Highlights
-
•
Limited information is known about treatment outcomes for squamous cell carcinoma of the bladder (SqCC).
-
•
Treatment for SqCC is extrapolated from urothelial carcinoma.
-
•
Outcomes of chemoRT for SqCC are very limited.
-
•
Following chemoRT, patients with SqCC do worse than counterparts with urothelial carcinoma.
Keywords: Squamous cell bladder cancer, Chemo-radiation, National cancer database
Abstract
Introduction
Squamous cell carcinoma (SqCC) is the second most common histology of primary bladder cancer, but still very limited information is known about its treatment outcomes. Most bladder cancer trials have excluded SqCC, and the current treatment paradigm for localized SqCC is extrapolated from results in urothelial carcinoma (UC). In particular, there is limited data on the efficacy of definitive chemo-radiotherapy (CRT). In this study, we compare overall survival outcomes between SqCC and UC patients treated with definitive CRT.
Materials/methods
We queried the National Cancer Database (NCDB) for muscle-invasive (cT2-T4 N0 M0) bladder cancer patients diagnosed from 2004 to 2013 who underwent concurrent CRT. Propensity matching was performed to match patients with SqCC to those with UC. OS was analyzed using the Kaplan-Meier survival method, and the log-rank test and Cox regression were used for analyses.
Results
3332 patients met inclusion criteria of which 79 (2.3%) had SqCC. 73.4% of SqCC patients had clinical T2 disease compared to 82.5% of UC patients. Unadjusted median OS for SqCC patients was 15.6 months (95% CI, 11.7–19.6) versus 29.1 months (95% CI, 27.5–30.7) for those with UC (P < 0.0001). On multivariable analysis, factors associated with worse OS included: SqCC histology [HR: 1.53 (95% CI, 1.19–1.97); P = 0.001], increasing age [HR: 1.02 (95% CI, 1.02–1.03); P < 0.0001], increasing clinical T-stage [HR: 1.21 (95% CI, 1.13–1.29); P < 0.0001], and Charlson-Deyo comorbidity index [HR: 1.26 (95% CI, 1.18–1.33); P < 0.0001]. Seventy-seven SqCC patients were included in the propensity-matched analysis (154 total patients) with a median OS for SqCC patients of 15.1 months (95% CI, 11.1–18.9) vs. 30.4 months (95% CI, 19.4–41.4) for patients with UC (P = 0.013).
Conclusions
This is the largest study to-date assessing survival outcomes for SqCC of the bladder treated with CRT. In this study, SqCC had worse overall survival compared to UC patients. Histology had a greater impact on survival than increasing T-stage, suggesting that histology should be an important factor when determining a patient’s treatment strategy and that treatment intensification in this subgroup may be warranted.
1. Introduction
Squamous cell carcinoma (SqCC) of the bladder is the second most common histologic variant of bladder cancer [1], [2]. Most bladder cancer trials have excluded SqCC, and the current treatment paradigm for localized SqCC is extrapolated from results in urothelial carcinoma (UC). There is limited data on the efficacy of these treatments in SqCC, particularly for definitive chemo-radiotherapy (CRT). In this study, we performed a propensity analysis to compare overall survival outcomes between SqCC and UC patients treated with definitive CRT.
2. Materials/methods
2.1. Data source and study population
Using the National Cancer Database (NCDB), we identified patients with clinical T2-4N0M0 bladder cancer diagnosed between 2004 and 2013 with complete demographic and treatment information [3]. All patients underwent transurethral resection of bladder tumor (TURBT) prior to definitive concurrent CRT. Patients who underwent cystectomy were excluded. Only patients receiving radiation therapy to the bladder or pelvis and total dose ≥40 Gy were included.
2.2. Statistical analysis
Overall survival (OS) was calculated from diagnosis until death, censoring at last follow-up for patients who were alive. The Kaplan-Meier method was used to estimate OS probabilities and Cox univariable and multivariable analyses were performed on all patients. The χ2 test and Fisher’s exact test were used to evaluate contingency tables, as appropriate. Variables with p-values <0.05 on univariable testing were entered into a multivariable analyses using the Cox proportional-hazards model. Propensity score analysis was performed to correct for baseline differences between histologic groups. A 1:1 matching algorithm including the variables used in univariable analysis was used with a caliper of 0.2 and without replacement. Significance was considered at a value of p < 0.05. SPSS v24 (IBM; Armonk, NY) was used.
3. Results
3.1. Demographics, patient and tumor characteristics
3332 CRT patients were identified with a median follow-up of 24.0 months (range, 1–142 months). 79 (2.3%) patients had SqCC and the remaining 3253 (97.7%) patients were diagnosed with UC. The median age was 78 years (range, 37–90) for SqCC patients and 77 years (range, 24–90) for UC patients. Patient demographic and clinical characteristics are summarized in Table 1. The majority of SqCC patients (54.4%) were female compared to 26.1% of patients with UC. 73.4% of SqCC patients had clinical T2 disease compared to 82.5% of UC patients. Median RT dose for patients with SqCC was 63 Gy (range, 40–84.6 Gy) and was not statistically different from patients with UC whose median dose was also 63 Gy (range, 44–74 Gy). The most common setting for treatment was either a comprehensive community cancer program (SqCC 50.6%; UC 48.3%) or an academic/research program (SqCC 24.1%; UC 26.1%).
Table 1.
Demographics and clinical characteristics.
| Number of Patients (%) |
||
|---|---|---|
| Squamous cell carcinoma | Urothelial carcinoma | |
| Age | ||
| ≤75y | 36 (45.6) | 1343 (41.3) |
| >75y | 43 (54.4) | 1910 (58.7) |
| Sex | ||
| Male | 36 (45.6) | 2405 (73.9) |
| Female | 43 (54.4) | 848 (26.1) |
| Race | ||
| White | 69 (87.3) | 2961 (91.0) |
| Black | 8 (10.1) | 206 (6.3) |
| Other | 2 (2.6) | 37 (1.2) |
| Unknown | 0 (0) | 49 (1.5) |
| Year of Diagnosis | ||
| 2004–2009 | 49 (62.0) | 1836 (56.4) |
| 2010–2013 | 30 (38.0) | 1417 (43.6) |
| Charlson Deyo Comorbidity | ||
| 0 | 54 (68.4) | 2165 (66.6) |
| 1 | 12 (15.2) | 769 (23.6) |
| >1 | 13 (16.5) | 319 (9.8) |
| Facility location | ||
| Central | 19 (24.1) | 913 (28.1) |
| Northeast | 18 (22.8) | 780 (24.0) |
| South/Southeast | 26 (32.9) | 981 (30.1) |
| West | 15 (18.9) | 576 (17.7) |
| Unknown | 1 (1.3) | 3 (0.1) |
| Facility Type | ||
| Academic/Research Program | 19 (24.1) | 849 (26.1) |
| Community Cancer Program | 9 (11.4) | 441 (13.6) |
| Comprehensive Community Cancer Program | 40 (50.6) | 1572 (48.3) |
| Integrated Network Cancer Program | 10 (12.7) | 388 (11.9) |
| Other | 1 (1.3) | 3 (0.1) |
| Insurance status | ||
| Medicaid | 4 (5.1) | 79 (2.4) |
| Medicare | 62 (78.5) | 2477 (76.1) |
| Not insured | 1 (1.3) | 48 (1.5) |
| Other government | 1 (1.3) | 53 (1.6) |
| Private | 11 (13.9) | 565 (17.4) |
| Unknown | 0 (0) | 31 (1.0) |
| Clinical T stage | ||
| T2 | 58 (73.4) | 2674 (82.2) |
| T3 | 11 (13.9) | 327 (10.1) |
| T4 | 10 (12.7) | 252 (7.7) |
3.2. Outcomes
The median OS of the entire cohort was 29.0 months (95% confidence interval [CI], 27.4–30.6 months). Patients with clinical T2 disease had median OS of 31.1 months (95% CI, 29.1–33.0) compared to 20.1 (95% CI, 17.8–23.6) and 20.7 (95% CI, 17.7–23.7) months for clinical T3 and T4 stage, respectively (P < 0.001 T2 versus T3 and T4). The unadjusted median overall survival for patients with SqCC was 15.6 months (95% CI, 11.7–19.6) versus 29.4 months (95% CI, 27.8–31.1) for those with UC (P < 0.0001) (Fig. 1). The estimated 3- and 5-year OS for SqCC were 27.5% and 20.5% compared to 43.8% and 29.5% for UC, respectively (P < 0.0001).
Fig. 1.
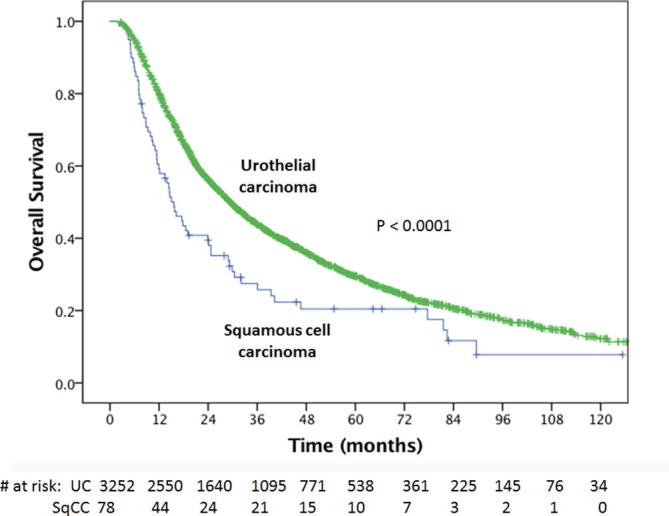
Kaplan Meier overall survival curves for squamous cell carcinoma versus urothelial cell carcinoma.
3.3. Univariable and multivariable analyses
On univariable analysis OS was affected by age, Charleson-Deyo comorbidity index (CCI), histology (UC versus SqCC), and clinical T-stage (Table 2). In the multivariable model, SqCC histology [HR: 1.53 (95% CI, 1.19–1.97); P = 0.001], increasing age [HR: 1.02 (95% CI, 1.02–1.03); P < 0.0001], increasing clinical T-stage [HR: 1.21 (95% CI, 1.13–1.29); P < 0.0001], and CCI [HR: 1.26 (95% CI, 1.18–1.33); P < 0.0001] were associated with worse OS.
Table 2.
Univariate and multivariate analysis.
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Histology | ||||
| UC | Reference group | Reference group | ||
| SCC | 1.60 (1.24–2.05) | <0.0001 | 1.51 (1.17–1.94) | 0.001 |
| Age (y) | ||||
| ≤75 | Reference group | Reference group | ||
| >75 | 1.46 (1.34–1.59) | <0.0001 | 1.47 (1.35–1.60) | <0.0001 |
| Sex | ||||
| Male | Reference group | – | ||
| Female | 1.09 (0.99–1.19) | 0.084 | – | |
| Race | ||||
| White | Reference group | – | ||
| Nonwhite | 1.00 (0.99–1.00) | 0.259 | – | |
| Year of Diagnosis | ||||
| 2004–2009 | Reference group | – | ||
| 2010–2013 | 1.024 (0.94–1.12) | 0.598 | – | |
| CCI | ||||
| 0 | Reference group | Reference group | ||
| 1 | 1.16 (1.05–1.28) | 0.003 | 1.17 (1.06–1.29) | 0.002 |
| >1 | 1.66 (1.48–1.92) | <0.0001 | 1.64 (1.44–1.87) | <0.0001 |
| Program Type | ||||
| Academic/Research Program | Reference group | – | ||
| Other | 1.01 (1.0–1.0) | 0.246 | – | |
| Clinical T stage | ||||
| T2 | Reference group | Reference group | ||
| T3 | 1.46 (1.28–1.66) | <0.0001 | 1.41 (1.24–1.61) | <0.0001 |
| T4 | 1.36 (1.17–1.58) | <0.0001 | 1.36 (1.17–1.58) | <0.0001 |
Bold = statistically significant (P < 0.05).
3.4. Matched cohort
Propensity score matching between the SqCC and UC histology groups was performed to address confounding patient, tumor, and demographic bias between the groups. This resulted in a successful match of 77 pairs of patients between SqCC and UC (154 total patients). There were no significant imbalances in matched variables in the resulting cohort and propensity scores were well matched (all standardized mean differences <0.2). For the matched cohort, the median OS for SqCC patients was 15.1 months (95% CI, 11.1–18.9) vs. 30.4 months (95% CI, 19.4–41.4) for patients with UC after propensity match adjusted analysis (P = 0.013, Fig. 2).
Fig. 2.

Kaplan Meier overall survival curves for squamous cell carcinoma versus urothelial cell carcinoma in the propensity matched cohort.
4. Discussion
Despite an overall increase in the incidence of bladder cancer in the USA over the last 40 years, the incidence of SqCC has declined but overall survival after therapy remains poor [1], [4]. An NCDB analysis demonstrated that patients with SqCC are more likely to be diagnosed with advanced disease than UC patients [1]. In a study from MD Anderson, SqCC patients were more likely to present with locally advanced disease and were more likely to fail loco-regionally [2]. Achieving local control for SqCC is therefore of particular importance, given the pattern of failure for SqCC and the morbidity and mortality of local recurrence [5], [6], [7]. The treatment strategies for localized SqCC are largely extrapolated from the treatment of urothelial bladder cancer and often include radiation therapy, radical cystectomy, and definitive CRT [4].
Several studies have reported worse survival outcomes for SqCC treated with either radical cystectomy or definitive RT alone compared to similarly treated patients with UC [8], [9], [10], [11], [12], [13], but there is limited data on SqCC patients treated with CRT. In one of the larger surgical series, Ehdaie et al. found that pure SCC (n = 78) was associated with significantly worse OS and disease-specific survival compared to UCC for patients treated with radical cystectomy [13]. In a study of SqCC patients treated with definitive RT alone from the early 1980s, Prempree et al. reported a 3-year disease-free survival of ∼16% for 33 SqCC patients treated to 60–65 Gy, results that compare unfavorably to contemporary series of RT alone for UC [11]. Given the unfavorable outcomes with radiation alone in SqCC and the results of randomized controlled trials in UC demonstrating improved survival with CRT vs. RT alone [14], [15], it seems reasonable to combine radiation therapy with chemotherapy for SqCC patients to take advantage of chemotherapy’s radiosensitizing effects, even though chemotherapy appears to have less benefit for SqCC than UC [16], [17], [18]. The BC2001 trial of CRT with 5-FU and mitomycin C vs. RT did allow SqCC patients on study, but only 2.7% (8 patients) had SqCC or adenocarcinoma [15]. Given the effectiveness and tolerability of 5-FU and mitomycin C in SqCC of the anus, the use of this regimen in SqCC of the bladder appears reasonable, especially for patients who are platinum-ineligible. Our purpose in this study was to determine the overall survival of SqCC patients treated with CRT and compare overall survival outcomes between SqCC and UC patients.
In our study, we found that SqCC patients treated with CRT had worse overall survival compared to UC patients in a multivariable model. The discrepancy in overall survival remained after propensity score matching between SqCC and UC. In light of the poor outcomes with definitive CRT in SqCC patients compared to UC patients, treatment intensification strategies for SqCC may be warranted, such as the use of immunotherapy, novel chemotherapeutic strategies, or radiation dose escalation/hypofractionation. Additionally, our findings are not a comparison of CRT versus radical cystectomy for this uncommon histology. Ehdaie et al. reported an estimated 5-year OS of 40% (95% CI, 28–51) for SqCC which is superior to the survival in our study, however many patients receiving CRT may have been inoperable due to co-morbidities or other various reasons [13]. This comparison, however, suggests a role for radical cystectomy in surgical candidates. Institutional retrospective and future prospective studies comparing CRT and radical cystectomy are encouraged.
There are several limitations to this retrospective analysis. In our study, the OS for patients with UC treated with CRT was worse than the OS reported in prior RCTs and large single-institution retrospective series. For example, the BC2001 trial reported a five-year OS of 48% compared to only 30% in our study [15]. This difference may be related to the older median age in our cohort and inclusion of patients with co-morbidities who would have been excluded from a RCT as well as heterogeneity in the radiation dose, treatment volumes, and chemotherapy administration. Importantly, the lack of details on chemotherapeutic regimens used in this study (e.g. chemotherapy agent(s), number of cycles administered, and dose reductions) is a major limitation of the database. Another limitation of this study is that while the size of the UC cohort treated with CRT is large, the size of the SqCC cohort is relatively small. Lastly, limitations include those inherent to the NCDB, such as the potential for unidentified confounding factors and missing/miscoded data.
5. Conclusions
This is the largest study to-date assessing survival outcomes for SqCC of the bladder treated with CRT. In this observational study, SqCC had worse overall survival compared to UC patients. On propensity analysis, OS remained significantly worse for the SqCC cohort. While the sample size is relatively small and subject to selection bias, the results suggest that treatment intensification strategies may be reasonable for this less common histologic subtype.
Funding sources
None.
Conflict of interest
John P. Christodouleas reports employee status at Elekta, Inc.
References
- 1.Royce T.J., Lin C.C., Gray P.J., Shipley W.U., Jemal A., Efstathiou J.A. Clinical characteristics and outcomes of nonurothelial cell carcinoma of the bladder: results from the National Cancer Data Base. Urol Oncol. 2018;36(2):78.e1–78.e12. doi: 10.1016/j.urolonc.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Kassouf W., Spiess P.E., Siefker-Radtke A. Outcome and patterns of recurrence of nonbilharzial pure squamous cell carcinoma of the bladder: a contemporary review of The University of Texas M D Anderson Cancer Center experience. Cancer. 2007;110(4):764–769. doi: 10.1002/cncr.22853. [DOI] [PubMed] [Google Scholar]
- 3.Fischer-Valuck B.W., Rao Y.J., Rudra S. Treatment patterns and overall survival outcomes of octogenarians with muscle invasive cancer of the bladder: an analysis of the national cancer database. J Urol. 2018;199(2):416–423. doi: 10.1016/j.juro.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Rahman O. Squamous cell carcinoma of the bladder: a SEER database analysis. Clin Genitourinary Cancer. 2017;15(3):e463–e468. doi: 10.1016/j.clgc.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Baumann B.C., Sargos P., Eapen L.J. The rationale for post-operative radiation in localized bladder cancer. Bladder Cancer. 2017;3(1):19–30. doi: 10.3233/BLC-160081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann B.C., Guzzo T.J., He J. Bladder cancer patterns of pelvic failure: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(2):363–369. doi: 10.1016/j.ijrobp.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Zaghloul M.S., Christodouleas J.P., Smith A. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg. 2018;153(1):e174591. doi: 10.1001/jamasurg.2017.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manunta A., Vincendeau S., Kiriakou G., Lobel B., Guille F. Non-transitional cell bladder carcinomas. BJU Int. 2005;95(4):497–502. doi: 10.1111/j.1464-410X.2005.05327.x. [DOI] [PubMed] [Google Scholar]
- 9.Rundle J.S., Hart A.J., McGeorge A., Smith J.S., Malcolm A.J., Smith P.M. Squamous cell carcinoma of bladder. A review of 114 patients. Br J Urol. 1982;54(5):522–526. doi: 10.1111/j.1464-410x.1982.tb13580.x. [DOI] [PubMed] [Google Scholar]
- 10.Bessette P.L., Abell M.R., Herwig K.R. A clinicopathologic study of squamous cell carcinoma of the bladder. J Urol. 1974;112(1):66–67. doi: 10.1016/s0022-5347(17)59644-5. [DOI] [PubMed] [Google Scholar]
- 11.Prempree T., Amornmarn R. Radiation management of squamous cell carcinoma of the bladder. Acta Radiol Oncol. 1984;23(1):37–42. doi: 10.3109/02841868409135983. [DOI] [PubMed] [Google Scholar]
- 12.Scosyrev E., Yao J., Messing E. Urothelial carcinoma versus squamous cell carcinoma of bladder: is survival different with stage adjustment? Urology. 2009;73(4):822–827. doi: 10.1016/j.urology.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Ehdaie B., Maschino A., Shariat S.F. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol. 2012;187(1):74–79. doi: 10.1016/j.juro.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppin C.M., Gospodarowicz M.K., James K. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14(11):2901–2907. doi: 10.1200/JCO.1996.14.11.2901. [DOI] [PubMed] [Google Scholar]
- 15.James N.D., Hussain S.A., Hall E. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 16.Serretta V., Pomara G., Piazza F., Gange E. Pure squamous cell carcinoma of the bladder in western countries. Report on 19 consecutive cases. Eur Urol. 2000;37(1):85–89. doi: 10.1159/000020105. [DOI] [PubMed] [Google Scholar]
- 17.Galsky M.D., Iasonos A., Mironov S. Prospective trial of ifosfamide, paclitaxel, and cisplatin in patients with advanced non-transitional cell carcinoma of the urothelial tract. Urology. 2007;69(2):255–259. doi: 10.1016/j.urology.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Zaghloul M.S., Christodouleas J.P., Smith A. A randomized clinical trial comparing adjuvant radiation versus chemo-RT versus chemotherapy alone after radical cystectomy for locally advanced bladder cancer. J Clin Oncol. 2016;34(2_suppl) abstract 356-356. [Google Scholar]


