Abstract
Objective
To develop and validate a 3-year recurrence prediction score (RPS) system for predicting the baseline risk of recurrence of stage I–II endometrial carcinoma.
Methods
We reviewed 427 patients with International Federation of Gynecology and Obstetrics staging I–II endometrial carcinoma underwent surgery without any adjuvant therapy from 2005 to 2013. The patients were divided into 2 groups: the test cohort (n=251) comprising those who underwent surgery in odd-numbered years, and the validation cohort (n=176) comprising those who underwent surgery in even-numbered years. Multivariate analysis was performed using 7 candidate predictors to identify the risk factors for 3-year recurrence-free interval (RFI) in the test cohort. Each risk factor was scored based on logistic regression analyses of the test data set, and the sum of the risk factor scores was defined as the RPS system. We then applied the system in the validation cohort.
Results
Multivariate analysis revealed that the significant risk factors were age ≥60 years, pathological type II, positive cervical stromal invasion, and positive peritoneal cytology. In the test cohort, the 3-year RFI rates were 100%, 95.8%, 79.9%, and 33.3% for RPSs of 0, 1, 2, and 3, respectively. In the validation cohort, the 3-year RFI was significantly higher in the low-RPS group (RPS 0 or 1) than in the high-RPS group (RPS 2 or 3) (95.2% vs. 79.9%, p<0.01).
Conclusions
The RPS system shows significant reproducibility for predicting the baseline risk of recurrence. The system could potentially impact the choice of adjuvant therapy for stage I–II endometrial carcinoma.
Keywords: Endometrial Neoplasms
INTRODUCTION
Endometrial cancer is a common gynecologic cancer. In 2009, approximately 10,000 women in Japan were diagnosed with gynecologic cancer, 1,600 of whom died [1]. The number of diagnosed patients has since gradually increased, with approximately 13,000 women diagnosed in 2013 [2]. The majority of women with endometrial cancer are diagnosed with early stage tumors (stage I–II) that are associated with good prognosis [3]. However, even patients with early stage tumors can develop recurrence. The risk factors for recurrence include older age, higher pathological tumor grade, myometrial invasion (MI), lymphovascular invasion (LVI), cervical stromal invasion, and positive peritoneal cytology [4,5,6,7,8,9].
Adjuvant therapy is generally considered for the patients at risk for recurrence. Several organizations have used patient-scored prognostic factors to categorize the risk of recurrence as low, intermediate, and high [10,11], which are then used as a basis for deciding the treatment.
However, the therapeutic strategies in Japan are different from that in western countries. The National Comprehensive Cancer Network Guideline recommends adjuvant radiotherapy, including vaginal brachytherapy and/or external beam radiotherapy, for patients with stage I–II endometrial cancer and with any risk factors for recurrence [12]. Meanwhile, Japanese gynecologic oncologists argue that local control must be accomplished with radical surgical procedure. The Japan Society of Obstetrics and Gynecology reported that only 1.5% of patients with endometrial cancer who underwent surgery were also given adjuvant radiotherapy in 2016 [13]. Japanese clinicians tend to prefer adjuvant chemotherapy for patients with intermediate to high risk. However, unlike in advanced stage, there is no clear evidence supporting that adjuvant chemotherapy yields a survival benefit for stage I–II endometrial carcinoma. As such, patients with stage I–II endometrial carcinoma do not receive any adjuvant chemotherapy or radiotherapy at our institution. Thus, our clinical data are suitable to assess the baseline risk of recurrence in stage I–II endometrial carcinoma.
The currently used guideline for risk stratification has not been validated and only considers limited risk factors; thus, it may not accurately reflect the risk of recurrence. Previously, we have reported that the baseline 5-year recurrence rate of early stage endometrial cancer was only 14% even in the high-risk group according to the European Society for Medical Oncology clinical practice guidelines and Japan Society of Gynecologic Oncology (JSGO) guidelines [3]. As such, a scoring system that includes multiple risk factors has to be established to determine the group with a higher risk of recurrence.
There is no established evidence regarding the optimal follow-up duration in patients with endometrial cancer, although a 3- to 5-year follow-up is commonly used. Frequent follow-ups until 3 years after treatment are recommended because the majority of recurrences occur during this period in patients with stage I–II endometrial cancer [14].
This study aimed to develop a 3-year recurrence prediction score (RPS) system and validate its capability for predicting the baseline risk of recurrence of stage I–II endometrial carcinoma.
MATERIALS AND METHODS
1. Patients
Among the patients with stage I–II endometrial carcinoma who underwent surgery between January 2005 and December 2013 at the National Cancer Center Hospital, those who met the following criteria were excluded: histological diagnosis of undifferentiated carcinoma, mixed epithelial and mesenchymal tumors, neuroendocrine features, history of adjuvant chemotherapy or radiotherapy, and death within 30 days of the surgery. Patients with histologically confirmed lymph node metastases were also excluded from this study. Surgical staging was according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 guidelines [15]. Stage II was defined as a state with pathologically diagnosed cervical stromal invasion. Estrogen-dependent (type 1) endometrial carcinoma was defined as G1 or G2 endometrioid adenocarcinoma, while the estrogen-independent (type 2) type was defined as G3 or non-endometrioid adenocarcinoma [16].
We divided the patients into 2 groups: the test cohort comprising those who underwent surgery in odd-numbered years, and the validation cohort comprising those who underwent surgery in even-numbered years. We then retrospectively collected the medical records of these patients from our institutional database. We confirmed our statistical consultant that the treated year is an independent factor and is valid as a study design to carry out internal validation.
The Institutional Review Board (IRB) of the National Cancer Center Hospital approved this study (IRB Approval No. 2015-259). All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was not required for this type of study.
2. Surgical procedure
All patients underwent at least abdominal total hysterectomy and bilateral salpingo-oophorectomy. Radical hysterectomy was performed for patients with suspicious cervical stromal invasion preoperatively. Pelvic lymph node biopsy and para-aortic examination were performed in patients with G1/2 disease or inner-half MI, and suspicious nodes were examined via rapid frozen section. Meanwhile, pelvic lymphadenectomy was performed in patients with outer-half MI, G3 disease, or non-endometrioid adenocarcinoma. Pelvic and para-aortic lymphadenectomy were performed when lymph node metastases were detected on preoperative examination, intraoperatively, or on frozen sections. In this study, we defined the patient who underwent retroperitoneal lymphadenectomy as the patient who underwent pelvic lymphadenectomy with or without para-aortic lymphadenectomy. Peritoneal lavage cytology was routinely performed, and omentectomy was performed in patients with non-endometrioid adenocarcinoma or positive peritoneal cytology.
3. Follow-up evaluation
All patients were followed up from the day of surgery. Postoperative follow-up procedures for the first 2 years comprised internal examination every 3 months and vaginal stump cytology as necessary. Thereafter, patients were followed up every 6 months for up to 5 postoperative years; computed tomography examination was performed annually or when any abnormal findings were recognized.
The patterns of recurrence were defined as follows: locoregional recurrence was defined as vaginal or intrapelvic recurrence, while distant recurrence included upper para-aortic lymph node metastasis, abdominal metastasis, and metastasis to other organs.
4. Adjuvant therapy
In our institution, only patients with stage III–IV endometrial adenocarcinoma (FIGO 2009), that is, residual tumor or extra-uterine metastasis, undergo adjuvant chemotherapy with doxorubicin-cisplatin. Positive peritoneal cytology alone is not considered an indication for adjuvant chemotherapy. Similar with most Japanese clinicians, we attained local control only surgically and do not routinely perform adjuvant radiotherapy. However, we recommend adjuvant brachytherapy to patients with pathological vaginal margin involvement. In either case, the patients who underwent adjuvant chemotherapy or radiotherapy were excluded from this study.
5. Recurrence prediction score
Because the majority of recurrences occur within the first 3 years after treatment in patients with stage I-II endometrial cancer, we developed a scoring system to predict the 3-year risk of recurrence [14]. We conducted a multivariate analysis of patients in the test cohort using all risk factors that were previously reported [4,5,6,7,8,9]: age, pathological type, MI, LVI, cervical stromal invasion, peritoneal cytology, and extent of retroperitoneal lymphadenectomy. Pathological type was defined as follows: type 1 group as G1 and G2 endometrioid adenocarcinoma and type 2 group as G3 endometrioid adenocarcinoma and other subtypes. We assigned scores to each risk factor based on the β coefficient calculated via logistic regression analysis for the 3-year recurrence of the test data set, and the sum of the risk factor scores was defined as the RPS. We then applied our RPS system to the validation cohort.
6. Statistical analysis
Summarized data are presented as numbers and percentages unless otherwise noted. Continuous data such as age were compared using the Wilcoxon rank sum test. Frequencies were compared using Fisher's exact test for categorical variables. The predictors of recurrence (age, pathological type, MI, LVI, cervical stromal invasion, peritoneal cytology, and the extent of retroperitoneal lymphadenectomy) were assessed via univariate and multivariate analyses using the Cox proportional hazards model in the test cohort. The candidate predictors were included in the logistic regression model, and prediction scores were assigned based on β coefficient. Logistic regression analysis was applied in the patients followed up for at least 3 years. We applied the RPS system in the validation cohort and analyzed the recurrence-free interval (RFI) via the Kaplan-Meier method using the log-rank test for internal validation. The RFI was defined as the interval between the date of surgery and recurrence. If no events occurred, the last observation was censored. We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and C-index for recurrence within the first 3 years. Finally, we compared the distribution and RFI according to the risk classification of the JSGO guideline and our system.
The value of p<0.05 was considered significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [17].
RESULTS
1. Patient characteristics
A total of 427 patients were eligible for inclusion in this study. The clinical features of the patients in the test and validation cohorts are provided in Table 1. There were no significant differences between the characteristics of the 2 groups.
Table 1. Baseline characteristics of the test cohort and the validation cohort.
| Characteristics | Test cohort (n=251) | Validation cohort (n=176) | p value | |
|---|---|---|---|---|
| Age (yr) | 0.787 | |||
| Median (range) | 57 (28–88) | 56.5 (30–80) | ||
| <60 | 152 (60.6%) | 103 (58.5%) | 0.689 | |
| ≥60 | 99 (39.4%) | 73 (41.5%) | ||
| Pathological type | 0.898 | |||
| Type 1 | 207 (82.5%) | 144 (81.8%) | ||
| Type 2 | 44 (17.5%) | 32 (18.2%) | ||
| MI | 0.112 | |||
| <1/2 | 193 (76.9%) | 147 (83.5%) | ||
| ≥1/2 | 58 (23.1%) | 29 (16.5%) | ||
| LVI | 0.362 | |||
| Negative | 185 (73.7%) | 137 (77.8%) | ||
| Positive | 66 (26.3%) | 39 (22.2%) | ||
| Cervical stromal invasion | 0.664 | |||
| Negative | 236 (94%) | 168 (95.5%) | ||
| Positive | 15 (6%) | 8 (4.5%) | ||
| Peritoneal cytology | 1 | |||
| Negative | 221 (88%) | 155 (88.1%) | ||
| Positive | 30 (12%) | 21 (11.9%) | ||
| Lymphadenectomy | 0.311 | |||
| Negative | 209 (83.3%) | 139 (79.0%) | ||
| Positive | 42 (16.7%) | 37 (21.0%) | ||
LVI, lymphovascular invasion; MI, myometrial invasion.
2. Test cohort
A total of 251 patients were enrolled in the test cohort. The median follow-up duration was 5.1 years (range, 0.6–11.3 years), and 14 patients (5.6%) developed recurrence within 3 years after the start of treatment. The distribution of the recurrence pattern was as follows: 8 patients (3.2%) showed locoregional recurrence, 8 patients (3.2%) showed distant recurrence, and 2 patients (0.7%) showed both locoregional and distant recurrence.
Univariate analysis revealed that every risk factor significantly influenced recurrence. The risk factor with the highest hazard ratio (HR) in univariate analysis was MI ≥1/2 (HR=13.5, 95% confidence interval [CI]=3.77–48.5). However, the patients who underwent lymphadenectomy showed worse RFI (HR=5.19, 95% CI=1.82–014.8). We therefore conducted multivariate analysis using all risk factors and found that the significant risk factors were age ≥60 years (HR=7.49, 95% CI=1.80–31.1), pathological type II (HR=3.66, 95% CI=1.02–13.1), positive cervical stromal invasion (HR=4.69, 95% CI=1.24–17.7), and positive ascites on cytology (HR=19.3, 95% CI=4.45–84.0; Table 2). The risk factor with the highest HR in multivariate analysis was positive ascites on cytology.
Table 2. Hazard ratios for recurrence-free survival in univariate and multivariate analyses in the test cohort (n=251).
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (≥60 years) | 5.86 | 1.63–21 | 0.007 | 7.49 | 1.80–31.1 | 0.006 |
| Pathological type (type 2) | 6.72 | 2.33–19.4 | <0.001 | 3.66 | 1.02–13.1 | 0.046 |
| MI (≥1/2) | 13.5 | 3.77–48.5 | <0.001 | 4.26 | 0.86–21.3 | 0.077 |
| LVI (positive) | 7.57 | 2.37–24.1 | <0.001 | 3.43 | 0.75–15.7 | 0.111 |
| Cervical stromal invasion (positive) | 6.71 | 2.10–21.4 | 0.001 | 4.69 | 1.24–17.7 | 0.023 |
| Peritoneal cytology (positive) | 3.25 | 1.02–10.4 | 0.047 | 19.3 | 4.45–84.0 | <0.001 |
| Lymphadenectomy (negative) | 0.19 | 0.06–0.55 | 0.002 | 0.68 | 0.20–2.26 | 0.525 |
CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion; MI, myometrial invasion.
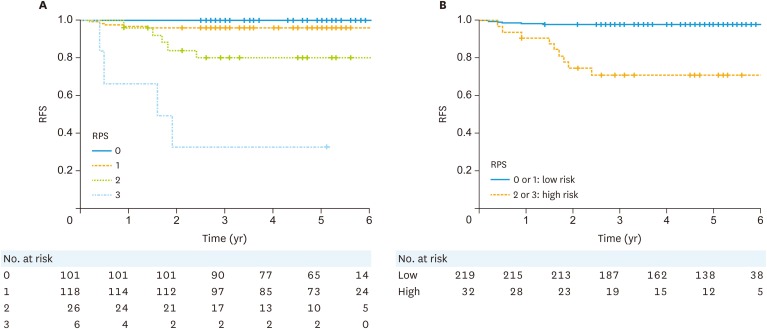
Logistic regression analysis for 3-year recurrence yielded almost similar results. The regression coefficients of logistic regression analysis were as follows: age ≥60 years, 2.49; pathological type 2, 1.61; cervical stromal invasion positive, 2.10; and positive peritoneal cytology, 2.89. From the result of logistic regression analysis, we defined each score as 1. In the test cohort, the 3-year RFI rates for each RPS score were as follows: RPS 0, 100%; RPS 1, 95.8%; RPS 2, 79.9%; and RPS 3, 33.3% (Fig. 1A). No patient had an RPS score of 4. We defined the low-RPS group as having RPS scores of 0 or 1, and the high-RPS group as having RPS scores of 2 or 3. The 3-year RFI was significant different between these two groups in the test cohort: 97.7% in the low-RPS group and 71.1% in the high-RPS group (p<0.001, Fig. 1B). The HR of recurrence in the low-RPS group was 13.5 (95% CI=4.5–40.3).
Fig. 1. RFI of patients in the test cohort. Survival curves according to the (A) overall recurrence prediction score and (B) high or low risk for recurrence score.
RFI, recurrence-free interval; RFS, recurrence-free survival; RPS, recurrence prediction score.
3. Validation cohort
A total of 176 patients were enrolled in the validation cohort. The median follow-up duration was 5.2 years (range, 1.7–10.1 years), and 13 patients (7.4%) developed recurrence within 3 years after the start of treatment. The distribution of the recurrence pattern was as follows: 5 patients (2.8%) showed locoregional recurrence, 11 patients (6.3%) showed distant recurrence, and 3 patients (1.7%) showed both locoregional and distant recurrence. We applied our RPS system to the validation cohort, and the score distributions are shown in Table 3.
Table 3. Distribution of RPS score in the test cohort and the validation cohort.
| RPS score | Risk group | Test cohort (n=251) | Validation cohort (n=176) | ||
|---|---|---|---|---|---|
| Score | Group | Score | Group | ||
| 0 | Low risk | 101 (40.2%) | 219 (87.3%) | 78 (44.3%) | 146 (83.0%) |
| 1 | Low risk | 118 (47.0%) | 219 (87.3%) | 68 (38.6%) | 146 (83.0%) |
| 2 | High risk | 26 (10.4%) | 32 (12.7%) | 24 (13.6%) | 30 (17.0%) |
| 3 | High risk | 6 (2.4%) | 32 (12.7%) | 6 (3.4%) | 30 (17.0%) |
The risk factors in the RPS system were age ≥60 years, pathological type 2, positive cervical stromal invasion, and positive ascites on cytology.
RPS, recurrence prediction score.
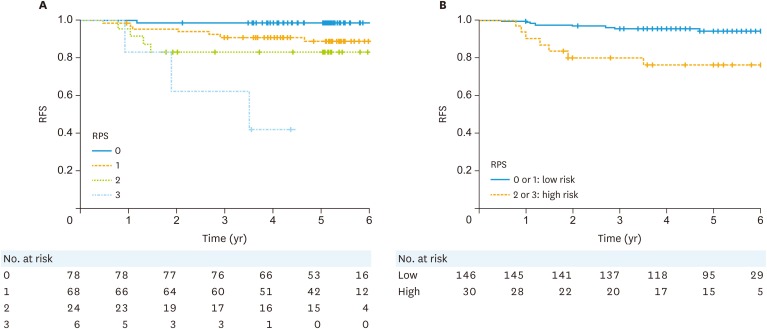
The Kaplan-Meier curve for RFI according to the RPS system is shown in Fig. 2. The 3-year RFI was significantly higher in the low-RPS group than that in the high-RPS group (95.2% vs. 79.9%, p<0.0001). The HR of recurrence in the low-RPS group was 5.5 (95% CI=2.0–15.1). The sensitivity, specificity, PPV, NPV, and C-index for 3-year survival in the test cohort and the validation cohort 64.3% and 46.2%, 90.6% and 87.2%, 32.1% and 23.1%, 97.3 and 95.1%, and 0.774 and 0.618, respectively (Table 4). The sensitivity and PPV are low because of the very low rate of recurrence in our cohort. As such, our system is considered to have good discrimination capability.
Fig. 2. RFI of the patients in the validation cohort. Survival curves according to the (A) overall recurrence prediction score and (B) high or low risk for recurrence score.
RFI, recurrence-free interval; RFS, recurrence-free survival; RPS, recurrence prediction score.
Table 4. Sensitivity, PPV, NPV, and C-index for recurrence within the first 3 years in the patients followed up for at least 3 years.
| Variables | Test cohort | Validation cohort |
|---|---|---|
| Sensitivity | 64.3% | 46.2% |
| Specificity | 90.6% | 87.2% |
| PPV | 32.1% | 23.1% |
| NPV | 97.3% | 95.1% |
| C-index | 0.774 | 0.618 |
NPV, negative predictive value; PPV, positive predictive value.
4. Comparison with the JSGO guideline
According to the risk classification of the JSGO guideline, the test and validation cohort were divided into 3 groups. In the test cohort, the low-, intermediate-, and high-risk group comprised 141 (56.2%), 72 (28.7%), and 38 (15.1%) patients, respectively, and the 3-year RFI was 98.6%, 94.4%, and 78.9%, respectively. In the validation cohort, the low-, intermediate-, and high-risk group comprised 96 (54.5%), 43 (24.4%), and 37 (21.0%), respectively, and the 3-year RFI was 97.9%, 90.6%, and 80.3%, respectively.
Meanwhile, the patients were divided into 2 groups according to our RPS system. The low- and high-risk groups in the test cohort comprised 219 (87.3%) and 32 (12.7%) patients, respectively, and the 3-year RFI was 97.7% and 71.1%, respectively. In the validation cohort, the low- and high-risk group comprised 146 patients (83.0%) and 30 (17.0%) patients, respectively, and the 3-year RFI was 95.2% and 79.9%, respectively.
DISCUSSION
Our findings showed that our RPS system has a good predictive capability and significant reproducibility for the baseline risk of recurrence in stage I–II endometrial cancer. We also found that the patients in the high RPS group had poor prognosis; thus, they may be good candidates for adjuvant therapy.
Prior studies have attempted to classify stage I–II endometrial carcinoma according to pathological risk factors [7,18]. However, no consensus has been achieved, and the scoring systems used have not been validated. In this trial, we constructed the RPS system based on postoperative risk factors in a test cohort, and then validated the system in a validation cohort.
The patients in the high RPS group had poor prognosis; thus, it is possible that the therapeutic effect of adjuvant therapy exceeds the risk of adverse effect in this group. In patients with stage III–IV endometrial cancer, chemotherapy with doxorubicin–cisplatin significantly improved progression-free survival and overall survival compared with whole-abdominal irradiation [19]. Meanwhile, there is no strong evidence that radiotherapy or chemotherapy improves survival after surgery in stage I–II endometrial carcinoma. Although adjuvant brachytherapy improves local control after surgery, it is rarely performed in Japan because surgery is the preferred modality for achieving local control. At our institution, we do not administer any adjuvant therapy to patients with uterine body cancer who have no residual tumor or extra-uterine metastasis. Meanwhile, we considered that the risk factors for relapse should be determined because we encountered patients with stage I–II who had recurrence. In our study, the patients in the high RPS group had a poor 3-year RFI (71.1% in the test cohort and 75.9% in the validation cohort). These patients had the potential to obtain greater absolute risk reduction from adjuvant therapy compared with the low RPS group. Our results showed that recurrence is not only limited to locoregional recurrence, indicating that systemic chemotherapy must be considered to control distant recurrence. However, there is limited evidence about the clinical benefit of adjuvant chemotherapy for patients with stage I–II endometrial carcinoma. As such, clinical trials to evaluate the role of adjuvant chemotherapy for patients with high RPS are warranted.
Older age, higher pathological tumor grade, MI, LVI, cervical stromal invasion, and peritoneal cytology are the prognostic factors for endometrial cancer [4,5,6,7,8,9]. Our multivariate analysis showed that except for myometrial and LVI, all these were significant predictive factors. In particular, positive peritoneal cytology correlated the most strongly with poor prognosis (HR=18.9). In the FIGO 2009 staging system, peritoneal cytology is not associated with clinical stage, and positive peritoneal cytology is indeed not considered as an independent prognostic factor in endometrial cancer. However, some reports have shown a relation between positive peritoneal cytology and poor prognosis in patients with early stage endometrial carcinoma [9,20]. Although MI is considered to be among the most important prognostic factors for endometrial cancer, multivariate analysis in our study showed no association between MI and prognosis. This finding was similar to that of a prior study in which deep MI had no significant effect on prognosis in multivariate analysis [8].
The extent of lymphadenectomy did not affect RFI in our study, and the findings of prior research on the effect of extent lymphadenectomy was unclear [21,22]. We hypothesized that the effect of extent lymphadenectomy was unlikely to have a significant impact on recurrence in our cohort. Moreover, there were few patients who had recurrence in the pelvic or para-aortic area (8/427, 1.9%).
A novelty of this study is that this is the first RPS system for predicting the baseline risk of recurrence of stage I–II endometrial carcinoma based on prognostic factors. Moreover, the system developed was validated in another cohort. Although the sensitivity of our system is not very high, the recurrence rate of stage I–II endometrioid carcinoma is very low, and our system is not inferior to the risk classification of the JSGO guideline. Moreover, more patients, whose 3-year RFI was very high, could be better classified into the low RPS group than based on the JSGO guideline (56.2% vs. 87.3% in test cohort, 54.5% vs. 83% in the validation cohort). Identifying the patients who did not need adjuvant therapy is important in clinical practice, and our system is applicable and can be used to this end. Meanwhile, some reports suggest that the genomic features of endometrial carcinomas permit a reclassification of the risk for recurrence [23]. Currently, genomic array- and sequencing-based technologies are not commonly used. Thus, it may be necessary to develop a new recurrence prediction system that includes both clinicopathological prognostic factors and genomic prognostic factors, and our system can be a basis for developing a more accurate recurrence prediction system.
One of the limitations of this study was that it was a single-institutional analysis. We designed this study suitable to carry out internal validation. Actually, we need to analysis and perform external validation with larger cohort. Although a multi-institutional prospective study would allow better analysis, it was difficult to conduct because postoperative treatments have been performed in recent trials and clinical practice. Second, we did not evaluate the overall survival in this study because of the relatively short follow-up period.
In conclusion, our RPS system is simple and easy to use in clinical practice. Its high predictive value and reproducibility are helpful in deciding the need for adjuvant therapy of patients with stage I–II endometrial cancer. Further studies are needed to prove that adjuvant therapy significantly improves survival in patients with high RPS scores.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Aya Kuchiba for statistical consultant. And the authors would like to thank Dr. Mitsuya Ishikawa, Dr Shun-ichi Ikeda. Dr. Tatunori Shimoi, Dr. Kan Yonemori, and Dr. Yasuhiro Fujiwara for supporting to make manuscript.
Footnotes
Presentation: We reported this report at the 69th Annual Congress of the Japan Society of Obstetrics and Gynecology and European Gynaecological Oncology Congress 2017.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.T., M.Y.
- Data curation: K.T., M.Y., S.S., Y.T.
- Formal analysis: K.T., M.Y.
- Funding acquisition: K.T.
- Investigation: K.T, M.Y, S.S., Y.T.
- Methodology: K.T., M.Y.
- Project administration: K.T.
- Resources: K.T., T.K.
- Software: K.T., M.Y.
- Supervision: N.M., T.K., K.T.
- Validation: K.T., M.Y.
- Visualization: K.T.
- Writing - original draft: K.T.
- Writing - review & editing: M.Y., S.S., N.M., T.K., K.T.
References
- 1.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Center. Center for Cancer Control and Information Services, Cancer Registry and Statistics [Internet] Tokyo: National Cancer Center; c2018. [cited 2018 May 21]. Available from: https://ganjoho.jp/reg_stat/index.html. [Google Scholar]
- 3.Sasada S, Yunokawa M, Takehara Y, Ishikawa M, Ikeda S, Kato T, et al. Baseline risk of recurrence in stage I–II endometrial carcinoma. J Gynecol Oncol. 2018;29:e9. doi: 10.3802/jgo.2018.29.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roma AA, Rybicki LA, Barbuto D, Euscher E, Djordjevic B, Frauenhoffer E, et al. Risk factor analysis of recurrence in low-grade endometrial adenocarcinoma. Hum Pathol. 2015;46:1529–1539. doi: 10.1016/j.humpath.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Jolly S, Vargas CE, Kumar T, Weiner SA, Brabbins DS, Chen PY, et al. The impact of age on long-term outcome in patients with endometrial cancer treated with postoperative radiation. Gynecol Oncol. 2006;103:87–93. doi: 10.1016/j.ygyno.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Gemer O, Arie AB, Levy T, Gdalevich M, Lorian M, Barak F, et al. Lymphvascular space involvement compromises the survival of patients with stage I endometrial cancer: results of a multicenter study. Eur J Surg Oncol. 2007;33:644–647. doi: 10.1016/j.ejso.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Dunn EF, Geye H, Platta CS, Gondi V, Rose S, Bradley KA, et al. Predictive factors of recurrence following adjuvant vaginal cuff brachytherapy alone for stage I endometrial cancer. Gynecol Oncol. 2014;133:494–498. doi: 10.1016/j.ygyno.2014.03.554. [DOI] [PubMed] [Google Scholar]
- 9.Garg G, Gao F, Wright JD, Hagemann AR, Mutch DG, Powell MA. Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol. 2013;128:77–82. doi: 10.1016/j.ygyno.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 11.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016;21:419–434. doi: 10.1007/s10147-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 12.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 13.Japan Society of Obstetrics and Gynecology. Gynecologic Oncology Committee [Internet] Tokyo: Japan Society of Obstetrics and Gynecology; c2018. [cited 2018 May 21]. Available from: http://plaza.umin.ac.jp/~jsog-go/ [Google Scholar]
- 14.Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T, et al. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101:520–529. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 17.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 19.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Suh DH, Kim K, No JH, Kim YB. Influence of positive peritoneal cytology on prognostic factors and survival in early-stage endometrial cancer: a systematic review and meta-analysis. Jpn J Clin Oncol. 2016;46:711–717. doi: 10.1093/jjco/hyw063. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 22.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK ASTEC Study Group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]