) | Other respiratory virus‐positive (n = 670) | Unadjusted linear regression (influenza‐positive vs other virus‐positive) | Multivariate linear regressiona (influenza‐positive vs other virus‐positive) | |||
|---|---|---|---|---|---|---|---|
| B (95% CI) | P value | B (95% CI) | P value | ||||
| Duration of ILI (d) | 9.54 ± 5.28 | 8.24 ± 4.79 | 8.50 ± 4.80 | 1.05 (0.25‐1.84) | 0.01 | 1.26 (0.37, 2.14) | 0.005 |
| Duration of medication use (d) | |||||||
| Paracetamol | 5.25 ± 3.04 | 4.51 ± 3.65 | 4.54 ± 3.92 | 0.72 (0.03‐1.40) | 0.042 | 0.90 (0.12, 1.67) | 0.023 |
| Ibuprofen | 4.91 ± 3.07 | 4.27 ± 3.09 | 4.24 ± 3.05 | 0.68 (−0.02‐1.38 | 0.058 | 0.75 (0.01, 1.49) | 0.046 |
| Decongestant/cough medicine | 5.32 ± 5.41 | 3.96 ± 2.49 | 4.17 ± 2.82 | 1.15 (−0.43‐2.73) | 0.153 | 0.09 (0.03, 0.14) | 0.003 |
| Antibiotics | 5.89 ± 3.04 | 7.22 ± 5.57 | 7.40 ± 6.39 | −1.51 (−2.98 to −0.04) | 0.044 | ‐1.09 (−2.63, 0.45) | 0.164 |
| Duration of absenteeism (h) | |||||||
| Child (school/day care) | 23.1 ± 19.8 | 17.3 ± 18.4 | 17.3 ± 18.4 | 5.78 (2.29‐9.26) | 0.001 | 4.79 (0.95, 8.63) | 0.015 |
| Parent (work) | 28.5 ± 27.8 | 23.0 ± 23.9 | 22.7 ± 24.1 | 5.77 (0.26‐11.29) | 0.040 | 8.24 (1.85, 14.62) | 0.012 |
| Duration of hospitalisation (d) | 2.28 ± 2.33 | 2.09 ± 1.82 | 2.10 ± 1.65 | 0.18 (−0.45‐0.81) | 0.571 | 0.57 (−0.61, 0.74) | 0.85 |
Adjusted for age, sex, Aboriginal and/or Torres Strait Islander status, any comorbidity, prematurity, attendance at day care/school >4 h/wk and TIV vaccination status.
The majority of children with influenza were given antipyretics (paracetamol and/or ibuprofen; 87.8%) with an average duration of 5.25 days and 4.91 days, respectively. Antibiotics were administered in 41% and decongestant/cough medicine in 22% (Tables 2, 3). Antipyretic and decongestant/cough medicines were used more frequently in children with influenza than with any other viruses (antipyretics: adjusted OR [aOR]: 2.05, 95% CI: 1.24‐3.38; decongestant aOR: 1.82, 95% CI: 1.19‐2.80 [Table 2]). Duration of antipyretic use was significantly longer in children with influenza, but this difference was <1 day of use (paracetamol B = 0.90, 95% CI 0.12‐1.67, P = 0.023; ibuprofen B = 0.75, 95% CI 0.01‐1.49, P = 0.046 [Table 3]). No significant difference was seen in either the odds or duration of antibiotic use. Antivirals (eg oseltamivir phosphate) were reported being used in only nine children: six with influenza and three children without.
Overall, 28.6% of influenza‐positive children were reported by parents to be diagnosed with a secondary infection, with otitis media most commonly reported (10.8%). The only significant difference between influenza and other virus‐positive groups was seen in the diagnosis of croup, being significantly higher in children with influenza (aOR: 3.53, 95% CI: 1.43‐8.70 [Table 2]).
Children with influenza were absent from school/day care in 65.4% of cases (mean 23.1 hours or approximately 2.9 days; Tables 2, 3), and parents of these children took time off work to care for the child in 53.4% of cases (mean 28.5 hours or approximately 3.5 workdays). Although no significant differences in the frequency of absenteeism were observed (Table 2), the duration of absenteeism was 4.79 hours longer in those with influenza (Table 3). Similarly, among parents who reported absenteeism, parents of children with influenza missed 8.24 hours more work (95% CI 1.85‐14.62) than parents of children with another respiratory virus (P = 0.012; Table 3).
Children with influenza visited ED on average 1.27 times (range: 1‐4 times, SD: 0.64) with 19% requiring more than one visit (Table 2). Two‐thirds of children with influenza were reported to have visited a GP or out of hours GP (Table 2), with over half (51.3%) of those visiting the GP requiring ≥2 visits. No differences were seen in the number and type of health care visit between influenza and other respiratory viruses. Hospitalisation occurred in 16% of influenza‐positive children with an average of 2.28 days (range: <24 hours to 9 days; SD: 2.33; Tables 2, 3). When all children were considered, children with influenza had lower odds than those with other respiratory viruses of requiring hospital admission (aOR: 0.61, 95% CI: 0.40‐0.93). Among children recruited in ED only, there was no significant difference between the proportion being hospitalised (11.6% of influenza‐positive children and 18.6% of other respiratory virus‐positive children; aOR: 0.71, 95% CI: 0.43‐1.17). There were no ICU admissions for children in either group.
Among children with influenza, 26.3% were reported to have a diagnostic test or procedure, including blood tests (11.4%) and chest X‐ray (10.2%). No significant differences were detected between exposure groups for the use of diagnostic tests or procedures (Table 2).
4. DISCUSSION
This is the first Australian study to quantify the impact of laboratory‐confirmed influenza infection in preschool children over several consecutive influenza seasons. Our findings illustrate that influenza causes a substantial impact on young children, their families and medical services. Although minor, differences exist between the impact of influenza and other common respiratory viruses presenting as ILI.
Mean duration of influenza illness was found to be 9.5 days, which was approximately 1 day longer than other respiratory viruses. The increased duration of the use of antipyretics and decongestant/cough medicine, and of child and parental absenteeism observed, is likely to be associated with the longer duration of illness.
The use of OTC medicines such as antipyretics, decongestant and cough medicines was frequently administered to minimise the discomfort of influenza, although they are unlikely to have an impact on the course of the illness.31 This study demonstrated that the use of antipyretics was very common, being used in 87.8% of children with influenza, for approximately 5 days. This finding is consistent with other studies, with the use of antipyretics in the existing literature ranging from 76.4%17 to 99.3%10 in influenza‐positive children, albeit with a shorter duration of use than demonstrated here (3.2‐3.9 days, respectively).10, 17 A lack of awareness or compliance with guidelines on the use of decongestant/cough medicines in children was noted. Decongestant/cough medicines should not be given to children under 2 years.32 Nevertheless, such medicines were given to 9% of children with ILI and 14% of children with influenza under 2 years. Similarly in 2013/14, 6% of children with ILI and 8% of children with influenza were given these medicines despite the contraindication being extended to include children under 6 years.33
Among children with influenza, 40.9% were treated with antibiotics despite only 42.3% of those treated having a reported bacterial infection. There are significant implications associated with inappropriate prescription of antibiotics, including a significant cost, unnecessary adverse side effects34 and the increasing prevalence of antibiotic resistance.35 In contrast, the infrequent use of antivirals highlights that empiric antiviral therapy, as opposed to antibacterial therapy, is uncommon in WA, particularly those seen in the emergency department. Of the nine children prescribed oseltamivir, six were shown to have influenza and three did not. Only 13% of children hospitalised with influenza were treated with antivirals. The use of rapid diagnostic tests, which are infrequently used in Australia, may increase the use of antivirals, decrease antibiotic prescriptions and reduce the influenza burden in those children at risk of severe disease and mortality.36, 37
A substantial social impact of influenza on the family was demonstrated with 65.4% of children having time off school or day care for an average of 3 days, and 53.4% of parents having time off work to care for the ill child. It was also demonstrated that of the children with influenza who attended school or day care, 77.6% were reported to have at least one absence. As the employment status of parents was unknown, it was not possible to determine the proportion of working parents who were absent, but in families with two working parents, the social impact is likely to be greater than in families with one or no working parents. The duration of absence estimate is somewhat higher than in the existing literature, with estimates by Tsolia et al and Principi et al14, 17 being 1.3 days and 1.2 days. These differences may be attributable to variations in the working patterns or in the virulence of the influenza strains circulating between the populations studied.
Multiple visits to medical practitioners were required in many cases: around two‐thirds of the children with influenza who presented to ED were taken to the GP for the same illness and a third required ≥2 GP visits. In addition, around one‐fifth required two or more ED visits. Those re‐presenting to ED were significantly younger both among those with influenza and overall (data not shown). This represents a high burden on health services with associated economic costs. The rate of hospitalisation for children with influenza and other viruses must be interpreted with caution, as children presenting to ED and hospitalised were both actively recruited. The higher rate of hospitalisation seen with other respiratory viruses was not significant when only children enrolled in ED prior to hospitalisation were included. Despite the limitations, these data are still useful in comparing the impact between influenza and non‐influenza ILI, and also for ascertaining average duration of hospitalisation among children admitted with influenza, which was found to be 2.23 days.
As the study population was limited to children presenting to ED or hospitalised at a single metropolitan tertiary hospital, there are limitations on the generalisability of the findings. Enrolled children had a higher rate of comorbidities and prematurity than the general population. For example, while only 8.5% of babies born in Australia were preterm in 2012,38 14.5% of our sample were reported as preterm. Also, parents of children who were severely unwell may not have been approached by research assistants or less willing to participate, thereby affecting the overall disease severity of the sample. Other limitations included data collection being via parental report rather than through medical record verification, decreasing accuracy of the reported clinical outcomes. Additionally, due to clinical duty of care, parents were informed whether their child was influenza‐positive or influenza‐negative prior to completion of the second questionnaire, which may have affected the reported outcomes.
These data indicate a high impact of influenza illness in WA children and can be used to re‐assess the current economic impact of influenza in young children in Australia.15, 39
5. CONCLUSION
This study demonstrates a significant impact of influenza in young children in WA, in particular showing the high use of antipyretics, antibiotics, common diagnosis of otitis media and high rates of absenteeism for both child and their parents, and the high use of outpatient health care services. In addition, these data demonstrate that influenza has a greater impact than other respiratory viruses causing ILI in terms of the use of antipyretic and decongestant/cough medicines, the diagnosis of croup, the duration of the illness, the duration of absence from school/day care for the child and the duration of absence from work for the parent. Demonstrated preventative strategies, such as universal influenza immunisation in young children, are therefore likely to have a significant impact and should be examined as a priority for inclusion on the Australian immunisation schedule.
CONFLICT OF INTEREST
Gabriela Willis, Peter Richmond and Christopher Blyth are members of the Vaccine Trials Group, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute. The Vaccine Trials Group has received funding from vaccine manufacturers for conducting clinical trials, and for travel support to attend immunisation conferences, although not in relation to this study.
Gabriela Willis has received travel support from Pfizer Australia to present the findings of this research at a scientific meeting.
Peter Richmond has previously served on scientific advisory boards regarding influenza vaccines for CSL Ltd. Sanofi, Janssen and GlaxoSmithKline (though has not received personal honorarium), has received travel support from Baxter and GlaxoSmithKline to present at scientific meetings and received institutional funding for investigator‐led research from GlaxoSmithKline and CSL Ltd.
David Smith is a director and board member for the Asia‐Pacific Alliance for the Control of Influenza. It is a not‐for‐profit organisation controlled by an independent board that receives pharmaceutical company funding. He does not receive any payment, only reimbursement of expenses. Prof Smith was a director and board member of a similar organisation, the Australian Influenza Specialist Group, until 36 months ago and has received reimbursement for expenses for attendance at their Annual Scientific Meeting. The other authors have no conflict of interests relevant to this article to disclose.
AUTHOR CONTRIBUTIONS
Dr Willis assisted in designing the study, cleaned and analysed the data and wrote the first draft of the manuscript. Dr Blyth supervised the project, analysed the data and assisted in writing the manuscript. Professor Preen supervised data cleaning and analysis, and assisted in writing the manuscript. Mr Jacoby assisted in designing the study, supervised the analysis and assisted with writing the manuscript. Professors Effler, Smith and Richmond designed the study, supervised analysis and assisted in writing the manuscript. Ms Robins enrolled participants, supervised research assistants, collated and cleaned the data and assisted with writing the manuscript. Dr Borland supervised the enrolment of participants and assisted with writing the manuscript. Dr Levy coordinated virologic studies, analysed results, collated and cleaned the data and assisted with writing the manuscript. Dr Keil assisted in designing the study, supervised laboratory processing and assisted with writing the manuscript; and all authors reviewed and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
CLINICAL TRIAL REGISTRATION
None.
ACKNOWLEDGEMENTS
The Western Australian Influenza Vaccine Effectiveness (WAIVE) study team includes Christopher Blyth, Meredith Borland, Dale Carcione, Paul Effler, Cazz Finucane, Gary Geelhoed, Peter Jacoby, Anthony Keil, Heath Kelly, Jennifer Kent, Alan Leeb, Avram Levy, Katie Lindsay, Hannah Moore, Christine Robins, Peter Richmond, David Smith, Simone Tempone, Paul van Buynder, Simon Williams and Gabriela Willis.
The authors thank all the nurses and research assistants of the Vaccine Trials Group who recruited children for this study as well as all the study participants and their parents. The authors also thank staff of the Emergency, General Pediatrics and Microbiology Departments at Princess Margaret Hospital for Children, Perth, WA. The authors thank all staff from PathWest Laboratory Medicine, WA, involved in processing and reporting study samples.
Dr Blyth is supported by a NHMRC Career Development Fellowship (APP1111596) and a Western Australian Health/Raine Medical Research Foundation Clinician Research Fellowship.
Trivalent influenza vaccination was kindly provided for the Western Australian Preschool Vaccination Program by Sanofi‐Pasteur (2008‐2012) and CSL Biotherapies (bioCSL; 2008‐2010).
The WAIVE study is funded by the Department of Health of Western Australia.
Willis GA, Preen DB, Richmond PC, et al; on behalf of the WAIVE Study Team . The impact of influenza infection on young children, their family and the health care system. Influenza Other Respi Viruses. 2019;13:18–27. 10.1111/irv.12604
Funding information
The Department of Health of Western Australia.
REFERENCES
- 1. Fraaij PL, Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine. 2011;29(43):7524‐7528. [DOI] [PubMed] [Google Scholar]
- 2. Chiu C, Dey A, Wang H, et al. Vaccine preventable diseases in Australia, 2005 to 2007. Commun Dis Intell Q Rep. 2010;34(Supp):S1‐S167. [PubMed] [Google Scholar]
- 3. Monto AS. Epidemiology of influenza. Vaccine. 2008;26(Suppl 4):D45‐D48. [DOI] [PubMed] [Google Scholar]
- 4. Conway NT, Wake ZV, Richmond PC, et al. Clinical predictors of influenza in young children: the limitations of “influenza‐like illness”. J Pediatric Infect Dis Soc. 2013;2(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muscatello DJ, Newall AT, Dwyer DE, Macintyre CR. Mortality attributable to seasonal and pandemic influenza, Australia, 2003 to 2009, using a novel time series smoothing approach. PLoS ONE. 2014;8(6):e64734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heikkinen T, Silvennoinen H, Peltola V, et al. Burden of influenza in children in the community. J Infect Dis. 2004;190(8):1369‐1373. [DOI] [PubMed] [Google Scholar]
- 7. Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355(1):31‐40. [DOI] [PubMed] [Google Scholar]
- 8. Antonova EN, Rycroft CE, Ambrose CS, Heikkinen T, Principi N. Burden of paediatric influenza in Western Europe: a systematic review. BMC Public Health. 2012;12:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilkes JJ, Leckerman KH, Coffin SE, et al. Use of antibiotics in children hospitalized with community‐acquired, laboratory‐confirmed influenza. J Pediatr. 2009;154(3):447‐449. [DOI] [PubMed] [Google Scholar]
- 10. Esposito S, Cantarutti L, Molteni CG, et al. Clinical manifestations and socio‐economic impact of influenza among healthy children in the community. J Infect. 2011;62(5):379‐387. [DOI] [PubMed] [Google Scholar]
- 11. Esposito S, Gasparini R, Bosis S, et al. Clinical and socio‐economic impact of influenza and respiratory syncytial virus infection on healthy children and their households. Clin Microbiol Infect. 2005;11(11):933‐936. [DOI] [PubMed] [Google Scholar]
- 12. Esposito S, Marchisio P, Bosis S, et al. Clinical and economic impact of influenza vaccination on healthy children aged 2‐5 years. Vaccine. 2006;24(5):629‐635. [DOI] [PubMed] [Google Scholar]
- 13. Salo H, Kilpi T, Sintonen H, Linna M, Peltola V, Heikkinen T. Cost‐effectiveness of influenza vaccination of healthy children. Vaccine. 2006;24(23):4934‐4941. [DOI] [PubMed] [Google Scholar]
- 14. Tsolia MN, Logotheti I, Papadopoulos NG, et al. Impact of influenza infection in healthy children examined as outpatients and their families. Vaccine. 2006;24(33–34):5970‐5976. [DOI] [PubMed] [Google Scholar]
- 15. Newall AT, Scuffham PA. Influenza‐related disease: the cost to the Australian healthcare system. Vaccine. 2008;26(52):6818‐6823. [DOI] [PubMed] [Google Scholar]
- 16. Yin JK, Salkeld G, Lambert SB, et al. Estimates and determinants of economic impacts from influenza‐like illnesses caused by respiratory viruses in Australian children attending childcare: a cohort study. Influenza Other Respir Viruses. 2013;7(6):1103‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Principi N, Esposito S, Gasparini R, Marchisio P, Crovari P, Flu‐Flu Study G . Burden of influenza in healthy children and their households. Arch Dis Child. 2004;89(11):1002‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Iskander M, Kesson A, Dwyer D, et al. The burden of influenza in children under 5 years admitted to the Children's Hospital at Westmead in the winter of 2006. J Paediatr Child Health. 2009;45(12):698‐703. [DOI] [PubMed] [Google Scholar]
- 19. Khandaker G, Zurynski Y, Ridley G, et al. Clinical epidemiology and predictors of outcome in children hospitalised with influenza A(H1N1)pdm09 in 2009: a prospective national study. Influenza Other Respir Viruses. 2014;8(6):636‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lambert SB, Allen KM, Druce JD, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool‐aged children using parent‐collected specimens. Pediatrics. 2007;120(4):e929‐e937. [DOI] [PubMed] [Google Scholar]
- 21. Australian Government Department of Health . Immunise Australia Program: Free Vaccines for Aboriginal and Torres Strait Islander Children this Flu Season. [Internet]. 2015; http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/news-20151405. Accessed 9 July, 2015.
- 22. Blyth CC, Jacoby P, Effler PV, et al. Influenza vaccine effectiveness and uptake in children at risk of severe disease. Pediatr Infect Dis J. 2016;35(3):309‐315. [DOI] [PubMed] [Google Scholar]
- 23. Blyth CC, Macartney KK, Hewagama S, et al. Influenza epidemiology, vaccine coverage and vaccine effectiveness in children admitted to sentinel Australian hospitals in 2014: the Influenza Complications Alert Network (FluCAN). Euro Surveill. 2016;21(30):pii: 30301. 10.2807/1560-7917.ES.2016.21.30.30301. [DOI] [PubMed] [Google Scholar]
- 24. Blyth CC, Jacoby P, Effler PV, et al. Effectiveness of trivalent flu vaccine in healthy young children. Pediatrics. 2014;133(5):e1218‐e1225. [DOI] [PubMed] [Google Scholar]
- 25. Dixon GA, Moore HC, Kelly H, et al. Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory‐confirmed influenza in hospitalised children aged 6‐59 months. Influenza Other Respir Viruses. 2010;4(4):231‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelly H, Jacoby P, Dixon GA, et al. Vaccine effectiveness against laboratory‐confirmed influenza in healthy young children: a case‐control study. Pediatr Infect Dis J. 2011;30(2):107‐111. [DOI] [PubMed] [Google Scholar]
- 27. Australian Bureau of Statistics. Regional Population Growth , 2014‐15, Western Australia. [Internet]. 2016; http://www.abs.gov.au/AUSSTATS/abs@.nsf/Latestproducts/3218.0Main%20Features402014-15?opendocument%26tabname=Summary%26prodno=3218.0%26issue=2014-15%26num=%26view=. Accessed 15 Sep, 2016.
- 28. Chidlow G, Harnett G, Williams S, Levy A, Speers D, Smith DW. Duplex real‐time reverse transcriptase PCR assays for rapid detection and identification of pandemic (H1N1) 2009 and seasonal influenza A/H1, A/H3, and B viruses. J Clin Microbiol. 2010;48(3):862‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chidlow GR, Harnett GB, Shellam GR, Smith DW. An economical tandem multiplex real‐time PCR technique for the detection of a comprehensive range of respiratory pathogens. Viruses. 2009;1(1):42‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chidlow GR, Harnett GB, Williams SH, et al. The detection of oseltamivir‐resistant pandemic influenza A/H1N1 2009 viruses using a real‐time RT‐PCR assay. J Virol Methods. 2010;169(1):47‐51. [DOI] [PubMed] [Google Scholar]
- 31. Klepser ME. Socioeconomic impact of seasonal (epidemic) influenza and the role of over‐the‐counter medicines. Drugs. 2014;74(13):1467‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Australian Government Department of Health: Therapeutic Goods Administration . Cough and cold medicines in children. [Internet]. 2008; https://www.tga.gov.au/media-release/cough-and-cold-medicines-children. Accessed 23 June, 2015.
- 33. Australian Government Department of Health: Therapeutic Goods Administration . OTC cough and cold medicines for children‐ Final outcomes of TGA review. [Internet]. 2012; https://www.tga.gov.au/otc-cough-and-cold-medicines-children-final-outcomes-tga-review. Accessed 23 June, 2015.
- 34. Royal Children's Hospital Melbourne . About your medication: Phenoxymethylpenicillin or amoxycillin. [Internet]. 2010; http://www.rch.org.au/uploadedFiles/Main/Content/pharmacy/Amoxycillin_and_Phenoxymethylpenicillin.pdf. Accessed 9 July, 2015.
- 35. Tenover FC, Hughes JM. The challenges of emerging infectious diseases. Development and spread of multiply‐resistant bacterial pathogens. JAMA. 1996;275(4):300‐304. [PubMed] [Google Scholar]
- 36. Blyth CC, Booy R, Dwyer DE. Point of care testing: diagnosis outside the virology laboratory. Methods Mol Biol. 2011;665:415‐433. [DOI] [PubMed] [Google Scholar]
- 37. Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision‐making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363‐367. [DOI] [PubMed] [Google Scholar]
- 38. Hilder L, Zhichao Z, Parker M, Jahan S, Chambers G. Australia's Mothers and Babies 2012 [Internet]. Canberra, ACT: AIHW; 2014. [Google Scholar]
- 39. Newall AT, Jit M, Beutels P. Economic evaluations of childhood influenza vaccination: a critical review. Pharmacoeconomics. 2012;30(8):647‐660. [DOI] [PubMed] [Google Scholar]


