Abstract
Sepsis is a life-threatening condition caused by infection and represents a substantial global health burden. Recent epidemiological studies showed that sepsis mortality rates have decreased, but that the incidence has continued to increase. Although a mortality benefit from early-goal directed therapy (EGDT) in patients with severe sepsis or septic shock was reported in 2001, three subsequent multicenter randomized studies showed no benefits of EGDT versus usual care. Nonetheless, the early administration of antibiotics and intravenous fluids is considered crucial for the treatment of sepsis. In 2016, new sepsis definitions (Sepsis-3) were issued, in which organ failure was emphasized and use of the terms “systemic inflammatory response syndrome” and “severe sepsis” was discouraged. However, early detection of sepsis with timely, appropriate interventions increases the likelihood of survival for patients with sepsis. Also, performance improvement programs have been associated with a significant increase in compliance with the sepsis bundles and a reduction in mortality. To improve sepsis management and reduce its burden, in 2017, the World Health Assembly and World Health Organization adopted a resolution that urged governments and healthcare workers to implement appropriate measures to address sepsis. Sepsis should be considered a medical emergency, and increasing the level of awareness of sepsis is essential.
Keywords: Compliance, Mortality, Sepsis, Treatment
Introduction
Sepsis is a major cause of death from infection and represents a substantial healthcare burden, accounting for 6.2% of total hospital costs in the United States 20111. The estimated annual incidence of sepsis in the United States was 751,000 cases (3 cases/1,000 population) and the estimated number of deaths was 215,0002. Recent large-scale epidemiological studies showed that the mortality rate of sepsis has decreased but its incidence continues to increase3,4. However, the true incidence of sepsis is likely to be underestimated.
On May 2017, the World Health Assembly (WHA) and World Health Organization (WHO) made sepsis a global health priority and adopted a resolution that urged the 194 United Nations Member States to improve the prevention, diagnosis, and management of sepsis5. Accordingly, to improve patient outcomes, strategies that incorporate early recognition and timely management of sepsis in hospitals are being implemented5,6,7,8.
In 2001, Rivers et al.9 reported the groundbreaking study on early-goal directed therapy (EGDT). However, three subsequent multicenter randomized controlled trials (RCTs) did not show that EGDT reduced the sepsis mortality rate compared to usual care10,11,12. Recently, new sepsis definitions were issued by the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) for screening and early identification. However, their benefits have yet to be validated by prospective studies3,4,13,14, and experts continue to place emphasis on the early administration of antibiotics and fluids for the initial resuscitation of patients with sepsis.
Definitions and Early Identification of Sepsis
1. Change in sepsis definitions
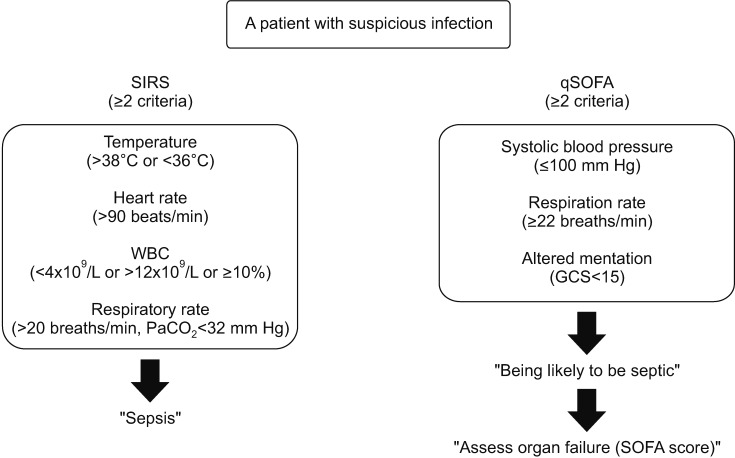
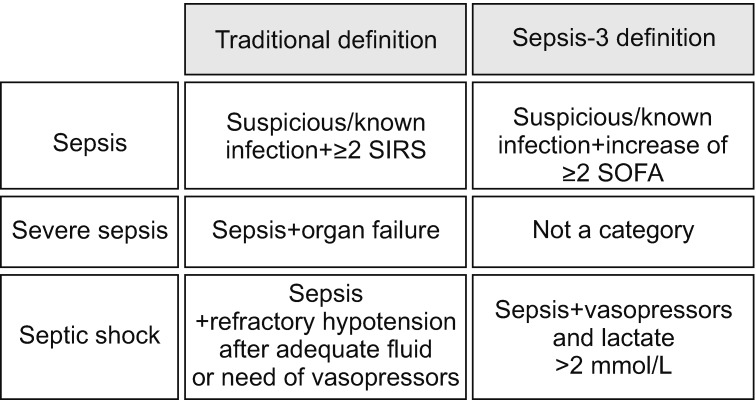
The definition of sepsis has changed several times since 199215,16. The SCCM and ESICM revised the definition of sepsis and septic shock in 2016. The new definitions (Sepsis-3) focused on a dysregulated host response to infection and organ dysfunction. Sepsis is defined as infected patients with an increase of ≥2 Sequential Organ Failure Score (SOFA) points17. Septic shock is defined as refractory hypotension requiring vasopressors with concurrent hyperlactemia (>2 mmol/L) despite adequate fluid resuscitation (Figure 1). Severe sepsis was excluded from the guidelines, and quick SOFA (qSOFA), instead of the systemic inflammatory response syndrome (SIRS), was adopted for screening purposes (Figure 2).
Figure 1. Definitions for SIRS and qSOFA. SIRS: systemic inflammatory response syndrome; qSOFA: Sequential Organ Failure Assessment; WBC: white blood cell.
Figure 2. Comparison of traditional and revised (Sepsis-3) definitions for sepsis. SIRS: systemic inflammatory response syndrome; SOFA: Sequential Organ Failure Assessment.
The Sepsis-3 definitions were based on a large database and were the first to be tested in derivation and validation datasets. However, the definitions were not endorsed by some organizations and there are several issues associated with them6. First, lactate was not retained in the sepsis definition. Hence, by the Sepsis-3 definitions, patients with an increased lactate level but no hypotension (or compensated septic shock) can be missed. In other words, we may miss patients in the early phase of sepsis. The prevalence of this phenotype (i.e., normotensive patients with hyperlactemia) was 26% in a previous multicenter trial11. In the Sepsis-3 datasets, the prevalence of normotensive hyperlactemia (>4 mmol/L) was 9.9% but their mortality rate was not low (29.9%). Therefore, the validity of the Sepsis-3 definitions is suspect. Second, using the Sepsis-3 definitions, two components (the need for vasopressors and hyperlactemia) are needed concurrently to diagnose septic shock. That is, the lactate level is not a component of the definitions until the patient becomes hypotensive. Also, an infected patient with hypotension might not be considered to be in septic shock unless the lactate level was known. This implies that the utility of the Sepsis-3 definitions is limited in low-resource settings, where lactate levels are not frequently available. Therefore, further prospective studies are needed to demonstrate the validity of the Sepsis-3 definitions. Until then, it seems acceptable to use the pre-existing sepsis definitions.
2. Sepsis screening
Sepsis screening is reportedly associated with a decreased mortality rate18,19. The surviving sepsis campaign (SSC) guidelines of 2016, as well as those of 2012, emphasize routine screening of potentially infected patients who are likely to be septic to improve the early identification and treatment of sepsis. They recommend that hospitals should have a performance improvement program that involves early recognition and management of sepsis8.
The SIRS criteria have, since 1992, been used to screen and identify sepsis patients20. To diagnose sepsis, at least two of the four SIRS criteria must be met. However, because SIRS can be triggered by a variety of infectious and noninfectious causes, it is insufficiently sensitive, and certainly not specific for sepsis. Hence, some patients who satisfy the SIRS criteria may not have sepsis, and vice versa21. In this context, the Sepsis-3 Task Force discarded the concept of SIRS and introduced, instead, qSOFA for sepsis screening17. The qSOFA is a simplified version of the SOFA score that comprises only three variables, and patients with a qSOFA score of ≥2 should be considered for the possibility of sepsis (Figure 2). The qSOFA is a readily available bedside tool without laboratory tests, and has better performance in non-intensive care unit (ICU) than ICU settings (area under the curve value, 0.81 vs. 0.66)13. The Sepsis-3 Task Force recommended that it be used to identify infected patients outside the ICU who are likely to be septic. However, a recent prospective study showed that a qSOFA score of ≥2 has high specificity (96.1% vs. 61.0% for SIRS ≥2) for organ dysfunctions but its poor sensitivity (29.7% vs. 72.1% for SIRS ≥2) may limit its use as a bedside tool22. The authors insisted that the SIRS criteria can be still useful.
Clinical evidence indicates that patients with acute deterioration or sepsis manifest clinical signs or symptoms several hours before the condition worsens. Early warning scores, such as the Modified Early Warning Score (MEWS), Early Warning Scoring System (EWSS), or National Early Warning Score (NEWS), were developed to screen patients at high risk of deterioration23,24,25,26. Although strong evidence based on robust data is lacking, these scores showed a trend toward improved outcomes and, when coupled with an outreach service (i.e., rapid response teams or medical emergency teams), it facilitates timely initiation of the optimal treatments upon recognition of septic patients25. Although respiratory or cardiac problems were the most common trigger for activations of such outreach teams27,28, one study showed that sepsis as a cause of activations accounted for 19.9%, and EGDT was undertaken in 22.7%28. Interestingly, Churpek et al.29 compared several early warning scores, including qSOFA, among patients outside the ICU. qSOFA had a higher specificity and lower sensitivity than SIRS, MEWS, and NEWS for predicting in-hospital death or ICU transfer. The SIRS criteria (≥2) were more rapid than qSOFA for identifying patients. Therefore, use of qSOFA may be premature, and the SIRS criteria are a sensitive and useful bedside tool for sepsis screening outside the ICU.
Early Treatments and Optimal Resuscitation
The EGDT was designed for the early detection of sepsis and timely optimization of hemodynamic parameters by continuous monitoring of central venous oxygen saturation (ScvO2, >70%), central venous pressure (8–12 mm Hg), mean arterial pressure (MAP, ≥65 mm Hg), and urine output (>0.5 mL/kg/h)6,9. This protocolized treatment, when administered to patients with severe sepsis or septic shock before admission to the ICU, reduced the incidence of multi-organ dysfunction and significantly decreased the in-hospital mortality rate compared with standard care9.
However, three international multicenter trials (Protocolized Care for Early Septic Shock [ProCESS]12, Australasian Resuscitation in Sepsis Evaluation [ARISE]11, and Protocolized Management in Sepsis [ProMISe]30) did not show any significant survival benefit compared to usual care (Table 1). Also, in a meta-analysis of individual participants in the three RCTs, the EGDT did not result in better outcomes than usual care but was associated with increased hospitalization costs31. Therefore, the EGDT concept was weakened in the 2016 guidelines8. However, initial fluid resuscitation with crystalloids is still being emphasized, and patients in the usual care groups received a considerable volume of fluids in these three RCTs (Table 1). Application of balanced crystalloids significantly decreased the rates of all-cause mortality, persistent renal insufficiency, and new dialysis treatments, compared to saline32. However, the 2016 guidelines emphasize, instead, the re-evaluation of volume status and tissue perfusion after the initial fluid resuscitation. This is because the persistence of a positive daily fluid balance over time was strongly associated with a higher mortality rate in patients with sepsis33. In this regard, the guidelines have recommended either repeated assessments of vital signs, cardiopulmonary status, capillary refill time, pulse, and skin findings, or measurement of the two of followings: CVP, ScvO₂, bedside cardiovascular ultrasound, and dynamic assessment of fluid responsiveness with passive leg raise or fluid challenge.
Table 1. Comparison of enrollment, treatment, and outcome of EGDT studies.
| EGDT | ProCESS | ARISE | ProMISe | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EGDT | Control | EGDT | Protocol-based standard Therapy | Usual care | EGDT | Usual care | EGDT | Usual care | |
| Location | United States | United States | Australia, New Zealand, Finland, Ireland, and Hong Kong | United Kingdom | |||||
| Enrolled patients | 130 | 133 | 439 | 446 | 456 | 793 | 798 | 625 | 626 |
| Age, yr | 67.1±17.4 | 64.4±17.1 | 60±16.4 | 61±16.1 | 62±16.0 | 62.7±16.4 | 63.1±16.5 | 66.4±14.6 | 64.3±15.5 |
| APACHE II score (baseline) | 21.4±6.9 | 20.4±7.4 | 20.8±8.1 | 20.6±7.4 | 20.7±7.5 | 15.4±6.5 | 15.8±6.5 | 18.7±7.1 | 18.0±7.1 |
| Arterial catheter insertion, % | Required | Required | Required | - | - | 91.4 | 76.3 | 74.2 | 62.2 |
| Central vein catheterization, % | Required | Required | 93.6 | 56.5 | 57.9 | 90 | 61.9 | 92.1 | 50.9 |
| Initial lactate >4 mmol/L , % | 79 | 59 | 59.2 | 60.7 | 46 | 46.5 | 65.4 | 63.7 | |
| Total fluid within 6 hr, mL | 4,981±2,984 | 3,499±2,438 | 5,059 | 5,511 | 4,362 | 4,479 | 4,304 | 4,100 | 4,074 |
| Fluid prior to randomization (at ED)* | - | - | 2,254±1,472 | 2,226±1,363 | 2,083±1,405 | 2,515±1,244 | 2,591±1,331 | 1,600 (1,000–2,500) | 1,790 (1,000–2,500) |
| Use of vasopressors within 6 hr, % | 27.4 | 30.3 | 54.9 | 52.2 | 44.1 | 66.6 | 57.8 | 53.3 | 46.6 |
| RBC transfusion within 6 hr, % | 64.1 | 18.5 | 14.4 | 8.3 | 7.5 | 13.6 | 7 | 8.8 | 3.8 |
| Length of ICU stay, day* | - | - | 5.1±6.3 | 5.1±7.1 | 4.7±5.8 | 2.8 (1.4–5.1) | 2.8 (1.5–5.7) | 2.6 (1.0–5.8) | 2.2 (0.0–5.3) |
| Hospital mortality, % | 30.5 | 46.5 | 21 | 18.2 | 18.9 | 14.5 | 15.7 | 25.6 | 24.6 |
*Mean±standard deviation or median (interquartile range).
EGDT: early goal-directed therapy; ProCESS: Protocolized Care for Early Septic Shock; ARISE: Australasian Resuscitation in Sepsis Evaluation; ProMISe: Protocolized Management in Sepsis; APACHE II: Acute Physiology and Chronic Health Evaluation II; ED: emergency department; RBC: red blood cell; ICU: intensive care unit.
A population-based study in the United States reported that early central vein catheterization was associated with a lower in-hospital mortality rate34. However, the three RCTs of EGDT highlighted that there was no benefit of invasive hemodynamic monitoring involving CVP and ScvO2 if initial fluids and adequate antibiotics were administered to septic patients in a timely manner. Accordingly, the 2016 SSC guidelines do not contain pre-specified treatment CVP and ScvO2 targets. CVP does not reflect intravascular volume status precisely and is not predictive of the fluid response35,36. Instead, repeated measurements of lactate (i.e., lactate clearance) enables evaluation of the responsiveness to initial resuscitation37, and echocardiography is a noninvasive method of assessing the volume status in patients on mechanical ventilator support38.
Delayed administration of empirical antibiotics after sepsis identification increases the in-hospital mortality rate5,7. Liu et al.39 recently reported that a 1-hour delay in antibiotic initiation was associated with an increased odds of in-hospital mortality among patients who received antibiotics within 6 hours. Therefore, the SSC guidelines recommend the intravenous administration of empiric antibiotics after obtaining blood culture results within 1 hour. Treatment with one or two broad-spectrum antibiotics and early de-escalation after clinical improvement or pathogen non-detection are recommended8.
Early administration of vasopressors is associated with an increased survival rate in patients with septic shock40 and is a component of the 6-hour sepsis bundle. Thus, norepinephrine, as the first choice, should be administered early to maintain a MAP ≥65 mm Hg, when hypotension does not respond to initial fluid resuscitation. In an open-label RCT, targeting a MAP of 80–85 mm Hg rather than 65–70 mm Hg did not increase the survival rate of patients with septic shock41. Thus, the target MAP should be determined according to the patients' condition; a higher target may be needed for patients with chronic hypertension and a lower target for those with uncontrolled bleeding with trauma42.
The SSC guidelines do not include a target heart rate for patients with septic shock43. However, due to the many adverse effects of tachycardia, such as diastolic dysfunction and myocardial ischemia, the heart rate should be maintained within the normal range in patients with septic shock44. Recently, Morelli et al.45,46 demonstrated that esmolol can be safely used to reduce the heart rate (target rate, 80–94 beats/min), without increased adverse effects, and was associated with a reduced dose of norephinephrine and a lower mortality rate in patients with septic shock compared to the the controls. These results should be verified by further large-scale studies.
Further Efforts to Decrease the Sepsis-Related Mortality Mate
1. Increasing sepsis awareness
Although sepsis dates back to at least the time of Hippocrates, the term “sepsis” is not well known47. This has led to avoidable mortality and morbidity worldwide. On May 24, 2017, Sir Liam Donaldson, the WHO envoy for patient safety, reported that awareness of sepsis by the public and politicians must be increased during a WHA Side Event on sepsis48. He said further that sepsis is an important issue that has been addressed effectively by clinicians and scientists, but is invisible to the public, political leaders, and leaders of healthcare systems.
Increased awareness, which leads to early presentation to hospital, can decrease the sepsis mortality rate by enabling timely and appropriate treatment. Experts recommend that the term “sepsis” be used frequently by healthcare professionals and patients to increase the level of awareness of the general public49. However, despite the significant impact, public awareness is currently very low; a survey in 2009 reported that 88% of respondents had never heard the term “sepsis”50. Since 2012, the Global Sepsis Alliance (GSA) has organized the annual World Sepsis Day, and many organizations or countries are undertaking sepsis awareness campaigns51. In healthcare facilities, continuous training and education are also needed to increase the level of awareness of sepsis on the part of healthcare providers, who must understand that the condition is a real medical emergency.
Most estimates of sepsis are based on studies in high-income countries, and data on low- and middle-income (or resource-limited) countries are scarce. So, increasing the awareness of sepsis is essential for controlling the sepsis burden in those countries52,53. In South Korea, on “World Sepsis Day”, annual symposia and field events have taken place since 201254. However, further promotional or educational activities for the public, as well as support from the political leadership, are required to improve the situation.
2. Building a sepsis registry
Sepsis is frequently handled like a “garbage code” in the Global Burden of Disease Statistics. Most deaths due to sepsis are classified as their underlying infections, rather than sepsis itself48. Therefore, the burden of sepsis is likely to be underestimated. Hence, improving the coding process for sepsis may facilitate estimation of the true burden.
Nationwide (or statewide) registry data collected prospectively can facilitate accurate estimation of the incidence of sepsis, which can be used to improve performance and formulate future policies. A good example is the New York State sepsis registry. Beginning in 2014, all hospitals in New York State adopted sepsis protocols based on Rory's regulations for the early diagnosis and treatment of sepsis5,48. They are required to report their performance (compliance), as well as clinical information, to the New York State Department of Health5,48. Surprisingly, after the onset of the initiative, the average rate of protocol compliance has increased progressively from 73.7% in the second quarter of 2014 to 84.7% of adult sepsis patients in the third quarter of 20165. This compliance is in contrast to Asian countries; a large multinational study of Asian ICUs reported rates of compliance with the resuscitation and management bundles of 7.6% and 3.5%, respectively55.
The Core Outcome and Resource Evaluation (CORE) committee is a component of the Australia and New Zealand Intensive Care Society (ANZICS)56. All ICUs in Australia and New Zealand were invited to contribute to the ANZICS CORE registries in 1992. These registries have four registry domains: adult patients, pediatric patients, critical care resources, and central line-associated bloodstream infections. The CORE committee collects comprehensive data on various aspects of ICUs and reports back to the contributing ICUs. They also audit and analyze the performance of ICUs for quality assurance purposes.
Therefore, Asian countries, including South Korea, need to benchmark the successful stories of Western countries. This would eventually lead to the performance improvement of healthcare workers and the reduction of sepsis mortality.
3. Implementation of performance improvement programs and sepsis care bundles
When several individual effective treatments are applied concurrently, we anticipate better outcomes than any of the individual treatments alone; e.g., the ventilator-associated pneumonia or central-line infection prevention bundles57,58. In 2004, the SSC group, in partnership with the Institute for Healthcare Improvement, developed the SSC bundle with the goal of reducing the mortality rate by 25%59. In 2012 and 2016, the 3-hour and 6-hour SSC bundles were introduced but, due to the negative results of three RCTs on EGDT, invasive monitoring, such as CVP and ScvO2, was excluded from the 6-hour bundle in 20168,60. More recently, based on the 2016 SSC guidelines, a revised hour-1 bundle (2018 bundle) with five key elements was developed (Table 2)61.
Table 2. Hour-1 surviving sepsis campaign bundle of care.
| The five key elements of hour-1 bundle |
|---|
| 1. Measure lactate level. Remeasure if initial lactate is >2 mmol/L. |
| 2. Obtain blood cultures prior to administration of antibiotics. |
| 3. Administer broad-spectrum antibiotics. |
| 4. Begin rapid administration of 30 mL/kg crystalloid for hypotension or lactate ≥4 mmol/L. |
| 5. Apply vasopressors if patient is hypotensive during or after fluid resuscitation to maintain MAP ≥65 mm Hg. |
“Time zero” or “time of presentation” is defined as the time of triage in the Emergency Department, or if presenting from another care venue, from the earliest chart annotation consistent with all elements of sepsis (formerly severe sepsis) or septic shock ascertained through chart review.
Adapted from Levy et al. Crit Care Med. 2018;46:997-1000, with permission of Society of Critical Care Medicine61.
MAP: mean arterial pressure.
The implementation of sepsis bundles is a cornerstone of sepsis performance improvement programs, which are associated with a significant increase in compliance with the sepsis bundles and a reduction in the mortality rate. Levy et al.62 reported low mortality rates in high-compliance hospitals during a 7.5-year observation. Analysis of the New York State registry also demonstrated that the compliance rate of the 3-hour sepsis bundle was associated with a lower risk-adjusted in-hospital mortality rate5. Among various factors, high-income countries, surgical ICUs, long duration of implementation, and presentation to an Emergency Department were associated with a high rate of compliance55,62, and lactate seemed frequently a non-compliant variable62,63. However, the rate of compliance should continue to increase during the first 2 years of implementation62, and the mortality rate may decrease even if bundle completion is delayed in sepsis patients64,65.
A large Asian study reported a low rate of compliance with SSC bundles. In South Korea, lack of critical care personnel was significantly associated with low compliance rates (e.g., total compliance of 5.6%)66. Thus, sufficient critical care personnel (e.g., intensivists and nurses) is an important factor for improving performance. Further studies should seek to identify methods of improving bundle compliance, as well as ways to overcome other barriers.
Conclusion
Prevention and early recognition of sepsis are of paramount importance until novel emerging drugs (or interventions) are demonstrated to be effective. Early application of the optimal treatments and improved compliance with sepsis bundles are pre-requisites for improving patients' outcomes. The validity of the Sepsis-3 definitions and that of qSOFA need to be demonstrated in large-scale prospective trials.
Footnotes
- Conceptualization: Kim HI, Park S.
- Methodology: Kim HI, Park S.
- Formal analysis: Kim HI, Park S.
- Original draft preparation: Kim HI, Park S.
- Review and editing: Kim HI, Park S.
- Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013. Statistical Brief #204. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2006. [cited 2018 Apr 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK368492/ [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborn TM. Severe sepsis and septic shock trials (ProCESS, ARISE, ProMISe): what is optimal resuscitation? Crit Care Clin. 2017;33:323–344. doi: 10.1016/j.ccc.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Pruinelli L, Westra BL, Yadav P, Hoff A, Steinbach M, Kumar V, et al. Delay within the 3-hour surviving sepsis campaign guideline on mortality for patients with severe sepsis and septic shock. Crit Care Med. 2018;46:500–505. doi: 10.1097/CCM.0000000000002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.Lilly CM. The ProCESS trial: a new era of sepsis management. N Engl J Med. 2014;370:1750–1751. doi: 10.1056/NEJMe1402564. [DOI] [PubMed] [Google Scholar]
- 11.ARISE Investigators; ANZICS Clinical Trials Group. Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 12.ProCESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsi. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatewood MO, Wemple M, Greco S, Kritek PA, Durvasula R. A quality improvement project to improve early sepsis care in the emergency department. BMJ Qual Saf. 2015;24:787–795. doi: 10.1136/bmjqs-2014-003552. [DOI] [PubMed] [Google Scholar]
- 19.Hayden GE, Tuuri RE, Scott R, Losek JD, Blackshaw AM, Schoenling AJ, et al. Triage sepsis alert and sepsis protocol lower times to fluids and antibiotics in the ED. Am J Emerg Med. 2016;34:1–9. doi: 10.1016/j.ajem.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 21.Beesley SJ, Lanspa MJ. Why we need a new definition of sepsis. Ann Transl Med. 2015;3:296. doi: 10.3978/j.issn.2305-5839.2015.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JM, Greenslade JH, McKenzie JV, Chu K, Brown AFT, Lipman J. Systemic inflammatory response syndrome, quick sequential organ function assessment, and organ dysfunction: insights from a prospective database of ED patients with infection. Chest. 2017;151:586–596. doi: 10.1016/j.chest.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 23.Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88:571–575. doi: 10.1308/003588406X130615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Roney JK, Whitley BE, Maples JC, Futrell LS, Stunkard KA, Long JD. Modified early warning scoring (MEWS): evaluating the evidence for tool inclusion of sepsis screening criteria and impact on mortality and failure to rescue. J Clin Nurs. 2015;24:3343–3354. doi: 10.1111/jocn.12952. [DOI] [PubMed] [Google Scholar]
- 26.Stark AP, Maciel RC, Sheppard W, Sacks G, Hines OJ. An early warning score predicts risk of death after in-hospital cardiopulmonary arrest in surgical patients. Am Surg. 2015;81:916–921. [PubMed] [Google Scholar]
- 27.Al-Qahtani S, Al-Dorzi HM, Tamim HM, Hussain S, Fong L, Taher S, et al. Impact of an intensivist-led multidisciplinary extended rapid response team on hospital-wide cardiopulmonary arrests and mortality. Crit Care Med. 2013;41:506–517. doi: 10.1097/CCM.0b013e318271440b. [DOI] [PubMed] [Google Scholar]
- 28.Huh JW, Lim CM, Koh Y, Lee J, Jung YK, Seo HS, et al. Activation of a medical emergency team using an electronic medical recording-based screening system*. Crit Care Med. 2014;42:801–808. doi: 10.1097/CCM.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 29.Churpek MM, Snyder A, Han X, Sokol S, Pettit N, Howell MD, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017;195:906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 31.PRISM Investigators. Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, et al. Early, goal-directed therapy for septic shock: a patient-level meta-analysis. N Engl J Med. 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 32.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell KH, Carlbom D, Caldwell E, Leary PJ, Himmelfarb J, Hough CL. Volume overload: prevalence, risk factors, and functional outcome in survivors of septic shock. Ann Am Thorac Soc. 2015;12:1837–1844. doi: 10.1513/AnnalsATS.201504-187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: a population-based study. Crit Care Med. 2013;41:1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 36.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 39.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai X, Yu W, Ji W, Lin Z, Tan S, Duan K, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care. 2014;18:532. doi: 10.1186/s13054-014-0532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 42.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DellaVolpe JD, Moore JE, Pinsky MR. Arterial blood pressure and heart rate regulation in shock state. Curr Opin Crit Care. 2015;21:376–380. doi: 10.1097/MCC.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 44.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 45.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–1691. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 46.Morelli A, Singer M, Ranieri VM, D'Egidio A, Mascia L, Orecchioni A, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med. 2016;42:1528–1534. doi: 10.1007/s00134-016-4351-2. [DOI] [PubMed] [Google Scholar]
- 47.Majno G. The ancient riddle of sigma eta psi iota sigma (sepsis) J Infect Dis. 1991;163:937–945. doi: 10.1093/infdis/163.5.937. [DOI] [PubMed] [Google Scholar]
- 48.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority: a WHO resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 49.Just say sepsis! A review of the process of care received by patients with sepsis [Internet] London: National Confidential Enquiry into Patient Outcome and Death; 2015. [cited 2018 Apr 3]. Available from: http://www.ncepod.org.uk/2015report2/downloads/JustSaySepsis_FullReport.pdf. [Google Scholar]
- 50.Rubulotta FM, Ramsay G, Parker MM, Dellinger RP, Levy MM, Poeze M, et al. An international survey: public awareness and perception of sepsis. Crit Care Med. 2009;37:167–170. doi: 10.1097/ccm.0b013e3181926883. [DOI] [PubMed] [Google Scholar]
- 51.Vincent JL. Increasing awareness of sepsis: World Sepsis Day. Crit Care. 2012;16:152. doi: 10.1186/cc11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thwaites CL, Lundeg G, Dondorp AM sepsis in resourcelimited settings-expert consensus recommendations group of the European Society of Intensive Care Medicine (ESCICM), the Mahidol-Oxford Research Unit (MORU) in Bangkok, Thailand. Infection management in patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. 2016;42:2117–2118. doi: 10.1007/s00134-016-4547-5. [DOI] [PubMed] [Google Scholar]
- 53.Schultz MJ, Dunser MW, Dondorp AM, Adhikari NK, Iyer S, Kwizera A, et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med. 2017;43:612–624. doi: 10.1007/s00134-017-4750-z. [DOI] [PubMed] [Google Scholar]
- 54.World Sepsis Day [Internet] Seoul: Korean Society of Critical Care Medicine; 2018. [cited 2018 Apr 3]. Available from: http://www.ksccm.org/#%2Fboard%2Flist.kin%3Fmenu_main%3D6%26menu_sub%3D29%261522741514642. [Google Scholar]
- 55.Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. 2011;342:d3245. doi: 10.1136/bmj.d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Center for Outcome and Resource Evaluation. ANZICS [Internet] Camberwell: Australian and New Zealand Intensive Care Society; 2018. [cited 2018 Apr 3]. Available from: http://www.anzics.com.au/Pages/CORE/About-CORE.aspx. [Google Scholar]
- 57.Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59:96–105. doi: 10.1093/cid/ciu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eom JS, Lee MS, Chun HK, Choi HJ, Jung SY, Kim YS, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: a multicenter study. Am J Infect Control. 2014;42:34–37. doi: 10.1016/j.ajic.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 59.Levy MM, Pronovost PJ, Dellinger RP, Townsend S, Resar RK, Clemmer TP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(11 Suppl):S595–S597. doi: 10.1097/01.ccm.0000147016.53607.c4. [DOI] [PubMed] [Google Scholar]
- 60.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 61.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46:997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 62.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40:1623–1633. doi: 10.1007/s00134-014-3496-0. [DOI] [PubMed] [Google Scholar]
- 63.Chen YC, Chang SC, Pu C, Tang GJ. The impact of nationwide education program on clinical practice in sepsis care and mortality of severe sepsis: a population-based study in Taiwan. PLoS One. 2013;8:e77414. doi: 10.1371/journal.pone.0077414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coba V, Whitmill M, Mooney R, Horst HM, Brandt MM, Digiovine B, et al. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med. 2011;26:304–313. doi: 10.1177/0885066610392499. [DOI] [PubMed] [Google Scholar]
- 65.Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodriguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36:542–547. doi: 10.1097/SHK.0b013e3182360f7c. [DOI] [PubMed] [Google Scholar]
- 66.Kim JH, Hong SK, Kim KC, Lee MG, Lee KM, Jung SS, et al. Influence of full-time intensivist and the nurse-to-patient ratio on the implementation of severe sepsis bundles in Korean intensive care units. J Crit Care. 2012;27:414.e11–414.e21. doi: 10.1016/j.jcrc.2012.03.010. [DOI] [PubMed] [Google Scholar]