Abstract
Intrahepatic cholestasis of pregnancy (ICP) is related to cholestatic disorder in pregnancy. Total urinary sulfated bile acids (SBAs) were found increased in ICP. We distinguished the metabolic profiling of urinary SBAs in ICP to find potential biomarkers for the diagnosis and grading of ICP. The targeted metabolomics based on high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) was used to analyze urinary SBAs profiling in mild and severe ICP cases, as well as healthy controls. 16 kinds of urinary SBAs were determined by HPLC-MS/MS. Sulfated dihydroxy glycine bile acid (di-GBA-S), glycine cholic acid 3-sulfate (GCA-3S), sulfated dihydroxy taurine bile acid (di-TBA-S) and taurine cholic acid 3-sulfate (TCA-3S) increased significantly in ICP group compared with the control group. Seven kinds of SBAs were significantly different (p < 0.05) between the ICP group and the control group, with the variable importance in the projection (VIP) value more than one by the orthogonal partial least squares discriminant analysis (OPLS-DA). GCA-3S was well-suited to be used as the biomarker for the diagnosis of ICP with the sensitivity of 100% and specificity of 95.5%. A multi-variable logistic regression containing GCA-3S and di-GBA-S-1 was constructed to distinguish severe ICP from mild ICP, with the sensitivity of 94.4% and specificity of 100%. The developed HPLC-MS/MS method is suitable for the measurement of urinary SBAs profiling. Moreover, the urinary SBAs in the metabolomic profiling have the potential to be used as non-intrusive biomarkers for the diagnosis and grading of ICP.
Keywords: Biomarker, HPLC-MS/MS, Intrahepatic cholestasis of pregnancy, Sulfated bile acids, Targeted metabolomics, Urine
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-associated liver disease with onset mainly in the second or third trimester of pregnancy. ICP is characterized by pruritus, cholestasis with raised maternal serum bile acids (BAs) and transaminases.1, 2 The incidence of ICP is reported to be from 0.2% to 2%. Whereas, it varies widely with the ethnicity and geographic location.3 ICP is associated with an increased risk of adverse pregnancy outcomes, including spontaneous preterm delivery, fetal distress, meconium staining of the amniotic fluid, fetal bradycardia, pneumonia, and unexplained intrauterine fetal demise (IUFD),4, 5, 6 which particularly occur in those severe ICP cases with serum total bile acid (TBA) level exceeding 40 μmol/L.7
Currently, serum TBA is the main laboratory index for the diagnosis and grading of ICP in clinics. However, the range of serum TBAs levels in ICP overlaps with that in healthy pregnant women.8 Thus, it is difficult to make a reliable diagnosis of ICP by serum TBA. Sulfation is an important detoxification pathway of BAs and may play an important role in maintaining BAs homeostasis under pathologic conditions.9 Sulfated bile acids (SBAs) have good water solubility and can be easily excreted in urine under cholestatic conditions, which could decrease the liver damnification. Urinary SBA was considered to be a useful indicator of hepatic fibrosis superior to TBA in patients with chronic hepatitis C.10 Urinary SBA was also beneficial to the early detection of fibrosis in primary biliary cirrhosis (PBC) and biliary atresia in infants.11, 12 In addition, total urinary SBA was suggested to be more selective and specific in the diagnosis of ICP than serum TBA.13
In our previously research, serum BAs profiling could be used to the diagnosis and clinical grading of ICP.14, 15 Masubuchi et al16 found serum BAs profiling and serum SBA levels were likely to be used as important biomarkers for the discriminating diagnosis of liver injury types in adult male SD rats. The urinary BAs profiling were characterized by different kinds of individual BAs, which were probably used as diagnostic biomarkers in hepatobiliary diseases.17 Liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was widely used for the quantification of SBAs.17, 18, 19, 20, 21, 22, 23 Nevertheless, urinary SBAs profiling of ICP has not been investigated.
We devote ourselves to identify most of the urinary SBAs in this study, some even without commercialized standards. A novel and sensitive high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method was developed and validated to investigate the metabonomic profiling of urinary SBAs in ICP in comparison with healthy pregnant women. Enhanced product ion (EPI) scan and multiple reaction monitoring (MRM) were used to identify urinary SBAs without standards. Ultimately, urinary SBAs profiling was applied for the diagnosis and grading of ICP.
Experimental
Chemicals and reagents
SBA standards of lithocholic acid 3-sulfate (LCA-3S), glycolithocholic acid 3-sulfate (GLCA-3S) and taurolithocholic acid 3-sulfate (TLCA-3S) were purchased from Sigma–Aldrich (St. Louis, MO, USA). The authentic compounds of five kinds of unconjugated BAs, including cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), ursodeoxycholic acid (UDCA), and hyodeoxycholic acid (HDCA), and four kinds of glycine conjugated BAs including GCA, GCDCA, GDCA and GUDCA, and four kinds of taurine conjugated BAs including TCA, TCDCA, TDCA, and TUDCA were all obtained from Sigma–Aldrich (St. Louis, MO, USA). 5β-cholanicacid-3α, 6β, 7α-triol, as internal standard (IS), was purchased from Steraloids Chemical (Newport, Rhode Island, USA). Water was prepared by a MilliQ™ System (Millipore, Milford, MA, USA). HPLC-grade methanol (MeOH) and acetonitrile (ACN) were obtained from Merck KGaA (Merck KGaA, Darmstadt, Germany).
Biological sample collection and pretreatment
Pregnant women diagnosed as ICP and healthy volunteers were recruited from May 1, 2013 to March 1, 2014 in the First Affiliated Hospital of Chongqing Medical University. Informed consent was obtained from each participant. 29 women with ICP and 22 healthy pregnant women as controls in all cases were enrolled at the same gestation period. 11 cases were clinically diagnosed as mild ICP and 18 cases were diagnosed as severe ICP in the 29 ICP patients. The diagnosis of ICP is based on the presence of pruritus, elevated serum TBAs level, and/or elevated serum transaminases as well as spontaneous relief of signs and symptoms within four to six weeks after delivery. The enrollment criteria of ICP and exclusion criteria of healthy pregnant women, and the criteria for the diagnosis and grading of ICP were described in our previous study.15
Urine samples from the patients with ICP were collected at the first visit for the definite diagnosis without any drug treatment. And urine samples from normal pregnant women were also collected as controls. The samples were stored at −80 °C before analysis. The sample preparation method was according to the published literature.18, 24 200 μL of urine samples was diluted with 800 μL of 50 mmol/L PBS (pH 7.0, containing 0.19 μg/mL IS). And the mixed solution was loaded onto an Oasis HLB SPE cartridge (30 mg/mL, Waters, Milford, MA, USA) which had been preconditioned with 1 mL of MeOH and 2 mL of water successively. And then the loaded cartridge was washed with 1 mL of water and eluted with 1.5 mL of 50% MeOH. The eluate was evaporated under a nitrogen stream below 40 °C and reconstituted in 1.0 mL of 0.1% formic acid-MeOH (9:1, v/v) solution. 10 μL of the aliquot was injected into the LC-MS/MS system.
LC-MS/MS analysis
LC-MS/MS analysis was performed with an API 4000 Q-Trap hybrid triple quadrupole linear ion-trap (QqQLIT) mass spectrometer (Applied Biosystems/MDS SCIEX; Concord, ON, Canada) connected to a Shimadzu LC system (Shimadzu, Tokyo, Japan). This system was consisted of an LC-20ADxp pump, an SIL-20AC autosampler, a CTO-20AC control instrument. The mass spectrometer was equipped with an electrospray ionization (ESI) source, controlled by Analyst 1.5 software (Applied Biosystems, Foster City, CA, USA). A Luna C18 column (150 mm × 2.00 mm, 3 μm, Phenomenex, Torrance, CA, USA) was used for the chromatographic separation.
The mobile phase was composed of (A) 2 mmol/L ammonium acetate and 0.1% formic acid in water and (B) 2 mmol/L ammonium acetate and 0.1% formic acid in ACN. The mobile phase was delivered at a flow rate of 0.2 mL/min. The gradient elution program was set as follows. The mobile phase B was increased linearly from 30% to 100% over 8 min, and then held at 100% for 4 min, and ultimately brought back to 30% in 1 min followed by an equilibrium of 5 min.
Urine SBAs were monitored by MRM in the negative ion mode. Ion source-dependent parameters were set as follows. The ion spray voltage was set at −4500 V. The ion source temperature was set at 500 °C. The curtain gas was set at 25 (arbitrary units). The ion source gas1 (GS1) was set at 50 (arbitrary units) and the ion source gas2 (GS2) was also set at 50 (arbitrary units). The optimum MRM transitions of three kinds of authentic SBAs (LCA-3S, GLCA-3S, TLCA-3S), 13 kinds of the identified SBAs and IS, as well as the scheduled MRM operation parameters for each analyte were shown in Table 1.
Table 1.
MRM transitions and MS parameters of identified SBAs and IS.
| Analyte | MRM transition (m/z) | DP(V) | EP(V) | CEP(V) | CE(V) | CXP(V) |
|---|---|---|---|---|---|---|
| LCA-3S | 455.2 → 97.0 | −80 | −10 | −35 | −90 | −10 |
| GLCA-3S | 432.2 → 97.0 | −65 | −10 | −20 | −100 | −10 |
| TLCA-3S | 562.2 → 482.2 | −45 | −8 | −20 | −140 | −9 |
| CA-3S | 487.2 → 97.0 | −50 | −10 | −30 | −80 | −8 |
| GCA-3S | 544.2 → 464.2 | −60 | −9 | −25 | −85 | −10 |
| TCA-3S | 594.1 → 514.1 | −40 | −10 | −30 | −100 | −10 |
| di-BA-S | 471.1 → 97.0 | −50 | −9 | −35 | −80 | −9 |
| di-GBA-S | 528.0 → 448.1 | −55 | −10 | −20 | −90 | −9 |
| di-TBA-S | 578.0 → 498.4 | −90 | −8 | −30 | −110 | −10 |
| IS | 401 → 248.9 | −80 | −9 | −34 | −40 | −10 |
Due to the complexity of BAs profiling in urine, the unknown compounds with the same m/z may have different structure. So, there may be multiple peaks from HPLC according to a certain m/z. Since most of authentic SBAs were not available, we need identify each peak caught by MRM using EPI. Compared with non-enhanced basic product ion scan, the EPI scan delivers high sensitivity, high mass accuracy, and fast scanning. Furthermore, the chromatographic behaviors and MS fragmentation informations were investigated to identify the SBAs without standards. The detailed procedure was described in the results and discussion.
Data collection and multivariate statistical analysis
The statistical analysis was performed using the Statistical Product and Service Solution (SPSS) software, version 18 (IBM corporation, Armonk, New York). Results are expressed as median (25th and 75th percentiles). The Shapiro–Wilk test was performed to determine the normality of the data distribution. The SBAs profiling of the different groups were compared with the Kruskal–Wallis H test. If the p value was less than 0.05, the Wilcoxon test for nonparametric, independent, two-group comparison was subsequently performed and the significance level was adjusted to 0.017 using the Bonferroni method to avoid type I errors. Multivariate statistical analysis was performed using SIMCA-P software version 14.1 (Umetrics AB, Umea, Sweden). Principle component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were performed using the data from MS/MS. Receiver operating characteristic (ROC) analysis was performed using Medcalc 15.5 to assess the diagnostic performance of the analytes. Potential biomarkers were analyzed and identified comprehensively by the ROC curves and variable importance in the projection (VIP) values.
Results and discussion
Identification of urinary SBAs
Before the analysis of the profiling of urinary SBAs, we need identify them firstly. The identification of three authentic SBAs (LCA-3S, GLCA-3S, TLCA-3S) in urine was operated in terms of their retention time (tR) in LC and m/z in MS/MS.
Since commercial standards were not available for some SBAs, we tried some other strategies to identify them. 13 kinds of common BAs were used to study fragmentation patterns of BAs. Mass transitions and their optimum MS parameters were listed in the Supporting Information, Table S1. The use of specific precursor/product ion transitions facilitates increased the specificity and sensitivity of the quantitative analysis. Selected precursor ion scans (parents of 97 for sulfated conjugates) and neutral loss scans (loss of 80 for sulfated conjugates) were used to find possible SBAs in urine. And the characteristic ion pairs were determined by MRM transitions. And then, the EPI scans were used for the identification of SBAs without standards.
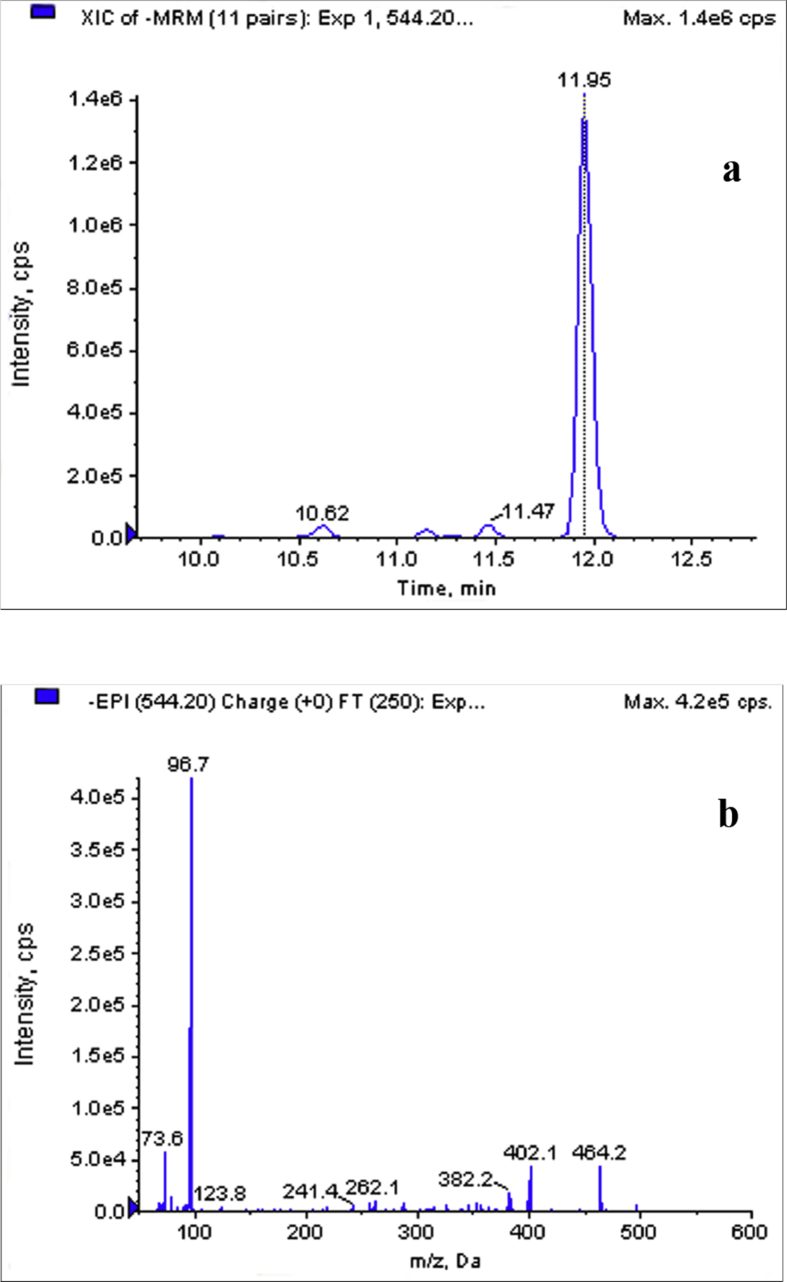
We took GCA-3S as an example to illustrate the identification process of SBAs without standards. Glycine-conjugated SBAs generated characteristic product ions at m/z of 74 (a fragment ion of the glycine moiety) and m/z of 96.8 (a fragment of sulfate moiety). The neutral losses of the sulfate moiety ([M-H-SO3]−) also can be seen in the CID spectrums of glycine-conjugated SBAs. So we used transition 544.2/464.2 as the MRM monitoring ions for GCA-3S. Fig. 1(a) shows the extracted ion chromatogram of MRM transition at m/z of 544.2 → 464.2 ([M-H-80]−). Due to the superior selectivity and sensitivity, MRM transitions were used as survey scans, and it triggered the acquisition of EPI spectra if the signal intensity of the survey scan exceeded a predefined threshold value. The analyte with a retention time of 11.95 min displayed a [M-H]− ion at m/z of 544.2. Representative EPI spectrum of precursor ion (m/z of 544.2) was shown in Fig. 1(b). The product ions at m/z of 96.7 and m/z of 73.6 were the characteristic fragments of sulfate moiety and glycine moiety, respectively. The presence of a product ion at m/z of 402.1 was probably generated by the losses of sulfate and CO2+H2O ([M-H-80-62]−). Another fragment ion at m/z of 464.2 was resulted from the [M-H]− ion with the loss of a sulfate unit. The structure of precursor ion (m/z of 544.2) was proposed based on both the signal from MRM transition and the EPI spectrum. The EPI spectrum shown in Fig. 1(b) matched well to the theoretical EPI spectrum of the parent compound. Hence, the analyte with a retention time of 11.95 min was identified to be GCA-3S.
Figure 1.
The MRM chromatogram and EPI spectrum of an identified BA obtained from a urinary sample. (a) Extracted ion chromatogram of MRM transition at m/z of 544.2 → 464.2 and (b) EPI spectrum of precursor ion at m/z of 544.2 with the retention time of 11.95 min.
Other SBAs without standards were all identified based on both of the signal from MRM transitions and the EPI spectra. However, CDCA-3S, DCA-3S and UDCA-3S isomers showed MS/MS spectra of product ion at of m/z 97 corresponding to sulfate moiety. Therefore, it would be impossible to distinguish the isomers by EPI spectra. It occurred in both glycine and taurine conjugated isomers. The separation by LC could discriminate the isomeric forms. The isomeric forms could not be qualified by the retention time due to the commercially unavailable standards of some SBAs. Three unknown peaks from LC were defined as sulfated dihydroxy BA-1 (di-BA-S-1), di-BA-S-2 and di-BA-S-3 by MS/MS spectra. The peaks of glycine and taurine conjugated isomers were named similarly. Nevertheless, four peaks with similar product fragments to sulfated dihydroxy glycine bile acid (di-GBA-S) were observed and indicated to be a new compound in urine. The MRM transitions and MS parameters of SBAs were listed in Table 1. It was also possible to identify the co-eluting components with SBAs specifically using the MRM mode to monitor different transitions.
Optimization of LC parameters
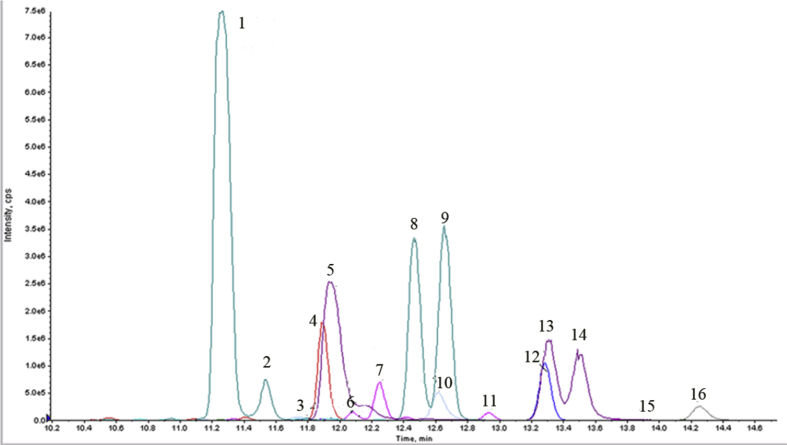
The separation of isomers is a crucial parameter for LC in method development. Different kinds of mobile phase were tested, according to the previously used method for the separation of SBAs.18, 25 0.1% (v/v) of formic acid was added as the modifier, to make the peak shape improved. Different gradient elution conditions were also investigated for the improved separation of SBAs in urine. Using the optimum chromatographic conditions, the separation of 16 kinds of SBAs with desirable peak shape in urine was achieved in 18 min including the re-equilibrium time. The chromatograms were shown in Fig. 2. Three groups of structure isomers, di-BA-S-1∼3, di-GBA-S-1∼4, and sulfated dihydroxy taurine bile acid (di-TBA-S-1∼3), were separated in each group. Some SBAs could not be separated completely. Nevertheless, they could be differentiated with different MRM modes. Two isomeric SBAs, di-TBA-S-2 and di-TBA-S-3, could not be totally separated. Fortunately, the reproducibility of these two SBAs was satisfactory.
Figure 2.
Chromatograms of 16 kinds of SBAs in urine. 1. di-GBA-S-1, 2. di-GBA-S-2, 3. CA-3S, 4. GCA-3S, 5. di-TBA-S-1, 6. di-BA-S-1, 7. di-BA-S-2, 8. di-GBA-S-3, 9. di-GBA-S-4, 10. TCA-3S, 11. di-BA-S-3, 12. GLCA-3S, 13. di-TBA-S-2, 14. di-TBA-S-3, 15. LCA-3S, 16. TLCA-3S.
Optimization of sample preparation procedure
The sample preparation of SBAs in human urine for LC/ESI-MS/MS analysis using solid phase extraction (SPE) was described as follows. Unsulfated BAs were eluted in MeOH as reported in previous work.24 SBAs have a lipophilic steroid nucleus and hydrophilic groups, such as hydroxyl and sulfate groups. Therefore, these sulfates were eluted in methanol-water solution because of their good hydrophilicity. The proportion of MeOH in the eluent was investigated by varying from 0 to 100% (v/v). When the proportion of MeOH was 50%, SBAs could be eluted with high efficiency.
Method validation
SBAs in the mixed urine samples from 20 healthy pregnant women were determined to estimate the accuracy and precision of the method. Measurements were repeated five times on the same day, and also on five consecutive days to investigate the intra-day and inter-day precisions, respectively. Results were shown as the ratio of the peak area of individual SBA to IS, expressed by A (SBA)/A (IS). To reduce the effect of urine volume on the concentration, the measured urinary SBAs levels were adjusted by the urinary creatinine concentration. The intra-day and inter-day precisions for 16 kinds of SBAs in urine were both less than 5% (Supporting Information, Table S2). The results indicated that the precisions of the method were satisfactory. The recoveries for the three authentic SBAs ranged from 93% to 97%, showing the recoveries were acceptable (Supporting Information, Table S3). The assay was linear over the tested concentration range of 9.0–1080.0, 9.3 to 1123.3 and 9.7–998.0 ng/mL for LCA-3S, GLCA-3S and TLCA-3S in urine, respectively. And the linear correlation coefficients for the three kinds of SBAs were all more than 0.999 (Supporting Information, Table S4). And the limit of detection for the individual SBAs was 5 μg/μL.
Characteristics of patients
The clinical informations on all of the participants were given in Supporting Information, Table S5. It showed no difference (p > 0.05) in maternal age and gestational age among the normal pregnancy, mild ICP and severe ICP groups. Glycocholic acid (CG), TBA, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevated significantly in the sera derived from both mild and severe ICP patients as compared with the healthy pregnant women. However, no significant difference was found in alkaline phosphatase (ALP) in the three groups. Direct bilirubin (DBIL) and total bilirubin (TBIL) increased significantly in severe ICP group compared with the control group.
Profiling of SBAs in human urine
Since the standards for most SBAs were unavailable commercially, relative quantitation was performed by determining the relative ratio of the peak area of the analytes and IS. The profiling of urinary SBAs in healthy pregnant women, mild ICP, and severe ICP groups were shown in Table 2. The level of total urinary SBAs was 6.0-fold (p < 0.017) higher in mild ICP group and 20.4-fold (p < 0.017) higher in severe ICP group than that in the healthy pregnant group. And the level of total GBA-S was 5.5-fold higher in mild ICP group and 17.9-fold higher in sever ICP group. Meanwhile, the level of total TBA-S was 8.5-fold higher in mild ICP group and 43.1-fold higher in severe ICP group, shown in Table 2. Furthermore, the peak area ratio of TBA-S to GBA-S, expressed as A (TBA-S)/A (GBA-S), was 0.38, 0.59 and 0.90 in healthy pregnancy, mild ICP and severe ICP group, respectively.
Table 2.
Profiling of urinary SBAs in women with normal pregnancy, mild ICP, and severe ICP (in peak area ratios of SBA and IS).
| SBAs | Normal pregnancy | ICP |
|
|---|---|---|---|
| Mild ICP | Severe ICP | ||
| 3S-LCA | 0.001 (0.000,0.001) | 0.001 (0.000,0.002) | 0.002 (0.001,0.006) |
| di-BA-S-1 | 0.028 (0.011, 0.060) | 0.098 (0.038, 0.143)a | 0.130 (0.037, 0.474)b |
| di-BA-S-2 | 0.009 (0.003, 0.032) | 0.023 (0.018, 0.056) | 0.181 (0.021, 5.374)b |
| di-BA-S-3 | 0.045 (0.018, 0.135) | 0.180 (0.101, 0.244) | 0.216 (0.092, 0.385)b |
| CA-3S | 0.090 (0.002, 0.864) | 1.849 (1.725, 2.044)a | 1.870 (1.198, 3.514)b |
| GLCA-3S | 0.201 (0.084 0.442) | 0.403 (0.213, 1.094) | 1.827 (0.243, 3.846)b |
| di-GBA-S-1 | 0.371 (0.117, 1.609) | 1.204 (0.515, 2.219) | 10.524 (0.892, 44.205)b |
| di-GBA-S-2 | 0.097 (0.046, 0.154) | 0.667 (0.239, 1.002)a | 0.766 (0.247, 1.746)b |
| di-GBA-S-3 | 0.552 (0.263, 1.124) | 5.903 (2.755, 6.587)a | 11.932 (5.989, 16.967)b,c |
| di-GBA-S-4 | 1.028 (0.428, 2.698) | 4.540 (2.184, 7.604)a | 9.513 (4.945, 18.579)b,c |
| GCA-3S | 0.077 (0.047, 0.215) | 1.457 (1.115, 3.326)a | 7.604 (3.679, 10.432)b,c |
| TLCA-3S | 0.111 (0.034, 0.211) | 0.348 (0.139, 0.498)a | 1.905 (0.292, 3.775)b |
| di-TBA-S-1 | 0.124 (0.036, 0.286) | 0.978 (0.299, 1.597)a | 1.861 (0.876, 23.245)b |
| di-TBA-S-2 | 0.208 (0.081, 0.407) | 3.616 (1.483, 4.424) | 12.563 (4.344, 22.159)b,c |
| di-TBA-S-3 | 0.499 (0.281, 1.252) | 3.142 (0.968, 5.543) | 7.881 (5.628, 13.853)b,c |
| TCA-3S | 0.070 (0.016, 0.137) | 1.350 (0.609, 2.446)a | 8.980 (2.492, 17.224)b,c |
| Total unconjugated SBA | 0.287 (0.095, 0.960) | 2.137 (1.884, 2.341)a | 3.606 (2.274, 7.949)b,c |
| Total GBA-S | 3.101 (1.242, 5.184) | 16.950 (11.396, 20.956)a | 55.520 (36.387, 80.127)b,c |
| Total TBA-S | 1.165 (0.689, 2.072) | 9.945 (6.983, 20.455)a | 50.240 (27.473, 74.890)b,c |
| Total SBAs | 4.938 (3.271, 7.992) | 29.407 (22.429, 38.275)a | 100.866 (80.760,178.595)b,c |
Results are expressed as median ((25th and 75th percentiles)). Wilcoxon test was used to assess the significance of difference between groups.
Means significantly different from the group of normal pregnancy.
Means significantly different from the group of normal pregnancy.
Means significantly different from the group of mild ICP.
TBA-S and GBA-S were the main components of SBAs, accounting for more than 90% in urine. The conjugation of BAs with glycine and taurine decreases their pKa, which increases their ionization and solubility and enhances their urinary elimination so that the toxicity decreases greatly.22, 26 The amidation with glycine is predominant over that with taurine in the serum of healthy volunteers in previous reports.21, 27, 28 In the present study, we found that GBA-S were the major SBAs in urine, which was consistent with Goto's report.18 Glycine was more abundant than taurine in peroxisomes, which may attribute to the predominance of glycine amidation in humans.29 Nevertheless, the increase in the proportion of TBA-S was more correlated to the severity of the disease than that of GBA-S. Taurine-amidated BAs are generally less cytotoxic than the glycine-amidated BAs. Sulfated unconjugated BAs were kept in low concentrations. And LCA-3S was the least abundant in the measured urinary SBAs. No obvious differences of LCA-3S were observed among the three groups, confirming the low level of LCA in serum in the previous reports.30
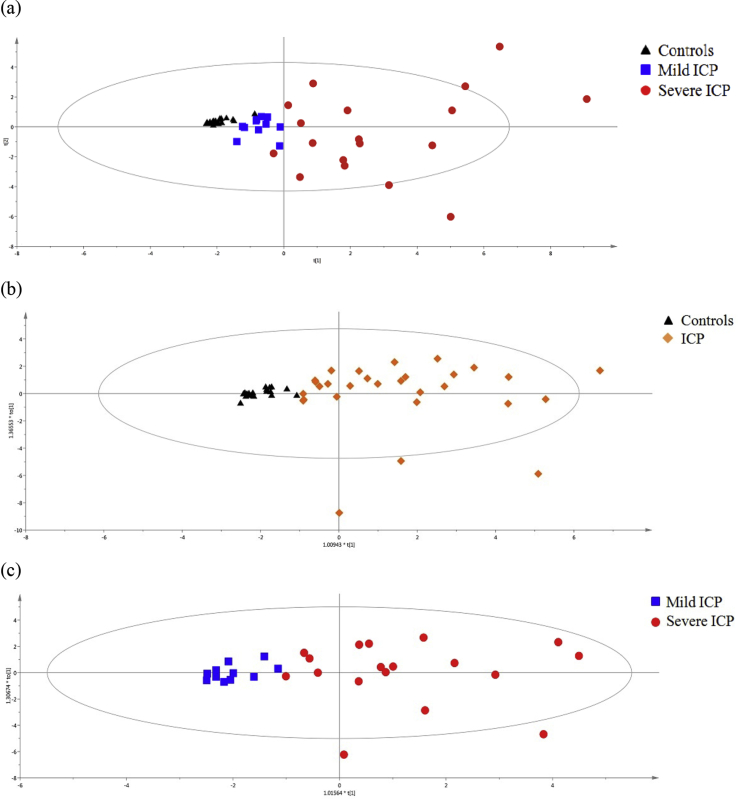
Furthermore, PCA was used as an unsupervised statistical method to study the metabolic differences among healthy pregnancy group, mild ICP group and severe ICP group. An obvious clustering effect was observed between the healthy pregnancy group and the severe ICP group, displayed in Fig. 3(a). OPLS-DA analysis (R2Xcum = 0.578, R2Ycum = 0.564, Q2Ycum = 0.407) was performed to discriminate the ICP patients from the healthy controls using the profiling of 16 kinds of SBAs, shown in Fig. 3(b). The correct rate of 88.24% was observed in the OPLS-DA model. The OPLS-DA (R2Xcum = 0.496, R2Ycum = 0.589, Q2Ycum = 0.348) was also performed between the mild ICP group and the severe ICP group, shown in Fig. 3(c). And the correct rate of 93.1% was obtained. None over-fitting was observed in the OPLS-DA models with 200 random permutations. The VIP value, calculated by the formula described in the user's guide of SIMCA-P, indicated the contribution of each metabolite ion in the classification. Variables with a VIP value more than one meant above average contribution to the explanation of the Y matrix (classification).
Figure 3.
Score plots of PCA and OPLS-DA based on SBAs profiling in urine of ICP patients and the controls. (a) PCA score plots for the controls, mild ICP and severe ICP patients. (b) OPLS-DA score plots for ICP patients and controls. (c) OPLS-DA score plots for mild ICP patients and severe ICP patients.
Potential biomarkers for ICP
The ROC curve was constructed by plotting the sensitivity against the corresponding false-positive rate, which was expressed as 1-specificity. The analytes with the area under curve (AUC) > 0.70 and with VIP value > 1.0 were selected as the potential biomarkers and were listed in Table 3. The sensitivity, specificity, Youden index and likelihood ratio for the selected cut-off value of each parameter were given in Table 3. As described in Table 3, seven kinds of SBAs including di-GBA-S-3, GCA-3S, di-TBA-S-3, di-TBA-S-2, TCA-3S, CA-3S, and TLCA-3S may be the potential biomarkers to distinguish ICP patients from normal pregnant women. Furthermore, GCA-3S had the highest AUC (>0.95) and Youden index (>0.95) among the seven possible biomarkers. The correct rate of 98% was obtained at the suggested cut-off value (0.49) of GCA-3S for the diagnosis of ICP. However, lower correct rate of 82.8% was obtained at the suggested cut-off value (3.46) of GCA-3S for the grading diagnosis of severe ICP group and mild ICP group. Fortunately, the correct rate of 93.1% was observed with the sensitivity of 94.4% and the specificity of 100% when multivariable logistic regression with the combination of GCA-3S and di-GBA-S-1 was applied for the grading diagnosis of ICP.
Table 3.
Potential biomarkers discovered by VIP value and ROC analysis.
| Biomarkers | VIP | AUC | p | Sensitivity (%) | Specificity (%) | Youden index | +LR | -LR | Cut-off | |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal-ICP | ||||||||||
| di-GBA-S-3 | 1.38 | 0.975 | <0.0001 | 96.6 | 90.9 | 0.875 | 10.6 | 0.04 | 1.66 | |
| GCA-3S | 1.37 | 0.997 | <0.0001 | 100 | 95.5 | 0.955 | 22.0 | 0 | 0.49 | |
| di-TBA-S-3 | 1.30 | 0.929 | <0.0001 | 86.2 | 100 | 0.862 | – | 0.14 | 1.60 | |
| di-TBA-S-2 | 1.25 | 0.983 | <0.0001 | 96.6 | 100 | 0.966 | – | 0.03 | 0.93 | |
| TCA-3S | 1.24 | 0.995 | <0.0001 | 100 | 95.5 | 0.955 | 22 | 0 | 0.37 | |
| CA-3S | 1.16 | 0.873 | <0.0001 | 79.3 | 95.5 | 0.748 | 17.5 | 0.22 | 1.21 | |
| TLCA-3S | 1.05 | 0.828 | <0.0001 | 69.0 | 90.9 | 0.599 | 7.59 | 0.34 | 0.34 | |
| Mild-severe ICP | ||||||||||
| di-GBA-S-3 | 1.40 | 0.773 | 0.0031 | 72.2 | 90.9 | 0.631 | 7.94 | 0.31 | 6.90 | |
| GCA-3S | 1.36 | 0.874 | <0.0001 | 77.8 | 90.9 | 0.687 | 8.56 | 0.24 | 3.46 | |
| di-TBA-S-2 | 1.30 | 0.768 | 0.0041 | 77.8 | 81.8 | 0.596 | 4.28 | 0.27 | 4.42 | |
| di-TBA-S-3 | 1.29 | 0.884 | <0.0001 | 83.3 | 90.9 | 0.742 | 9.17 | 0.18 | 5.63 | |
| TLCA-3S | 1.27 | 0.763 | 0.0057 | 72.2 | 90.9 | 0.631 | 7.94 | 0.31 | 0.67 | |
| TCA-3S | 1.19 | 0.803 | 0.0003 | 77.8 | 81.8 | 0.596 | 4.28 | 0.27 | 2.45 | |
| di-BA-S-2 | 1.04 | 0.707 | 0.0331 | 50 | 90.9 | 0.409 | 5.5 | 0.55 | 0.25 | |
| di-GBA-S-1 | 1.03 | 0.717 | 0.025 | 72.2 | 72.7 | 0.450 | 2.65 | 0.38 | 1.33 | |
Some SBAs such as TCA-3S, GCA-3S and sulfated conjugated dihydroxy BAs elevated significantly in severe ICP group compared to mild ICP group. These SBAs were abundant relatively in urine and can be detected easily, which made them the potential biomarkers for the grading diagnosis of ICP. From the results, we proposed that urinary SBAs could be a substitute to serum BAs for its non-intrusive sample collection in the diagnosis of ICP.
The total SBAs increased remarkably in ICP patients. More BAs were sulfated and excreted into urine with the development of ICP, which might be the adaptive response to cholestasis. Nevertheless, it was also considered that cholestasis was caused by the disfunction of the BAs sulfating. Whatever, it requires further research.
Conclusions
In this study, HPLC-MS/MS method was developed and applied for the identification and quantification of SBAs in human urine samples. The level of total urinary SBAs increased remarkably in ICP patients, especially for sulfated glycine-amidated and taurine-amidated BAs, indicating that sulfation was a major pathway for the elimination of BAs in human. The characteristics of the SBAs profiling might provide insights into the mechanisms of underlying fetal complications and the disease progression of ICP. It is also possible to further understand the change of urinary SBAs profiling in patients with ICP, which might contribute to the grading diagnosis and the clinical treatment strategies for ICP. Since a limited number of samples were included in this study, further research needs large sample size to confirm these observations.
Conflict of interest
None declared.
Acknowledgments
The present work was supported by the Chongqing Postdoctoral Science Foundation (Xm201313), National Natural Science Foundation of China (81471473), and Research Fund for the Doctoral Program of Higher Education of China (20115503110013).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.gendis.2018.01.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Geenes V., Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049–2066. doi: 10.3748/wjg.15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diken Z., Usta I.M., Nassar A.H. A clinical approach to intrahepatic cholestasis of pregnancy. Am J Perinatol. 2014;31(1):1–8. doi: 10.1055/s-0033-1333673. [DOI] [PubMed] [Google Scholar]
- 3.Williamson C., Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124(1):120–133. doi: 10.1097/AOG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 4.Kondrackiene J., Kupcinskas L. Intrahepatic cholestasis of pregnancy-current achievements and unsolved problems. World J Gastroenterol. 2008;14(38):5781–5788. doi: 10.3748/wjg.14.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pusl T., Beuers U. Intrahepatic cholestasis of pregnancy. Orphanet J Rare Dis. 2007;2:26. doi: 10.1186/1750-1172-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rook M., Vargas J., Caughey A. Fetal outcomes in pregnancies complicated by intrahepatic cholestasis of pregnancy in a Northern California cohort. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0028343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu W.B., Menon R., Xu Y.Y. Downregulation of peroxiredoxin-3 by hydrophobic bile acid induces mitochondrial dysfunction and cellular senescence in human trophoblasts. Sci Rep. 2016;6:38946. doi: 10.1038/srep38946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castano G., Lucangioli S., Sookoian S. Bile acid profiles by capillary electrophoresis in intrahepatic cholestasis of pregnancy. Clin Sci (Lond) 2006;110(4):459–465. doi: 10.1042/CS20050302. [DOI] [PubMed] [Google Scholar]
- 9.Alnouti Y. Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108(2):225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 10.Negoro S., Tanaka A., Takikawa H. Urinary bile acid sulfate levels in patients with hepatitis C virus-related chronic liver diseases. Hepatol Res. 2009;39(8):760–765. doi: 10.1111/j.1872-034X.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 11.Miura R., Tanaka A., Takikawa H. Urinary bile acid sulfate levels in patients with primary biliary cirrhosis. Hepatol Res. 2011;41(4):358–363. doi: 10.1111/j.1872-034X.2011.00779.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki M., Muraji T., Obatake M. Urinary sulfated bile acid analysis for the early detection of biliary atresia in infants. Pediatr Int. 2011;53(4):497–500. doi: 10.1111/j.1442-200X.2010.03268.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang W.M., Seubert D.E., Donnelly J.G. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. J Perinat Med. 2007;35(6):486–491. doi: 10.1515/JPM.2007.128. [DOI] [PubMed] [Google Scholar]
- 14.Ye L., Liu S., Wang M. High-performance liquid chromatography-tandem mass spectrometry for the analysis of bile acid profiles in serum of women with intrahepatic cholestasis of pregnancy. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):10–17. doi: 10.1016/j.jchromb.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Deng W., Wang J. Primary bile acids as potential biomarkers for the clinical grading of intrahepatic cholestasis of pregnancy. Int J Gynaecol Obstet. 2013;122(1):5–8. doi: 10.1016/j.ijgo.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Masubuchi N., Nishiya T., Imaoka M. Promising toxicological biomarkers for the diagnosis of liver injury types: bile acid metabolic profiles and oxidative stress marker as screening tools in drug development. Chem Biol Interact. 2016;255:74–82. doi: 10.1016/j.cbi.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Bathena S.P.R., Thakare R., Gautam N. Urinary bile acids as biomarkers for liver diseases II. Signature profiles in patients. Toxicol Sci. 2015;143(2):308–318. doi: 10.1093/toxsci/kfu228. [DOI] [PubMed] [Google Scholar]
- 18.Goto T., Myint K.T., Sato K. LC/ESI-tandem mass spectrometric determination of bile acid 3-sulfates in human urine 3beta-Sulfooxy-12alpha-hydroxy-5beta-cholanoic acid is an abundant nonamidated sulfate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846(1–2):69–77. doi: 10.1016/j.jchromb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Shinka T., Inoue Y., Ohse M. Simple and quantitative analysis of urinary sulfated tauro- and glycodihydroxycholic acids in infant with cholestasis by electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855(1):104–108. doi: 10.1016/j.jchromb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Huang J., Bathena S.P., Csanaky I.L. Simultaneous characterization of bile acids and their sulfate metabolites in mouse liver, plasma, bile, and urine using LC-MS/MS. J Pharmaceut Biomed Anal. 2011;55(5):1111–1119. doi: 10.1016/j.jpba.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Humbert L., Maubert M.A., Wolf C. Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;899:135–145. doi: 10.1016/j.jchromb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Bathena S.P., Mukherjee S., Olivera M. The profile of bile acids and their sulfate metabolites in human urine and serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;942–943:53–62. doi: 10.1016/j.jchromb.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Bathena S.P.R., Thakare R., Gautam N. Urinary bile acids as biomarkers for liver diseases I. Stability of the baseline profile in healthy subjects. Toxicol Sci. 2015;143(2):296–307. doi: 10.1093/toxsci/kfu227. [DOI] [PubMed] [Google Scholar]
- 24.Alnouti Y., Csanaky I.L., Klaassen C.D. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873(2):209–217. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikegawa S., Yanagihara T., Murao N. Separatory determination of bile acid 3-sulfates by liquid chromatography/electrospray ionization mass spectrometry. J Mass Spectrom. 1997;32(4):401–407. [Google Scholar]
- 26.Hofmann A.F., Hagey L.R. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65(16):2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burkard I., von Eckardstein A., Rentsch K.M. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;826(1–2):147–159. doi: 10.1016/j.jchromb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Scherer M., Gnewuch C., Schmitz G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(30):3920–3925. doi: 10.1016/j.jchromb.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Solaas K., Ulvestad A., Söreide O. Subcellular organization of bile acid amidation in human liver: a key issue in regulating the biosynthesis of bile salts. J Lipid Res. 2000;41(7):1154–1162. [PubMed] [Google Scholar]
- 30.Tribe R.M., Dann A.T., Kenyon A.P. Longitudinal profiles of 15 serum bile acids in patients with intrahepatic cholestasis of pregnancy. Am J Gastroenterol. 2010;105(3):585–595. doi: 10.1038/ajg.2009.633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.