Abstract
Objective
There is no definitive guideline for the significance and cut-off value of squamous-cell carcinoma antigen (SCC-Ag) in cervical cancer. Thus, we analyzed the significance and optimal cut-off value of SCC-Ag for predicting tumor recurrence and patient survival in squamous-cell carcinoma of uterine cervix.
Methods
From January 2010 to October 2016, we enrolled 304 cervical cancer patients with squamous-cell carcinoma staging International Federation of Gynecology and Obstetrics (FIGO) Ib–IVa and treated with definitive chemoradiotherapy (CRT) followed by intra-cavitary radiotherapy (ICR). The cut-off value of SCC-Ag level for tumor recurrence was calculated using the receiver operating characteristic (ROC) curve. The recurrence-free survival (RFS) and overall survival (OS) were assessed using Kaplan-Meier method to estimate the significance of SCC-Ag level.
Results
The optimal cut-off value of SCC-Ag level for predicting tumor recurrence was calculated and set at 4.0 ng/mL in the ROC curve. After a median follow-up period of 36.5 months, the 3-year RFS (56.6% vs. 80.2%, p<0.001) and OS (72.1% vs. 86.8%, p=0.005) were significantly lower in SCC-Ag ≥4 ng/mL arm than in <4 ng/mL arm. The 3-year locoregional recurrence (17.6% vs. 7.0%, p=0.012), distant metastasis (20.4% vs. 6.9%, p=0.002), and para-aortic recurrence (9.4% vs. 2.1%, p=0.012) rates were significantly higher in SCC-Ag ≥4 ng/mL arm than in SCC-Ag <4 ng/mL arm.
Conclusion
Pre-treatment SCC-Ag level higher than 4 ng/mL may be a useful predictor of tumor recurrence in patients with squamous-cell carcinoma of uterine cervix treated with definitive CRT and ICR.
Keywords: Cervical Cancer, Chemoradiotherapy, Recurrence, Squamous Cell Carcinoma Antigen
INTRODUCTION
Uterine cervix cancer is a malignancy with a high incidence in women, and it is the 4th common female tumor globally [1]. In early cervical cancer, the cure rate without recurrence based on surgery or chemoradiotherapy (CRT) is high, but the mortality rate increases in advanced stages or delayed detection of tumor recurrence [2,3]. Several studies reported clinical factors of tumor recurrence and patient survival for advanced cervical cancer. Known predictive factors of tumor recurrence in cervical cancer include primary tumor size and nodal involvement [4,5]. Postoperative pathological findings including surgical margin involvement and deep stromal and lymphovascular invasion are associated with patient's poor prognosis [6]. However, these factors are determined surgically. In the era when CRT with curative intent is mainly performed for advanced cervical cancer, several studies focused on the predictors of tumor recurrence and patient survival before treatment.
Until now, no definitive tumor marker is available at initial diagnosis or surveillance, to predict tumor recurrence in cervical cancer. In a few studies, squamous-cell carcinoma antigen (SCC-Ag) which was a subfraction of tumor-associated antigen and derived from the tissues of squamous-cell carcinoma exhibited higher sensitivity during initial diagnosis of squamous-cell cervical cancer than tissue polypeptide antigen (TPA), carcinoembryonic antigen (CEA), and cancer antigen 125 (CA 125) [7,8]. In addition, SCC-Ag level was associated with tumor stage and nodal involvement in cervical cancer [9,10]. Since 1990s, most studies set the cut-off value of SCC-Ag level for tumor recurrence at 1.5–2.5 ng/mL. However, no consensus is available regarding this value [11,12,13,14,15,16,17]. SCC-Ag is elevated in 28%–88% of all patients with squamous-cell carcinoma of uterine cervix, which is considered as a useful diagnostic tool, although it has a weak influence on prognosis of cervical cancer [8].
In this multi-center study, we determined the optimal cut-off value of SCC-Ag for the prediction of tumor recurrence in squamous-cell carcinoma of uterine cervix and analyzed its significance on patient survival.
MATERIALS AND METHODS
1. Patients
We performed a multi-institutional analysis of patients with cervical cancer treated with definitive CRT and intra-cavitary radiotherapy (ICR) as a radical approach. Eligible criteria were as follows: 1) squamous cell carcinoma of uterine cervix; 2) International Federation of Gynecology and Obstetrics (FIGO) stage Ib–IVa; and 3) lymph node (LN) localization in the pelvic regional area. Patients with distant metastasis (DM) or significant para-aortic lymph nodes (PANs) or adenocarcinoma of uterine cervix were excluded from this study. Staging workups included pelvic examination, flexible sigmoidoscopy, chest and abdomniopelvic computed tomography (CT), pelvic magnetic resonance imaging (MRI), and SCC-Ag measurements. The short-axis diameter of LN of >5 mm observed on MRI was considered positive [18]. Positron emission tomography (PET)-CT is optional in our study. We consider the short-axis diameter of PAN ≥1.0 cm in abdomen CT and/or positive PAN in PET-CT as M1. SCC-Ag levels were evaluated before and after radiotherapy. Serum SCC-Ag was measured using an immunoradiometric assay with the ARCHITECT SCC kit (Abbott Diagnostics, Chicago, IL, USA).
2. Treatment
Patients underwent three-dimensional or intensity-modulated external-beam radiotherapy followed by ICR. All patients underwent CT-based simulation for external-beam radiotherapy and received 45 to 66 Gy (median, 50.4 Gy) of radiation. 126 patients underwent boost irradiation up to 3.6–16 Gy (median, 9.0 Gy) for parametrial invasive tumor or pelvic metastatic LN after 45 to 50.4 Gy of pelvic irradiation. Concurrent 6 cycles of weekly cisplatin (40 mg/m2) was administered with radiotherapy. High-dose rate ICR of 20 to 30 Gy in 4 to 6 fractions (median 30 Gy in 6 fractions) was delivered following CRT.
3. Evaluation and follow-up
After the completion of radiotherapy, patients were followed up every 3 to 6 months for 5 years. The initial response was evaluated with the Response Evaluation Criteria in Solid Tumors (RECIST) criteria in the follow-up MRI at 3 months after completion of radiotherapy.
Locoregional recurrence (LRR) was defined as recurrence in the pelvic cavity. The stable or progressive disease on the follow-up MRI was assessed as LRR. DM was defined as nodal recurrence out of pelvic cavity or recurrence at other distant organs. Para-aortic recurrence (PAR) was defined as recurrence in para-aortic nodal area but not in distant organs. Recurrence-free survival (RFS) was defined as interval between the time of initial diagnosis and recurrence, last follow-up or death. Overall survival (OS) was defined as interval from the initial diagnosis to death or last follow-up.
4. Statistical analyses
The cut-off value of SCC-Ag for tumor recurrence was calculated using the receiver operating characteristic (ROC) curve. The recurrence and survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test between 2 groups in the univariate analysis. We evaluated the prognostic factors for recurrence and survival using Cox proportional hazards regression model in the multivariate analysis. Independent t-test was used to compare continuous variables and χ2 test was used to compare categorical variables between 2 groups. A p-value <0.05 was considered as statistically significant one. For multiple comparisons of recurrence and survival rates among 3 groups, the Bonferroni adjustment was applied. Statistical analyses were performed using SPSS statistical software (IBM Corp., Armonk, NY, USA) and R version 3.1.2 (R Development Core Team, Vienna, Austria).
RESULTS
From January 2010 to October 2016, a total of 305 patients were treated at five tertiary institutions, and 304 patients were finally included in this study, except one who failed to complete planned radiotherapy. The follow-up time ranged from 6 to 55.4 months (median 36.5 months). Patient characteristics are detailed in Table 1. Advanced FIGO stage higher than IIb was diagnosed in 254 (83.6%) out of 304 patients and 164 (53.9%) out of 304 patients had positive pelvic LNs. The median cervical tumor size was 4.5 cm and 156 (51.3%) out of 304 patients had a cervical mass greater than 4.5 cm.
Table 1. Patient characteristics (n=304).
| Characteristic | No. (%) | |
|---|---|---|
| Age (yr) | ||
| <60 | 168 (55.3) | |
| ≥60 | 136 (44.7) | |
| FIGO stage | ||
| Ib | 32 (10.5) | |
| IIa | 18 (5.9) | |
| IIb | 204 (67.1) | |
| IIIa | 9 (3.0) | |
| IIIb | 24 (7.9) | |
| IVa | 17 (5.6) | |
| Initial tumor size (cm) | ||
| <4.5 | 148 (48.7) | |
| ≥4.5 | 156 (51.3) | |
| Clinical nodal involvement | ||
| No | 140 (46.1) | |
| Yes | 164 (53.9) | |
| Initial SCC-Ag (ng/mL) | ||
| <1.5 | 49 (16.1) | |
| ≥1.5 | 255 (83.9) | |
FIGO, International Federation of Gynecologic Oncology; SCC-Ag, squamous-cell carcinoma antigen.
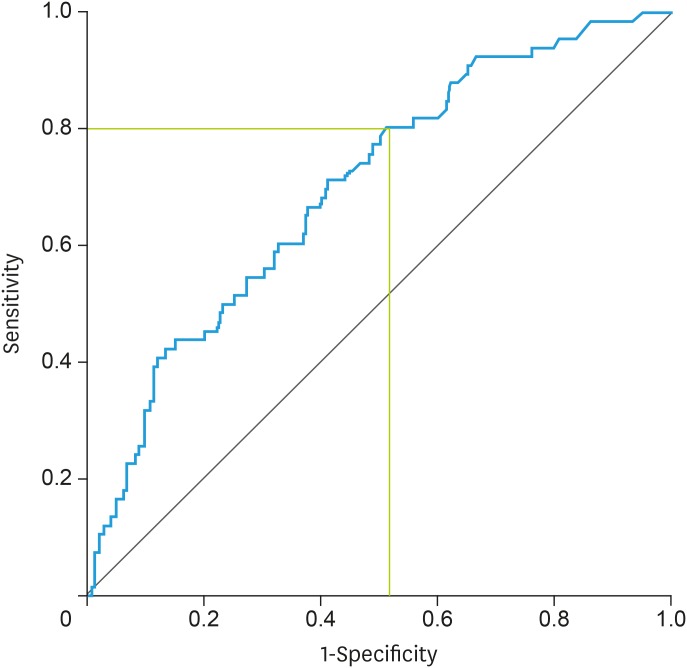
There were a total of 66 (21.7%) cases of tumor recurrence and 65 (21.4%) cases of death. The ROC curve was used to determine the optimal cut-off value of the SCC-Ag level associated with tumor recurrence. The threshold was calculated using the Youden index [maximum (sensitivity+specificity−1)] and the results showed a Youden index at 4.0 ng/mL (sensitivity, 0.803; specificity, 0.487; positive predictive value, 0.293; negative predictive value, 0.894; accuracy, 0.536; efficiency, 0.700; all p<0.001) (Fig. 1). Thus, the cut-off value of initial SCC-Ag level was set at 4.0 ng/mL. The number of patients with SCC-Ag ≥4 ng/mL and <4 ng/mL were 181 and 123, respectively. Patient characteristics of the two groups were compared when the cut-off value was 4 ng/mL in Table 2. SCC-Ag ≥4 ng/mL arm was associated with significantly higher FIGO stage (p=0.003), larger tumor size (p<0.001), and pelvic LN involvement (p=0.001) than SCC-Ag <4 ng/mL arm.
Fig. 1. ROC curve for calculating the cut-off value of SCC-Ag level on tumor recurrence.
ROC, receiver operating characteristic; SCC-Ag, squamous-cell carcinoma antigen.
Table 2. Comparison between SCC-Ag ≥4 ng/mL arm and SCC-Ag <4 ng/mL arm.
| Characteristic | SCC-Ag ≥4 ng/mL arm (n=181) | SCC-Ag <4 ng/mL arm (n=123) | p-value | |
|---|---|---|---|---|
| Age (yr) | 0.099 | |||
| <60 | 93 (51.4) | 75 (61.0) | ||
| ≥60 | 88 (48.6) | 48 (39.0) | ||
| FIGO stage | 0.003 | |||
| Ib–II | 141 (77.9) | 112 (91.1) | ||
| III–IVa | 40 (22.1) | 11 (8.9) | ||
| Initial tumor size (cm) | <0.001 | |||
| <4.5 | 68 (37.6) | 80 (65.0) | ||
| ≥4.5 | 113 (62.4) | 43 (35.0) | ||
| Clinical nodal involvement | 0.001 | |||
| No | 69 (38.1) | 71 (57.7) | ||
| Yes | 112 (61.9) | 52 (42.3) | ||
FIGO, International Federation of Gynecologic Oncology; SCC-Ag, squamous-cell carcinoma antigen.
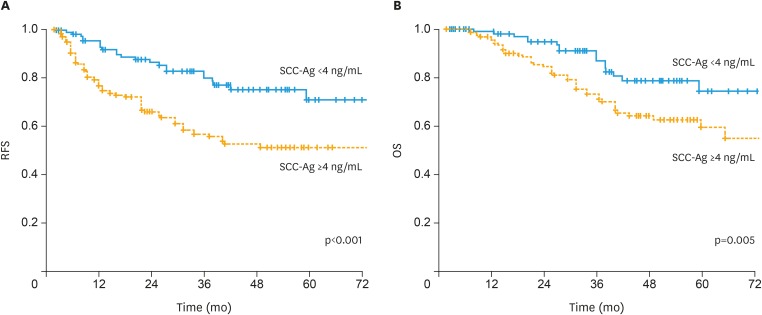
We divided enrolled patients into 3 groups using the data-derived method for selecting the optimal cut-off (based on pre-CRT SCC-Ag; group A: <1.5 ng/mL; group B: ≥1.5 and <4.0 ng/mL; and group C: ≥4.0 ng/mL). The 3-year RFS was significantly lower in group C than in groups A or B (91.3% vs. 74.4% vs. 56.6%, p<0.001: Bonferroni correction). The 3-year OS was significantly lower in group C than in groups A or B (94.7% vs. 83.2% vs. 72.1%, p=0.015: Bonferroni correction). However, there is no significant difference in RFS (p=0.456) and OS (p=0.472) between groups A and B. Thus, we categorize the enrolled patients into 2 groups with pre-CRT SCC-Ag <4 ng/mL and SCC-Ag ≥4 ng/mL arms which significantly differs in RFS and OS. The 3-year RFS and OS rates for all cohorts were 66.0% and 78.2%, respectively. The 3-year RFS (56.6% vs. 80.2%, p<0.001) and OS (72.1% vs. 86.8%, p=0.005) rates were significantly lower in the SCC-Ag ≥4 ng/mL arm than in the SCC-Ag <4 ng/mL arm (Fig. 2).
Fig. 2. (A) RFS and (B) OS in patients with SCC-Ag ≥4 ng/mL and SCC-Ag <4 ng/mL.
OS, overall survival; RFS, recurrence-free survival; SCC-Ag, squamous-cell carcinoma antigen.
The SCC-Ag level was re-evaluated at median 5.5 weeks (range, 4.0–12.0) after completion of radiotherapy. The median value of post-CRT SCC-Ag level was 1.1 ng/mL (range, 0.1–87.4). Patients were categorized according to the post-CRT SCC-Ag level (based on post-CRT SCC-Ag; group A: <1.5 ng/mL; group B: ≥1.5 and <4.0 ng/mL; group C: ≥4.0 ng/mL). The 3-year RFS was significantly higher in group A than in groups B or C (74.8% vs. 47.1% vs. 48.9%; p=0.002: Bonferroni correction). The 3-year OS was significantly higher in group A than in groups B or C (86.5% vs. % 62.7 vs. 53.7%; p=0.001: Bonferroni correction) (Supplementary Fig. 1). However, there is no significant difference in RFS (p=0.540) and OS (p=0.197) between groups B and C.
The factors associated with RFS after definitive CRT are summarized in Table 3. In the univariate analysis, initial FIGO stage (p=0.001), tumor size (p<0.001), and SCC-Ag level (p<0.001) were significant factors for RFS. In the multivariate analysis, tumor size (hazard ratio [HR]=2.23; 95% confidence interval [CI]=1.44–3.46; p=0.001) and initial SCC-Ag level (HR=2.02; 95% CI=1.26–3.24; p=0.004) retained their statistical significances.
Table 3. Prognostic factors associated with RFS in cervical cancer patients who received definitive CRT.
| Variable | No. | 3-year rate (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | ||||
| Age (yr) | 0.204 | 0.244 | ||||
| <60 | 168 | 61.7 | Reference | |||
| ≥60 | 136 | 71.9 | 1.33 (0.87–2.04) | |||
| FIGO stage | 0.001 | 0.162 | ||||
| Ib2–II | 253 | 70.7 | Reference | |||
| III–IVa | 51 | 44.0 | 1.45 (0.90–2.36) | |||
| Tumor size (cm) | <0.001 | 0.001 | ||||
| <4.5 | 148 | 79.9 | Reference | |||
| ≥4.5 | 156 | 52.7 | 2.23 (1.44–3.46) | |||
| Initial pelvic node | 0.103 | 0.976 | ||||
| Negative | 140 | 70.3 | Reference | |||
| Positive | 164 | 62.6 | 1.09 (0.70–1.69) | |||
| Initial SCC-Ag (ng/mL) | <0.001 | 0.004 | ||||
| <4 | 123 | 80.2 | Reference | |||
| ≥4 | 181 | 56.6 | 2.02 (1.26–3.24) | |||
CI, confidence interval; CRT, chemoradiotherapy; FIGO, International Federation of Gynecologic Oncology; RFS, recurrence-free survival; SCC-Ag, squamous-cell carcinoma antigen.
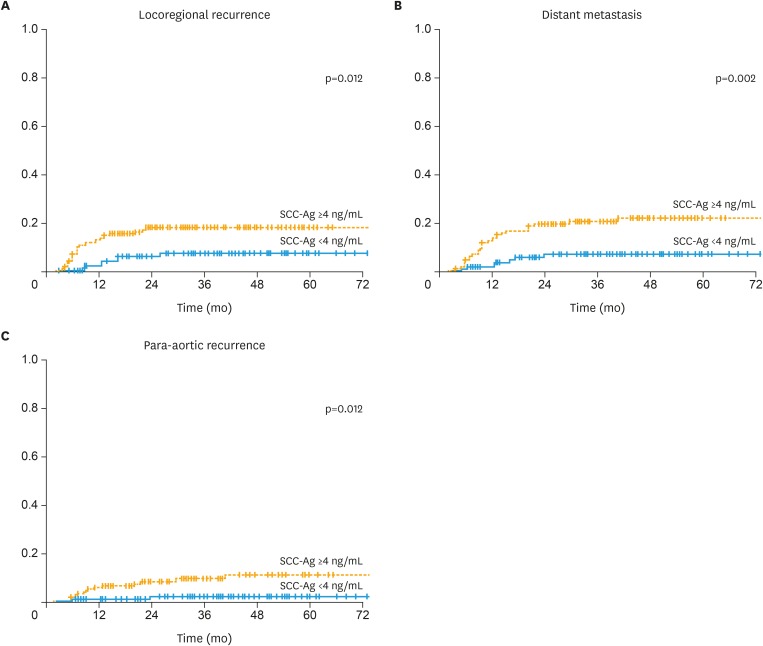
There were 37 (12.2%) cases for LRR, 40 (13.2%) for DM, and 17 (5.6%) for PAR among a total of 66 recurrences. The 3-year LRR rates were 17.6% in the SCC-Ag ≥4 ng/mL arm and 7.0% in the SCC-Ag <4 ng/mL arm, respectively (p=0.012; Fig. 3A). The 3-year DM rates were 20.4% in the SCC-Ag ≥4 ng/mL arm and 6.9% in the SCC-Ag <4 ng/mL arm, respectively (p=0.002; Fig. 3B). The 3-year PAR rates were 9.4% in the SCC-Ag ≥4 ng/mL arm and 2.1% in the SCC-Ag <4 ng/mL arm, respectively (p=0.012; Fig. 3C). In 66 patients who had relapsed, the median value of SCC-Ag level measured at disease recurrence was 3.4 ng/mL (range, 0.5–114.4). 37 (56.1%) had SCC-Ag level <4 ng/mL and 29 (43.9%) had SCC-Ag level ≥4 ng/mL.
Fig. 3. (A) LRR, (B) DM, (C) PAR rates in SCC-Ag ≥4 ng/mL and SCC-Ag <4 ng/mL arms.
DM, distant metastasis; LRR, locoregional recurrence; PAR, para-aortic recurrence; SCC-Ag, squamous-cell carcinoma antigen.
DISCUSSION
In a radioimmunoassay study for tumor antigens, SCC-Ag increased in squamous epithelium of uterine cervix during malignant transformation [7]. Currently, the levels of SCC-Ag are measured using an immunoradiometric assay in which anti-bead-coated antibodies are reacted non-competitively with labeled and radiolabeled antibodies [19]. SCC-Ag has been studied in other squamous-cell malignancies, including those of the lung, esophagus, head and neck, anal canal, and skin [19,20].
In our multi-institutional analysis, at an initial SCC-Ag concentration of 4 ng/mL, the difference in 3-year OS and RFS was statistically significant. Evidence was scarce to support that SCC-Ag was a significant predictor of RFS and OS at the cut-off value of 1.5–2.5 ng/mL used in clinical practice. In previous studies, there was no significant difference in OS and disease-free survival (DFS) when the cut-off value of initial SCC-Ag was set at 1.5 ng/mL [19,20,21]. Hong et al. [22] evaluated the 5-year disease-specific survival of each group by dividing the cut-off values of initial SCC-Ag into <2, 2–10, and >10 ng/mL groups, respectively, in FIGO stage I–IVa patients treated with radiotherapy. There was no significant difference among the results at <2, 2–10, and >10 ng/mL.
Ohno et al. [23] divided patients with squamous cell carcinoma of uterine cervix treated with radiotherapy in the initial FIGO stage I–IVa into the following groups: (A) 1.5–5 ng/mL, (B) 5–30 ng/mL, and (C) >30 ng/mL. Significant differences in DFS were found only in B and C groups. These studies did not use the cut-off values based on the ROC curve. Therefore, it was considered as a limitation in verifying the statistical significance of the sensitivity and specificity. In our study, we calculated the cut-off value of SCC-Ag using the Youden index in the ROC curve and analyzed the patients treated with concurrent CRT as standard treatment for localized uterine cervical cancer. The optimal cut-off of SCC-Ag level for LRR or PAR was at 4.25–4.35 ng/mL, which was similar to that of SCC-Ag level for all recurrences in our study (Supplementary Table 1). SCC-Ag level showed significant differences in tumor size, FIGO stage, and LN involvement between patients with SCC-Ag ≥4 ng/mL and those with <4 ng/mL. Duk et al. [9] demonstrated via t-test that an elevation in SCC-Ag was associated with unfavorable clinicopathological factors in early-stage SCC following radical hysterectomy. We performed a logistic regression analysis of the clinical factors associated with SCC-Ag in patients at all stages undergoing radical CRT.
Our results showed that SCC-Ag is an independent significant factor (HR=2.02; 95% CI=1.26–3.24; p=0.004) that affects the RFS irrespective of tumor size, nodal involvement, and FIGO stage, which are known as strong prognostic clinical factors. Our study also showed that large tumor size was a significant factor of RFS in the multivariate analysis. Especially, tumor size (p=0.001) showed a more significant p-value than SCC-Ag level (p=0.004). Our results were in accordance with previous reports that cervical tumor size was an independent prognostic factor for recurrence and survival [24,25]. In Perez et al. [25], increased tumor size (<3 cm vs. 3–5 cm vs. >5 cm) was significantly associated with decreased RFS in FIGO Ib to III cervical tumor.
Based on a cut-off value of 4 ng/mL, there were significant differences in all recurrences and metastases. Hirakawa et al. [26] found that the cut-off value of SCC-Ag, 1.5 ng/mL at initial diagnosis did not show significant differences in locoregional or distant recurrence. They just demonstrated that an identification of patients at high risk of DM is important and SCC-Ag level immediately after CRT showed a significant factor for the DM. In our study, the cut-off value, 4 ng/mL at initial diagnosis was a significant predictor of LRR, PAR, and DM, suggesting that initial SCC-Ag level was a valuable biomarker for the management of cervical tumor. In previous series, SCC-Ag decreased after 4–6 weeks after radiotherapy and SCC-Ag level within 4 weeks after radiotherapy had no predictive value for tumor recurrence in cervical cancer [27,28]. In our study, SCC-Ag level was re-evaluated between 4 and 12 weeks after radiotherapy and it was shown that the risk of disease relapse could be higher if it was maintained above 1.5 ng/mL during this period. Hong et al. [22] demonstrated that non-normalization of SCC-Ag level 3 months after radiotherapy was a significant predictor of reduced survival rate due to the DM.
There are several limitations in our study. This study was conducted as a retrospective one. Thus, our study should be understood in view of the inherent biases of a retrospective study design. In our retrospective study, patients' data from 5 institutions were not centrally reviewed. Thus, we acknowledge that substantial differences or disparities between local reporting and central assessment can exist in this multicenter study. However, there is no statistically significant difference in patient characteristics, treatment outcomes, and follow-up durations across five institutions (Supplementary Fig. 2). The median follow-up duration was relatively short of 36.5 months, which was just analyzed by 3-year RFS and OS. However, we undertook a multi-center study which enrolled a large cohort to minimize its biases. We used Youden index which was a frequently-used measure of the ROC curve for determining the cut-off point of SCC-Ag for tumor recurrence. It evaluates the effectiveness of a diagnostic marker and enables the selection of an optimal cut-off point for the marker [29]. The cut-off point may differ according to the combination of enrollments and the event that we select for ROC analysis. Recurrence basically harbors the time factor, which may be inappropriate in the time-independent ROC curve [30]. Thus, a validation study with external data set is necessary to assure our conclusions in the future time.
In conclusion, in this multi-institutional analysis, when the cut-off value of SCC-Ag level was set at 4.0 ng/mL, pre-treatment SCC-Ag level was a significant predictor of tumor recurrence and patient survival in squamous-cell carcinoma of uterine cervix. Additional long-term follow-up evaluation and prospective study will be indicated in the near future.
ACKNOWLEDGMENTS
The statistical analyses performed in this article were advised by Catholic Medical Center Clinical Research Coordinating Center.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.K.H., J.S., L.J.W., L.J.H.
- Data curation: L.S.W., J.S., L.J.W., L.J.H.
- Formal analysis: C.K.H., L.S.W., J.S., L.J.W., L.J.H.
- Investigation: Y.M., J.S., L.J.W., L.J.H.
- Methodology: C.K.H., Y.M., L.J.W., L.J.H.
- Project administration: L.J.H.
- Resources: L.S.W., Y.M., J.S., L.J.H.
- Software: C.K.H., L.J.H.
- Supervision: J.S., L.J.H.
- Validation: L.S.W., J.S., L.J.H.
- Visualization: L.J.H.
- Writing - original draft: C.K.H., Y.M., L.J.W., L.J.H.
- Writing - review & editing: C.K.H., L.J.H.
SUPPLEMENTARY MATERIALS
Classification analyses in LRR, PAR, DM, and survival
(A) RFS and (B) OS according to the post-radiotherapy SCC-Ag level.
SCC-Ag level of table (A) and box plot (B) and recurrence and survival (C) analyses in each institution.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 3.Lai CH. Management of recurrent cervical cancer. Chang Gung Med J. 2004;27:711–717. [PubMed] [Google Scholar]
- 4.Perez CA, Grigsby PW, Chao KS, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998;41:307–317. doi: 10.1016/s0360-3016(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 5.Kamura T, Tsukamoto N, Tsuruchi N, Saito T, Matsuyama T, Akazawa K, et al. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer. 1992;69:181–186. doi: 10.1002/1097-0142(19920101)69:1<181::aid-cncr2820690130>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan AN, Lee H, Hanson E, Berkowitz RS, Crum CP. Influence of margin status and radiation on recurrence after radical hysterectomy in stage IB cervical cancer. Int J Radiat Oncol Biol Phys. 2006;65:1501–1507. doi: 10.1016/j.ijrobp.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–1628. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66:10–20. doi: 10.1016/j.critrevonc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Duk JM, Groenier KH, de Bruijn HW, Hollema H, ten Hoor KA, van der Zee AG, et al. Pretreatment serum squamous cell carcinoma antigen: a newly identified prognostic factor in early-stage cervical carcinoma. J Clin Oncol. 1996;14:111–118. doi: 10.1200/JCO.1996.14.1.111. [DOI] [PubMed] [Google Scholar]
- 10.Gaarenstroom KN, Bonfrer JM, Kenter GG, Korse CM, Hart AA, Trimbos JB, et al. Clinical value of pretreatment serum Cyfra 21-1, tissue polypeptide antigen, and squamous cell carcinoma antigen levels in patients with cervical cancer. Cancer. 1995;76:807–813. doi: 10.1002/1097-0142(19950901)76:5<807::aid-cncr2820760515>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Lehtovirta P, Viinikka L, Ylikorkala O. Comparison between squamous cell carcinoma-associated antigen and CA-125 in patients with carcinoma of the cervix. Gynecol Oncol. 1990;37:276–278. doi: 10.1016/0090-8258(90)90347-n. [DOI] [PubMed] [Google Scholar]
- 12.Scambia G, Panici PB, Baiocchi G, Amoroso M, Foti E, Greggi S, et al. The value of squamous cell carcinoma antigen in patients with locally advanced cervical cancer undergoing neoadjuvant chemotherapy. Am J Obstet Gynecol. 1991;164:631–636. doi: 10.1016/s0002-9378(11)80037-2. [DOI] [PubMed] [Google Scholar]
- 13.Ngan HY, Cheung AN, Lauder IJ, Wong LC, Ma HK. Prognostic significance of serum tumour markers in carcinoma of the cervix. Eur J Gynaecol Oncol. 1996;17:512–517. [PubMed] [Google Scholar]
- 14.Massuger LF, Koper NP, Thomas CM, Dom KE, Schijf CP. Improvement of clinical staging in cervical cancer with serum squamous cell carcinoma antigen and CA 125 determinations. Gynecol Oncol. 1997;64:473–476. doi: 10.1006/gyno.1996.4581. [DOI] [PubMed] [Google Scholar]
- 15.Abe A, Nakano T, Morita S, Oka K. Clinical evaluation of serum and immunohistochemical expression of SCC and CA19-9 in radiation therapy for cervical cancer. Anticancer Res. 1999;19:829–836. [PubMed] [Google Scholar]
- 16.Micke O, Prott FJ, Schäfer U, Tangerding S, Pötter R, Willich N. The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res. 2000;20:5113–5115. [PubMed] [Google Scholar]
- 17.Pras E, Willemse PH, Canrinus AA, de Bruijn HW, Sluiter WJ, ten Hoor KA, et al. Serum squamous cell carcinoma antigen and CYFRA 21-1 in cervical cancer treatment. Int J Radiat Oncol Biol Phys. 2002;52:23–32. doi: 10.1016/s0360-3016(01)01805-3. [DOI] [PubMed] [Google Scholar]
- 18.Yamanoi K, Matsumura N, Kido A, Baba T, Hamanishi J, Yamaguchi K, et al. A novel diagnostic criterion for lymph node metastasis in cervical cancer using multi-detector computed tomography. Gynecol Oncol. 2013;131:701–707. doi: 10.1016/j.ygyno.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Jeong BK, Choi DH, Huh SJ, Park W, Bae DS, Kim BG. The role of squamous cell carcinoma antigen as a prognostic and predictive factor in carcinoma of uterine cervix. Radiat Oncol J. 2011;29:191–198. doi: 10.3857/roj.2011.29.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung W, Park KR, Lee KJ, Kim K, Lee J, Jeong S, et al. Value of imaging study in predicting pelvic lymph node metastases of uterine cervical cancer. Radiat Oncol J. 2017;35:340–348. doi: 10.3857/roj.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvatici M, Achilarre MT, Sandri MT, Boveri S, Vanna Z, Landoni F. Squamous cell carcinoma antigen (SCC-Ag) during follow-up of cervical cancer patients: Role in the early diagnosis of recurrence. Gynecol Oncol. 2016;142:115–119. doi: 10.1016/j.ygyno.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Hong JH, Tsai CS, Chang JT, Wang CC, Lai CH, Lee SP, et al. The prognostic significance of pre- and posttreatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:823–830. doi: 10.1016/s0360-3016(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 23.Ohno T, Nakayama Y, Nakamoto S, Kato S, Imai R, Nonaka T, et al. Measurement of serum squamous cell carcinoma antigen levels as a predictor of radiation response in patients with carcinoma of the uterine cervix. Cancer. 2003;97:3114–3120. doi: 10.1002/cncr.11453. [DOI] [PubMed] [Google Scholar]
- 24.Eifel PJ, Morris M, Wharton JT, Oswald MJ. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1994;29:9–16. doi: 10.1016/0360-3016(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 25.Perez CA, Grigsby PW, Nene SM, Camel HM, Galakatos A, Kao MS, et al. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992;69:2796–2806. doi: 10.1002/1097-0142(19920601)69:11<2796::aid-cncr2820691127>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa M, Nagai Y, Inamine M, Kamiyama K, Ogawa K, Toita T, et al. Predictive factor of distant recurrence in locally advanced squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy. Gynecol Oncol. 2008;108:126–129. doi: 10.1016/j.ygyno.2007.08.091. [DOI] [PubMed] [Google Scholar]
- 27.de Bruijn HW, Duk JM, van der Zee AG, Pras E, Willemse PH, Boonstra H, et al. The clinical value of squamous cell carcinoma antigen in cancer of the uterine cervix. Tumour Biol. 1998;19:505–516. doi: 10.1159/000030044. [DOI] [PubMed] [Google Scholar]
- 28.Ngan HY, Chan SY, Wong LC, Choy DT, Ma HK. Serum squamous cell carcinoma antigen in the monitoring of radiotherapy treatment response in carcinoma of the cervix. Gynecol Oncol. 1990;37:260–263. doi: 10.1016/0090-8258(90)90344-k. [DOI] [PubMed] [Google Scholar]
- 29.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 30.Yang K, Park W, Huh SJ, Bae DS, Kim BG, Lee JW. Clinical outcomes in patients treated with radiotherapy after surgery for cervical cancer. Radiat Oncol J. 2017;35:39–47. doi: 10.3857/roj.2016.01893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Classification analyses in LRR, PAR, DM, and survival
(A) RFS and (B) OS according to the post-radiotherapy SCC-Ag level.
SCC-Ag level of table (A) and box plot (B) and recurrence and survival (C) analyses in each institution.