Abstract
This article discusses research conducted on the sampling of two tick species: Ixodes ricinus and Rhipicephalus bursa. Ticks were collected in northern Algeria (El Tarf) in 2014 and studied for differences in abundance and seasonal distribution of population dynamics, as well as tested by PCR for the presence of Rickettsia spp. By molecular tools, four Rickettsia pathogens agents were detected: R. helvetica, R. monacensis, R. raoultii and R. massiliae.
Keywords: Algeria, Ixodes, PCR, Rhipicephalus, Rickettsia, ticks
Introduction
Ticks are obligatory haematophagous arthropods that parasitize all classes of vertebrates in almost all regions of the world. They are currently considered the second most common vector of human infectious diseases in the world after mosquitoes. Each tick species has privileged environmental conditions and biotopes that determine the geographical distribution of ticks and consequently the areas at risk of tick-borne diseases [1].
Ticks are limited to geographic locations where climatic conditions are conducive to the completion of their life cycle, and changing climatic conditions (temperature and precipitation) can alter the geographical extent and seasonal period of disease risk and subsequent transmission dynamics in endemic areas, leading to changes in spatial and temporal patterns of human disease [2]. The impact of ticks on human public health was recognized with the emergence of Lyme disease 25 years ago [3].
Since then, about 15 tick rickettsioses have appeared [4]. Tick rickettsiosis is an infection caused by a mandatory Gram-negative intracellular bacterium of the spotted fever group with the genus Rickettsia within the Rickettsiaceae family of the order Rickettsiales [5]. These zoonoses are now recognized as important and emerging vector-borne infections throughout the world. Rickettsial disease is widespread throughout the world, with a seasonal epidemic appearance. In a study of tick-borne encephalitis virus, Korenberg [6] reported that seasonal changes in the prevalence of infection in active, unfed adult ticks in a natural population are determined by the virus content of individual ticks at the time of their activation and also by the duration of subsequent virus persistence in ticks.
Knowledge of the seasonal abundance of ticks and the prevalence of tick-borne pathogens is therefore essential to describe in order to better understand the risk of tick-borne diseases. Many studies are therefore being conducted on the detection of Rickettsia in different tick species, but few are studying the dynamics of transmission of this bacterium. The Mediterranean region is a complex biogeographical area thanks to its the diversity of habitats and sudden landscape changes, which are the result of variations in altitude. This region is mainly characterized by a warm climatic season (summer), which coincides with the driest period of the year; the rainy season coincides with the coldest period of the year. The original forests of the Mediterranean region comprise Quercus spp. and Olea europaea, which define the region's main regions [7].
The variability of climatic characteristics provides a tick fauna rich in species, some of which are specific to the Mediterranean region. Because of these main climatic characteristics, the composition of tick fauna in the Mediterranean region is highly variable, and the distribution of the most important tick species can change significantly depending on the specific characteristics of the region concerned.
Algeria is one of the Mediterranean regions known for its great bioclimatic variety. Our objectives were to clarify the differences in abundance and dynamics of tick populations, and to determine the seasonal distribution of Rickettsia in the far east of Algeria by real-time PCR.
Materials and methods
Study area
Ticks were collected monthly between January and December 2014 from cattle in the El Tarf region of the northeastern border of Algeria (near the Tunisian border) (GPS coordinates 36°45′7.0 N; 8°10′0 E). The climate is Mediterranean, with a rainy season from autumn to spring, and a long dry and hot season in summer [8]. All ticks were adult. We identified them morphologically using the usual taxonomic keys of the species or genus. Ticks were stored in ethanol at 90°C at room temperature.
DNA extraction
The ticks were rinsed with distilled water for 10 minutes, dried on sterile filter paper in a laminar flow hood and individually ground in Eppendorf sterile tubes (Hamburg, Germany). Total genomic DNA of bacterial strains was extracted with the Qiagen QIAamp Blood Kit (Qiagen, Hilden, Germany).
Real-time PCR
In order to increase the size of the treated sample and to see if there was a seasonal effect on the prevalence of Rickettsia, a probe was specifically designed (Table 1).
Table 1.
List of primers and probes used for PCR
| Rickettsia sp. | Probe | Primers |
|---|---|---|
| R. massiliae | 6-FAM-GTGCAAGCAGCGGCACAACC-TAMARA | R: TTGGATGAGTGTGACGGACT F: TACCGGAACCGTTGCTTTAC |
| R. helvetica | 6-FAM-CCTGTGTAGACGATTCAAGAGGGATGA-TAMRA | R: GTTTAATGGGCATTCGGCTA F: ACTTGGATGAGGCAAACACC |
| R. raoultii | 6-FAM-TGGGGCTTTTTCATGTCCTAAGCACA-TAMRA | R: AAATTGATGGTGCAGGAGTGG F: CCAATACCTTGCCCAAAACA |
| R. monacensis | 6-FAM-AACTGTTGAGGTAGAAGCATTCTGCTCATGGTCTG-TAMRA | R: GTTCTCTTTCGGCATTTTAC F: GCAAAAGGGTTAGCTCCRA |
PCR was performed using a Smart Cycler instrument (Cepheid, Sunnyvale, CA, USA). The PCR mixture included a final volume of 20 μL with 10 μL from the Probe Master kit (Qiagen), 0.5 μL (10 pmol/μL) from each primer, 2 μL (2 μmol/μL) probe, 2 μL (2 μmol/μL) probe, 2 μL distilled water and 5 μL extracted DNA. The real-time quantitative PCR reactions were incubated in the Smart Cycler at 94°C for 2 minutes, followed by 50 cycles of a two-step amplification protocol of 94°C for 5 seconds and 60°C for 30 seconds. Fluorescence was monitored during the annealing phase of each cycle, and the results were analysed with Smart Cycler 2.0c (Cepheid). A negative control consisting of DNA extracted from uninfected laboratory ticks and a positive Rickettsia control (one positive control every 20 samples) was included in each test.
Results
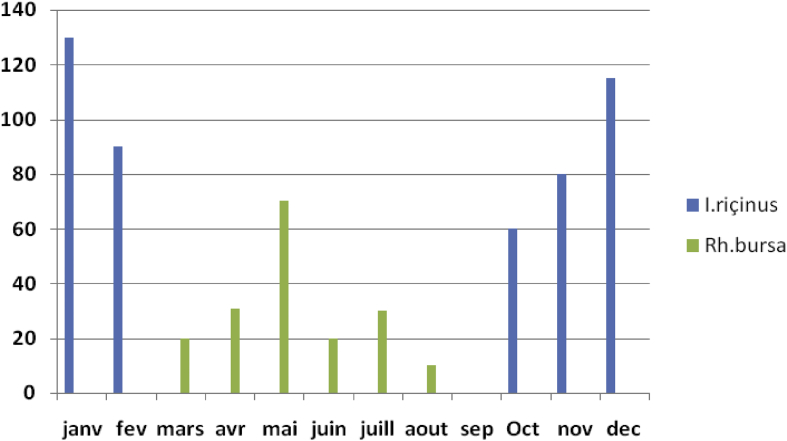
In this part of our survey, hard ticks were collected monthly between January and December 2014 from cattle in the El Tarf region (Fig. 1).
Fig. 1.
Seasonal dynamics of tick collection.
Several cattle farms were part of our survey. A total of 656 ticks were collected, 475 Ixodes ricinus and 181 Rhipicephalus bursa (Table 2).
Table 2.
Number of ticks collected by month
| Month | Ixodes ricinus | Rhipicephalus bursa |
|---|---|---|
| January | 130 | |
| February | 90 | |
| March | 20 | |
| April | 31 | |
| May | 70 | |
| June | 20 | |
| July | 30 | |
| August | 10 | |
| September | ||
| October | 60 | |
| November | 80 | |
| December | 115 | |
| Total | 475 | 181 |
All ticks collected were identified by entomologic keys [7].
A total of 120 I. ricinus individuals and 60 of Rh. bursa were analysed by molecular tools for the detection of Rickettsia pathogens (Table 3). Four species of Rickettsia were detected: R. helvetica, R. monacensis and R. raoultii from I. ricinus, and R. massiliae from Rh. bursa, with different prevalence during the fourth season of the year (Table 3, Table 4).
Table 3.
Seasonal prevalence of Rickettsia spp. in Ixodes ricinus
| I. ricinus |
R. helvetica |
R. monacensis |
R. raoultii |
|||
|---|---|---|---|---|---|---|
| Autumn | Winter | Autumn | Winter | Autumn | Winter | |
| No. of ticks analysed | 120 | 120 | 120 | 120 | 120 | 120 |
| No. of positive ticks | 24 | 52 | 32 | 64 | — | 36 |
| Prevalence of infection | 20% | 43.33% | 26.66% | 53.33% | — | 30% |
Table 4.
Seasonal prevalence of Rickettsia massiliae in Rhipicephalus bursa
| Characteristic |
R. massiliae |
|
|---|---|---|
| Spring | Summer | |
| No. of Rh. bursa ticks analysed | 60 | 60 |
| No. of positive ticks | 38 | 8 |
| Prevalence of infection | 63.33% | 13.33% |
Discussion
During the research phase, a total of 656 ticks were collected in the study area. Relative abundance analysis revealed that I. ricinus species ticks were dominant compared to Rh. bursa (475/181). The seasonal dynamics of ticks were assessed from January to December. I. ricinus had seasonal activity; its activity seemed to be limited to cooler months (autumn–winter), although the largest number of ticks was recorded in winter (n = 335) compared to autumn (n = 140). Rh. bursa has an activity adapted to the warmer months, and the number of species is higher in spring (n = 121) than in summer (n = 560).
In North Africa, the range of I. ricinus is limited to the humid climatic zone; the biotopes of this species essentially correspond to oak formations (Quercus faginea and Q. suber) [9], which explains the frequency of this species in the study area considered among the most humid in North Africa (1300 mm of rain per year). The maximum abundance of Rh. bursa has been reported in spring compared to summer. Rh. bursa habitat is formed by grasslands and forest edges, especially in areas of low hills [10].
In Tunisia, Bouattour et al. [9] showed that the adult activity of this tick varies from April to August. In this study, a total of 460 ticks were identified as I. ricinus or Rh. bursa, and were analysed by specific Rickettsia probes. The DNA of R. helvetica, R. monacensis and R. raoultii were detected in I. ricinus, and the DNA of R. massiliae was detected in Rh. bursa. The seasonal prevalence of Rickettsia detected showed 43.33% of I. ricinus were infected with R. helvetica in winter, but in autumn only 20% of this Rickettsia were detected. R. monacensis showed a high prevalence in winter (53.33%) compared to autumn (26.66%).
However, R. raoultii showed a limited presence in winter. Otherwise, I. ricinus is the main vector of the number of human pathogens, which is why R. monacensis was described when it was isolated from ticks of I. ricinus collected in 1998 from an urban park in Munich, Germany [11]. R. helvetica was isolated for the first time in Switzerland [12], [13] from I. ricinus (the main vector of Lyme borreliosis). DNA of R. raoultii was found on Ixodes spp. ticks in southern Poland and on Dermacentor spp. ticks in northeastern Poland.
Until recently, R. raoultii had only been reported on Dermacentor nuttalli, Rhipicephalus sp. and Dermacentor spp. in Europe and Asia (i.e. Siberia and Astrakhan area) [14], [15]. In our survey, R. massiliae was found with a prevalence of 31.66% in spring and 11.66% in summer on Rh. bursa.
Elsewhere, R. massiliae was isolated from ticks of Rhipicephalus sanguineus collected near Marseille in 1992. It was characterized as a distinct species within the spotted fever group of rickettsiae and named R. massiliae [16]. In Algeria, R. conorii, R. aeschlimannii and R. massiliae were detected by PCR on ticks [17]. Therefore, I. ricinus showed a high prevalence in 3 Rickettsia species in winter, but Rh. bursa showed a high prevalence rate in spring.
One of our main objectives was to understand what factors can influence the evolution of the intensity of tick infection by pathogens. This may include the density of the vector or the reactivation phenomenon. In fact, the relationships between ticks and Rickettsia are complex, and the mechanism used by Rickettsia to survive in unfed wintering ticks or during molting is poorly understood, although experiments have deciphered the phenomenon of reactivation of rickettsian virulence after infected ticks have taken a blood meal. The underlying molecular events after feeding have not yet been elucidated; a tick larva enters a rest period before becoming a searching nymph the following year. Even though the precise mechanism of Rickettsia reactivation is not known, it is believed that temperature variations and blood sampling reactivate Rickettsia. As in the Borrelia burgdorferi–Ixodes scapularis model, Rickettsia transmission probably cannot occur until 24 hours after tick fixation, which gives Rickettsia time to grow [18].
In another study, Korenberg [6] reported the seasonal populations dynamics of Ixodes ticks and tick-borne encephalitis virus, demonstrating that seasonal changes in the prevalence of infection in active nonfed adult ticks in a natural population are generally determined by the virus content of individual ticks at their activation and the duration of tick virus persistence or the rate of virus loss during the subsequent period.
In different regions, these processes may have some specific characteristics. In general, the infection parameters of adult ticks Ixodes persulcatus are initially higher and then gradually decrease during their activity period [19]. In addition, the seasonal dynamics of infectivity in adult I. persulcatus ticks can change from year to year, even in the same natural outbreak. For example, 2-year studies in European southern taiga forests on the same test plot, using the same tick collection and virologic analysis methods, yielded the following results.
During the first year, the frequency of tick-borne encephalitis virus isolation in May (15% of bioassays were positive) was about twice as high as in June and July. The following year, this parameter remained at about the same level throughout the tick activity period: 7.2% in May, 7.9% in June and 5.5% in July [20]. In I. ricinus ticks, the initial prevalence of infection in a new generation in the autumn is apparently more or less unchanged during the cold winter period. Therefore, studies conducted in regions with such a climate do not reveal any seasonal dynamics distinct from infection in nymphs and adult ticks during the period of their activity [21].
Conclusion
On the basis of our results, we conclude that there are few data to explain our results. Hence, studies on Rickettsia's seasonal dynamics remain poor, which requires further research.
Acknowledgements
We acknowledge all our contributors. My research was carried in the laboratory of IHU Méditerranée-Infection, Marseille, France and all products and primers used were financed by the laboratory.
Conflict of interest
None declared.
References
- 1.Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32(6):897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 2.Gage K.L., Burkot T.R., Eisen R.J., Hayes E.B. Climate and vectorborne diseases. Am J Prev Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Wormser G.P. Hematogenous dissemination in early Lyme disease. Wien Klin Wochenschr. 2006;118:634–637. doi: 10.1007/s00508-006-0688-9. [DOI] [PubMed] [Google Scholar]
- 4.Parola P., Paddock C., Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechah Y., Capo C., Mege J.L., Raoult D. Epidemic typhus. Lancet Infect Dis. 2008;8:417–426. doi: 10.1016/S1473-3099(08)70150-6. [DOI] [PubMed] [Google Scholar]
- 6.Korenberg E. Seasonal population dynamics of Ixodes ticks and tick-borne encephalitis virus. Exp Appl Acarol. 2000;24:665–681. doi: 10.1023/a:1010798518261. [DOI] [PubMed] [Google Scholar]
- 7.Estrada-Peña A., Bouattour A., Camicas J., Walker A.R. International Consortium for Ticks and Tick-Borne Diseases; Utrecht, The Netherlands: 2004. Ticks of domestic animals in the Mediterranean region: a guide to identification of species; pp. 52–55. [Google Scholar]
- 8.Emberger L. Travaux de botanique et d’écologie. Edition Masson et Cie; Paris: 1971. La végétation de la région méditerranéenne (Essai d’une classification des groupements végétaux) pp. 25–50. [Google Scholar]
- 9.Bouattour A., Darghouth M.A., Daoud A. Distribution and ecology of ticks (Acari: Ixodidae) infesting livestock in Tunisia: an overview of eighth years field collections. Parasitiologia. 1999;41:5–10. [PubMed] [Google Scholar]
- 10.Bourdeau P. Les tiques d’importance Vétérinaire et médicale. 2ère partie: principales espèces de tiques dures (Ixodidae et Amblyommidae) Point Vet. 1993;25:27–41. [Google Scholar]
- 11.Simser J.A., Palmer A.T., Fingerle V., Wilske B., Kurtti T.J., Munderloh U.G. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microbiol. 2002;68:4559–4566. doi: 10.1128/AEM.68.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beati L., Peter O., Burgdorfer W., Aeschlimann A., Raoult D. Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group of rickettsiae. Int J Syst Bacteriol. 1993;43:521–526. doi: 10.1099/00207713-43-3-521. [DOI] [PubMed] [Google Scholar]
- 13.Burgdorfer W., Aeschlimann A., Peter O., Hayes S.F., Philip R.N. Ixodes ricinus, vector of a hitherto undescribed spotted fever group agent in Switzerland. Acta Trop. 1979;36:357–367. [PubMed] [Google Scholar]
- 14.Mediannikov O., Matsumoto K., Samoylenko I., Drancourt M., Roux V., Rydkina E. Rickettsia raoultii sp. nov., a spotted fever group Rickettsia associated with Dermacentor ticks in Europe and Russia. Int J Syst Evol Microbiol. 2008;40:149–156. doi: 10.1099/ijs.0.64952-0. [DOI] [PubMed] [Google Scholar]
- 15.Dautel H., Dippel C., Oehme R., Hartelt K., Schettler E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int J Med Microbiol. 2006;40:149–156. doi: 10.1016/j.ijmm.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Beati L., Finidori J.P., Gilot B., Raoult D. Comparison of serologic typing, sodium dodecyl sulfate–polyacrylamide gel electrophoresis protein analysis, and genetic restriction fragment length polymorphism analysis for identification of rickettsiae: characterization of two new rickettsial strains. J Clin Microbiol. 1992;30:1922–1930. doi: 10.1128/jcm.30.8.1922-1930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitam I., Parola P., Matsumoto K., Rolain J.M., Baziz B., Boubidi S.C. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann N Y Acad Sci. 2006;1078:368–372. doi: 10.1196/annals.1374.073. [DOI] [PubMed] [Google Scholar]
- 18.Azad A.F., Beard C.B. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis. 1998;4:179–186. doi: 10.3201/eid0402.980205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katin A.A. Tick-borne encephalitis and Omsk hemorrhagic fever in the Tyumen region (in Russian) 1993. Specific spatiotemporal features in the life of natural tick-borne encephalitis foci in relation to the problem of predicting their epidemiological activity; pp. 22–42. Omsk. [Google Scholar]
- 20.Pchelkina A., Korenberg E.I., Zemskaya A.A., Suvorova L.G., Kovalevskii YuV. Studies on virus prevalence in Ixodes persulcatus P. Sch. ticks from tick-borne encephalitis foci located in East-European southern taiga forests. In: Markevich A., editor. Abstracts of the second acarological conference. Naukova Dumka; Kyiv, Ukraine: 1970. pp. 96–97. [Google Scholar]
- 21.Okulova N.M., Bannova G.G., Mikhailova I.S. Virus prevalence in vectors from a conjugate focus of tick-borne encephalitis and Kemerovo virus located in the southern Kemerovo region. Med Virusol. 1973;21:89–93. [Google Scholar]