Abstract
Objective
To evaluate the impact of age-adjusted Charlson comorbidity index (ACCI) in predicting disease-free survival (DFS), overall survival (OS), and cancer-specific survival (CSS) among surgically treated patients with vulvar carcinoma. The secondary aim is to evaluate its impact as a predictor of the pattern of recurrence.
Methods
We retrospectively evaluated data of patients that underwent surgical treatment for vulvar cancer from 1998 to 2016. ACCI at the time of primary surgery was evaluated and patients were classified as low (ACCI 0–1), intermediate (ACCI 2–3), and high risk (>3). DFS, OS and CSS were analyzed using the Kaplan-Meir and the Cox proportional hazard models. Logistic regression model was used to assess predictors of distant and local recurrence.
Results
Seventy-eight patients were included in the study. Twelve were classified as low, 36 as intermediate, and 30 as high risk according to their ACCI. Using multivariate analysis, ACCI class was an independent predictor of worse DFS (hazard ratio [HR]=3.04; 95% confidence interval [CI]=1.54–5.99; p<0.001), OS (HR=5.25; 95% CI=1.63–16.89; p=0.005) and CSS (HR=3.79; 95% CI=1.13–12.78; p=0.03). Positive nodal status (odds ratio=8.46; 95% CI=2.13–33.58; p=0.002) was the only parameter correlated with distant recurrence at logistic regression.
Conclusion
ACCI could be a useful tool in predicting prognosis in surgically treated vulvar cancer patients. Prospective multicenter trials assessing the role of ACCI in vulvar cancer patients are warranted.
Keywords: Vulvar Cancer, Comorbidity, Prognostic Factors, Elderly, Frailty
INTRODUCTION
Approximately half of vulvar cancer patients are aged 70 or older at the time of diagnosis [1]. Women's life expectancy has been increasing worldwide [2] and, consequently, so have age related comorbidities. Comorbidity seems to be correlated with poor prognosis in different tumors [3,4,5,6]; however, only few reports for gynecological tumors exist. Age-adjusted Charlson comorbidity index (ACCI) seems to be a predictor of survival in endometrial cancer [7] and ovarian cancer [8].
To our knowledge only one study has evaluated the effect of the Charlson comorbidity index (CCI) on overall survival (OS) in vulvar cancer patients [9]. No one study has evaluated the impact of the age-adjusted comorbidity index on survival (OS, disease-free survival [DFS] and cancer-specific survival [CSS]) in vulvar cancer patients.
Due to the scarcity of data examining the prognostic impact of medical comorbidity on survival for vulvar cancer, the primary aim of this study is to evaluate the impact of the age-adjusted Charlson comorbidity score in predicting DFS, OS, and CSS among surgically treated patients with vulvar carcinoma. The secondary aim of the study is to evaluate the impact of ACCI as a predictor of the pattern of recurrence.
MATERIALS AND METHODS
For the present study, our internal database was analyzed from 1998 to 2016. Following IRB approval (CDD034/2015 of 23/11/2015), we retrospectively reviewed our prospectively maintained database searching for patients that have undergone surgical treatment for vulvar cancer.
Inclusion criteria were histologically confirmed squamous carcinoma or adenocarcinoma of the vulva. Exclusion criteria were recurrent disease, pre-invasive lesions (VIN 1–3), or uncommon histology (melanoma, Paget's disease, basalioma).
Demographic data, operative and pathological reports, and clinical outcomes were extracted from the database. Missing data and data concerning pre-existing medical conditions present at the time of primary surgery were extracted from the patient's medical records. Patients whose medical records had insufficient data concerning their medical history were also excluded from the analysis.
ACCI was calculated by summing the weighted comorbidities and age of each patient. A binomial value (present or absent) was assigned to 19 different comorbidities. If present the following parameters were assigned 1 point: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease (cerebrovascular accident [CVA] with mild or no residua) or transient ischemic attack [TIA], dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, and diabetes without end organ damage. If present the following parameters were assigned 2 points: hemiplegia, moderate or severe renal disease, diabetes with end organ damage, solid tumor without metastases (exclude if >5 years from diagnosis), leukemia and lymphoma. If present the following parameters were assigned 3 points: moderate or severe liver disease. If present the following parameters were assigned 6 points: metastatic solid tumor and acquired immune deficiency syndrome (not just human immunodeficiency virus positive). Moreover, for each life decade after 40 years of age, a point was added until a maximum of 4 points (1 point for age 50–59, 2 points for age 60–69, 3 points for age 70–79, and 4 points for age ≥80).
Vulvar cancer was not considered in the score assessment (6 points for solid tumor), and any new medical conditions that developed after the date of surgical treatment were omitted. The overall score corresponds to the weighed sum of 19 medical conditions and patient age [10]. Patients were classified into 3 groups based on their ACCI score (ACCI 0–1= low; ACCI 2–3 = intermediate; ACCI >3 = high).
Standard statistical analysis was used to evaluate descriptive analysis such as mean, frequencies and percentages. Incidence of event was analyzed for statistical significance by using the Fisher exact test. Normality testing (D'Agostino and Pearson test) was performed to determine whether data were sampled from a Gaussian distribution. The t-test and Mann-Whitney U test were used to compare continuous parametric and non-parametric values, respectively.
Predicting variables were evaluated for their association with distant and local recurrences on the basis of logistic regression model. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated for each comparison.
DFS was calculated in months from the date of surgery to the last follow-up or date of first recurrence, OS was calculated in months from the date of surgery to the last follow-up or date of death, and CSS was calculated in months from the date of surgery to the last follow-up or date of death from disease.
Five-year DFS, OS, and CSS were estimated using the Kaplan-Meier curve. Cox regression analysis was used to calculate both univariate and multivariate analysis for variables influencing DFS, OS, and CSS. Multivariable models were performed for variables with a p value 0.10 based on univariate analysis. Associations were summarized by calculating hazard ratio (HR) and corresponding 95% CI. All calculated p values were 2-sided. The p value <0.05 were considered statistically significant. Statistical analysis was performed with IBM Microsoft's SPSS Statistics version 24.0 for Mac (IBM Corp., Armonk, NY, USA).
RESULTS
Seventy-eight patients met the inclusion criteria of this study. In Table 1 are reported the patients surgical and pathological characteristics. Mean age was 67.9±10.3 and mean BMI was 27.5±5.5. The most represented histology was squamous cell carcinoma (96.2%). Thirty-four patients (43.6%) were diagnosed at early stage (stage IA–IB), 27 (34.6%) at stages II and III, and 6 (7.7%) had advanced disease (stage IV). Twelve patients were classified as ACCI low risk 0–1 (15.4%), 36 as intermediate risk (46.2%), and 30 as high risk (38.5%).
Table 1. Surgical and pathological characteristics.
| Variables | Values | |
|---|---|---|
| Age at diagnosis | 67.9±10.3 | |
| BMI | 27.5±5.5 | |
| Histology | ||
| Squamous | 75 (96.2) | |
| Adenocarcinoma | 3 (3.8) | |
| Grade | ||
| 1 | 16 (20.5) | |
| 2 | 33 (42.3) | |
| 3 | 15 (19.2) | |
| Missing | 14 (17.9) | |
| FIGO stage | ||
| IA | 11 (14.1) | |
| IB | 23 (29.5) | |
| II | 13 (16.7) | |
| III | 14 (17.9) | |
| IV | 6 (7.7) | |
| Missing | 11 (14.1) | |
| Symptoms at diagnosis | ||
| Bleeding | 8 (10.3) | |
| Pruritus | 36 (46.2) | |
| Mass | 10 (12.8) | |
| ACCI score | ||
| Low risk (0–1) | 12 (15.4) | |
| Intermediate risk (2–3) | 36 (46.2) | |
| High risk (>3) | 30 (38.5) | |
| Surgical procedure | ||
| Skin vulvectomy | 3 (3.8) | |
| Local excision | 11 (14.1) | |
| Hemivulvectomy | 29 (37.2) | |
| Radical vulvectomy | 35 (44.9) | |
| Flap | 21 (26.9) | |
| Monolateral lymphadenectomy | 18 (23.1) | |
| Bilateral lymphadenectomy | 47 (60.3) | |
| Tumor size (mm) | 29.4±17.9 | |
| Tumor-free resection margins | ||
| >10 mm | 48 (61.5) | |
| >5 mm | 12 (15.4) | |
| 5 mm | 11 (14.1) | |
| Infiltrated | 7 (9) | |
| Number of removed lymph nodes | 16.6±11.9 | |
| Node positive patients | 19 (24.4) | |
| Number of positive nodes* | 3.3±3.4 | |
Values are presented as number of patients (%) or mean±standard deviation.
ACCI, age-adjusted Charlson comorbidity index; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics.
*Among positive-node patients.
Considering factors influencing DFS, we observed using univariate analysis that age >75 (HR=1.93; 95% CI=1.06–3.53; p=0.033), positive nodes >2 (HR=3.03; 95% CI=1.48–6.22; p=0.003), stage (HR=1.40; 95% CI=1.04–1.79; p=0.026), and ACCI (HR=1.80; 95% CI=1.19–2.72; p=0.005) were correlated with 5-year DFS (Table 2). Considering factors influencing DFS we observed using multivariate analysis that more than 2 positive nodes (HR=8.94; 95% CI=2.67–29.97; p<0.001) and ACCI (HR=3.04; 95% CI=1.54–5.99; p<0.001) were independent predictors (Table 2).
Table 2. Survival outcomes.
| Variables | DFS | OS | CSS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate Analysis | Univariate analysis | Multivariate Analysis | Univariate analysis | Multivariate Analysis | |||||||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age >75 yr | 1.93 | 1.06–3.53 | 0.033 | 1.73 | 0.76–3.96 | 0.19 | 2.78 | 1.24–6.26 | 0.013 | 1.13 | 0.36–3.51 | 0.84 | 3.69 | 1.61–8.47 | 0.002 | 1.65 | 0.47–5.79 | 0.44 |
| Obesity (BMI >30) | 0.34 | 0.11–1.01 | 0.09 | - | 0.56 | 0.13–2.50 | 0.45 | - | 0.52 | 0.12–2.31 | 0.40 | - | ||||||
| Positive node patients | 2.53 | 1.24–5.16 | 0.011 | - | 2.78 | 1.09–7.08 | 0.04 | - | 2.32 | 0.86–6.24 | 0.095 | - | ||||||
| Positive nodes >2 | 3.03 | 1.48–6.22 | 0.003 | 8.94 | 2.67–29.97 | <0.001 | 3.40 | 1.33–8.67 | 0.01 | 7.72 | 2.05–28.98 | 0.002 | 3.02 | 1.11–8.20 | 0.03 | 5.25 | 1.45–19.05 | 0.012 |
| Size of lesion (mm) | 0.99 | 0.84–1.19 | 0.99 | - | 1.02 | 0.81–1.30 | 0.88 | - | 1.00 | 0.79–1.28 | 0.99 | - | ||||||
| Positive margins | 1.57 | 0.77–3.22 | 0.22 | - | 1.76 | 0.68–4.56 | 0.25 | - | 0.99 | 0.35–2.78 | 0.98 | - | ||||||
| Grade 3 | 1.71 | 0.86–3.40 | 0.13 | - | 1.33 | 0.52–3.41 | 0.55 | - | 1.57 | 0.63–3.90 | 0.33 | - | ||||||
| Stage | 1.40 | 1.04–1.79 | 0.026 | 1.59 | 0.94–1.95 | 0.42 | 1.28 | 0.35–4.71 | 0.07 | 0.82 | 0.42–1.58 | 0.54 | 1.25 | 0.27–5.75 | 0.08 | 0.78 | 0.30–1.44 | 0.30 |
| NACT | 1.34 | 0.54–3.41 | 0.54 | - | 0.34 | 0.049–3.89 | 0.14 | - | 0.51 | 0.07–3.85 | 0.52 | - | ||||||
| Adjuvant therapy | 1.14 | 0.77–1.71 | 0.51 | - | 1.13 | 0.60–2.13 | 0.70 | - | 0.96 | 0.47–1.96 | 0.91 | - | ||||||
| ACCI | 1.80 | 1.19–2.72 | 0.005 | 3.04 | 1.54–5.99 | <0.001 | 3.93 | 1.86–8.31 | <0.0001 | 5.25 | 1.63–16.89 | 0.005 | 3.59 | 1.70–7.56 | 0.001 | 3.79 | 1.13–12.78 | 0.031 |
All variables with a p value inferior to 0.1 at univariate and multi appear in bold character.
ACCI, age-adjusted Charlson comorbidity index; BMI, body mass index; CI, confidence interval; CSS, cancer-specific survival; DFS, disease-free survival; HR, hazard ratio; NACT, neoadjuvant chemotherapy; OS, overall survival.
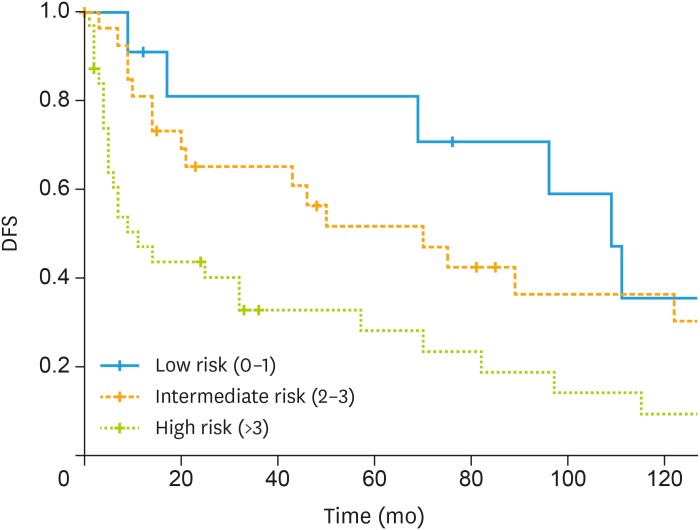
Five-year DFS was 45.8% for all patients. Negative nodal status had a 50.5% 5-year DFS, whereas it was 24.3% in positive node status (p=0.007). Five-year DFS was 80.8%, 46.9% and 28% for low ACCI, intermediate ACCI and high ACCI (p=0.009), respectively. Fig. 1 shows DFS according ACCI class.
Fig. 1. Kaplan-Meier curve of DFS according to ACCI class.
ACCI, age-adjusted Charlson comorbidity index; DFS, disease-free survival.
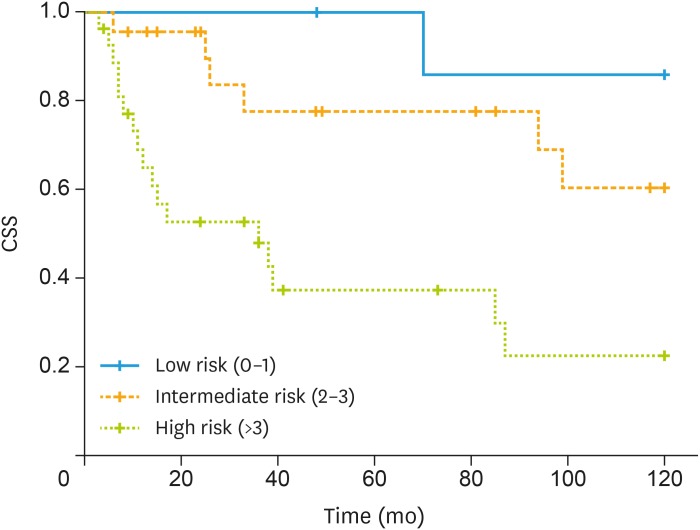
Considering factors influencing CSS we observed using univariate analysis that age >75 (HR=3.69; 95% CI=1.61–8.47; p=0.002), positive nodes >2 (HR=3.02; 95% CI=1.11–8.20; p=0.03), stage (HR=1.25; 95% CI=0.27–5.75; p=0.08), and ACCI (HR=3.59; 95% CI=1.7–5.56; p=0.001) were correlated with 5-year CSS (Table 2). Considering factors influencing CSS we observed using multivariate analysis that more than 2 positive nodes (HR=5.25; 95% CI=1.45–19.05; p=0.012) and ACCI (HR=3.79; 95% CI=1.13–12.78; p=0.031) were independent predictors (Table 2). Fig. 2 shows CSS according ACCI class.
Fig. 2. Kaplan-Meier curve of CSS according to ACCI class.
ACCI, age-adjusted Charlson comorbidity index; CSS, cancer-specific survival.
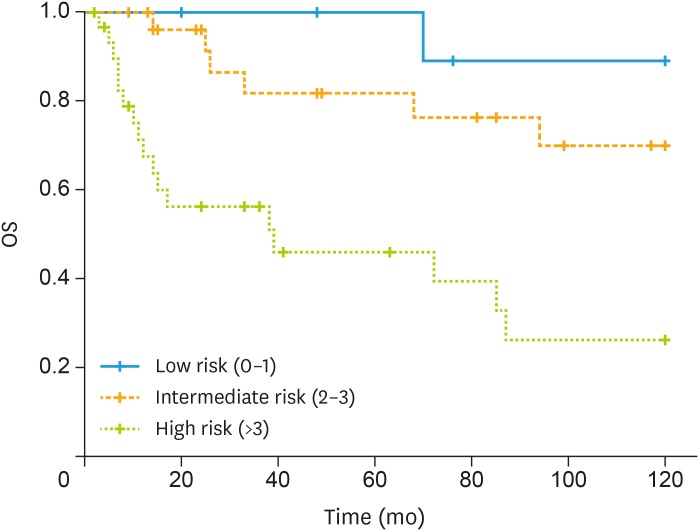
Considering factors influencing OS we observed that at univariate analysis that age >75 (HR=2.78; 95% CI=1.24–6.26; p=0.013), positive nodes >2 (HR=3.40; 95% CI=1.33–8.67; p=0.01), stage (HR=1.28; 95% CI=0.35–4.71; p=0.07), and ACCI (HR=3.93; 95% CI=1.86–8.31; p<0.001) were correlated with 5-year OS (Table 2). Considering factors influencing OS we observed that at multivariate analysis more than 2 positive nodes (HR=7.72; 95% CI=2.05–28.98; p=0.002) and ACCI (HR=5.25; 95% CI=1.63–16.89; p=0.005) were independent predictors (Table 2). Fig. 3 shows OS and according ACCI class.
Fig. 3. Kaplan-Meir curve of OS according to ACCI class.
ACCI, age-adjusted Charlson comorbidity index; OS, overall survival.
Among 49 patients that recurred, 32 (65.3%) were local, and 17 (34.7%) were distant. Considering factors influencing local recurrence, there was a non-statistical association with close margins at logistic regression (OR=2.89; 95% CI=0.87–9.54; p=0.08). The presence of 2 or more positive nodes independently correlated with the occurrences of distant recurrence (OR=8.46; 95% CI=2.13–33.58; p=0.002). There was a non-significant statistical association between obesity, adenocarcinoma histology and occurrence of distant recurrence.
DISCUSSION
Vulvar cancer is one of the least common gynecological cancers, and the role of its prognostic factors is far from clear [11,12]. The major finding of the present study is the correlation between ACCI and OS, CSS, and DFS.
The impact of ACCI on DFS is possibly associated with the increased probability of receiving suboptimal treatment in patients with multiple comorbidities making them at increased risk for disease recurrence and mortality. These findings seem to be in accordance with the literature [13,14,15,16,17,18].
A recent analysis of a SEER database has clearly demonstrated the importance of surgery in vulvar cancer treatment [19]. This study showed that patients who underwent surgical procedure had a better prognosis (HR=0.4; 95% CI=0.24–0.69; p<0.001). In our study, the patients analyzed had all been treated with curative intent, and presumably extensive demolition surgery had been avoided in patients with high comorbidities because of the inherent risk of increased complication. In support of this, we found that the presence of close margins (<5 mm) are more frequently found among patients with intermediate (35%) or high risk (38%) ACCI was compared to low risk (14%), although it should be noted that this difference is not statistically significant. Furthermore, some studies report that complication rates seem to be increased in patients with multiple comorbidities [20,21,22] and often their management delays the access to adjuvant treatment [23,24], if required. We also found that the ACCI was correlated with longer postoperative stay in patients submitted to more extensive surgical procedure. In our study patients submitted to radical vulvectomy with high ACCI score had a significant (p=0.007) longer postoperative stay (13.73±14.4) compared to those with intermediate/low ACCI score (9±4.80).
ACCI is an independent predictor of OS. Our study is the first to correlate the ACCI with OS in vulvar cancer. This finding is in line with a recent study that found that the CCI was correlated with increased risk of death [9]. According to the literature [19] age is considered an independent predictor of OS. However, in our study although age was a predictor of survival using univariate analysis, it was not confirmed using multivariate analysis, suggesting that, more than age itself, rather age-related comorbidity independently correlates with mortality rate. An even more interesting finding is the association between ACCI and CSS. This could be associated with a more aggressive behavior of the tumor affecting elderly patients with multiple comorbidities, this finding seems to be in line with the literature [25,26,27]. This suggests that treatment should not only focus on the cancer itself [28,29,30] but also on the various comorbidities that affect survival. The major limitation of this study is its retrospective nature and the number of patients included that limits the possibility to have firm conclusions. However, because vulvar cancer is a rare disease, it is very difficult to conduct large prospective evaluation of prognostic factors of this neoplasm. In the present study, presumably, more aggressive treatment had been avoided in patients with high comorbidities because of the inherent risk of increased complications. For that reason, even if tested using multivariate analysis, age could represent a confounding factor. Prospective multicenter trials assessing ACCI to validate our findings are therefore warranted.
In conclusion, to our knowledge our study is the first to evaluate the impact of the age adjusted comorbidity index on survival in vulvar cancer patients. ACCI evaluation could be a useful tool in predicting prognosis in surgically treated vulvar cancer patients. During initial patient assessment, ACCI could prove useful in assessing risk of mortality from cancer and could ultimately be used as a tool for a more tailored, individualized patient management.
ACKNOWLEDGMENTS
This study was supported the time and facilities of VDD (Sapienza University of Rome grant C26N15LL2Y/2015).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported
- Conceptualization: D.D.V., B.C., B.P.P.
- Data curation: D.D.V., T.F., M.A.
- Formal analysis: P.Z., B.C.
- Methodology: M.A., B.P.P.
- Supervision: T.F., M.A., P.G., B.P.P.
- Validation: P.G., B.P.P.
- Visualization: P.Z., T.F., M.A., B.P.P.
- Writing - original draft: D.D.V., P.Z., B.C., T.F., M.A., P.G.
- Writing - review & editing: B.C., P.G.
References
- 1.National Cancer Institute. SEER cancer statistics review, 1975??014 [Internet] Bethesda, MD: National Cancer Institute; 2017. [cited 2018 Jun 09]. Available from: https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 2.World Health Organization. World health statistics [Internet] place unknown: World Health Organization; 2016. [cited 2016 Sep 11]. Available from: http://www.who.int/gho/publications/world_health_statistics/en/ [Google Scholar]
- 3.Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–2392. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 4.Grose D, Morrison DS, Devereux G, Jones R, Sharma D, Selby C, et al. The impact of comorbidity upon determinants of outcome in patients with lung cancer. Lung Cancer. 2015;87:186–192. doi: 10.1016/j.lungcan.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Ather MH, Nazim SM. Impact of Charlson's comorbidity index on overall survival following tumor nephrectomy for renal cell carcinoma. Int Urol Nephrol. 2010;42:299–303. doi: 10.1007/s11255-009-9636-8. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Kim HS, Kim W, Kim JH, Kang SH, Han I. Comorbidity is independently associated with poor outcome in extremity soft tissue sarcoma. Clin Orthop Surg. 2015;7:120–130. doi: 10.4055/cios.2015.7.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins JR, Gayar OH, Zaki M, Mahan M, Buekers T, Elshaikh MA. Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer. Gynecol Oncol. 2013;131:593–597. doi: 10.1016/j.ygyno.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Suidan RS, Leitao MM, Jr, Zivanovic O, Gardner GJ, Long Roche KC, Sonoda Y, et al. Predictive value of the age-adjusted Charlson comorbidity index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2015;138:246–251. doi: 10.1016/j.ygyno.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghebre RG, Posthuma R, Vogel RI, Geller MA, Carson LF. Effect of age and comorbidity on the treatment and survival of older patients with vulvar cancer. Gynecol Oncol. 2011;121:595–599. doi: 10.1016/j.ygyno.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Panici PB, Tomao F, Domenici L, Giannini A, Giannarelli D, Palaia I, et al. Prognostic role of inguinal lymphadenectomy in vulvar squamous carcinoma: younger and older patients should be equally treated. A prospective study and literature review. Gynecol Oncol. 2015;137:373–379. doi: 10.1016/j.ygyno.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Bogani G, Cromi A, Serati M, Uccella S, Donato VD, Casarin J, et al. Predictors and patterns of local, regional, and distant failure in squamous cell carcinoma of the vulva. Am J Clin Oncol. 2017;40:235–240. doi: 10.1097/COC.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 13.Janssen-Heijnen ML, Maas HA, Houterman S, Lemmens VE, Rutten HJ, Coebergh JW. Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43:2179–2193. doi: 10.1016/j.ejca.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Vulto AJ, Lemmens VE, Louwman MW, Janssen-Heijnen ML, Poortmans PH, Lybeert ML, et al. The influence of age and comorbidity on receiving radiotherapy as part of primary treatment for cancer in South Netherlands, 1995 to 2002. Cancer. 2006;106:2734–2742. doi: 10.1002/cncr.21934. [DOI] [PubMed] [Google Scholar]
- 15.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 16.Hawfield A, Lovato J, Covington D, Kimmick G. Retrospective study of the effect of comorbidity on use of adjuvant chemotherapy in older women with breast cancer in a tertiary care setting. Crit Rev Oncol Hematol. 2006;59:250–255. doi: 10.1016/j.critrevonc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Lash TL, Silliman RA, Guadagnoli E, Mor V. The effect of less than definitive care on breast carcinoma recurrence and mortality. Cancer. 2000;89:1739–1747. doi: 10.1002/1097-0142(20001015)89:8<1739::aid-cncr14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 19.Ramanah R, Lesieur B, Ballester M, Darai E, Rouzier R. Trends in of late-stage squamous cell vulvar carcinomas: analysis of the surveillance, epidemiology, and end results (SEER) database. Int J Gynecol Cancer. 2012;22:854–859. doi: 10.1097/IGC.0b013e318249bce6. [DOI] [PubMed] [Google Scholar]
- 20.Chang CM, Yin WY, Wei CK, Wu CC, Su YC, Yu CH, et al. Adjusted age-adjusted Charlson comorbidity index score as a risk measure of perioperative mortality before cancer surgery. PLoS One. 2016;11:e0148076. doi: 10.1371/journal.pone.0148076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alibhai SM, Leach M, Tomlinson G, Krahn MD, Fleshner N, Holowaty E, et al. 30-day mortality and major complications after radical prostatectomy: influence of age and comorbidity. J Natl Cancer Inst. 2005;97:1525–1532. doi: 10.1093/jnci/dji313. [DOI] [PubMed] [Google Scholar]
- 22.Birim O, Maat AP, Kappetein AP, van Meerbeeck JP, Damhuis RA, Bogers AJ. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:30–34. doi: 10.1016/s1010-7940(02)00721-2. [DOI] [PubMed] [Google Scholar]
- 23.Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–377. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 24.Tevis SE, Kohlnhofer BM, Stringfield S, Foley EF, Harms BA, Heise CP, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum. 2013;56:1339–1348. doi: 10.1097/DCR.0b013e3182a857eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ording AG, Garne JP, Nyström PM, Frøslev T, Sørensen HT, Lash TL. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis--a Danish nationwide matched cohort study. PLoS One. 2013;8:e76013. doi: 10.1371/journal.pone.0076013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erichsen R, Horváth-Puhó E, Iversen LH, Lash TL, Sørensen HT. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer. 2013;109:2005–2013. doi: 10.1038/bjc.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Manwani B, Leng SX. Frailty, inflammation, and immunity. Aging Dis. 2011;2:466–473. [PMC free article] [PubMed] [Google Scholar]
- 29.Di Donato V, Bracchi C, Cigna E, Domenici L, Musella A, Giannini A, et al. Vulvo-vaginal reconstruction after radical excision for treatment of vulvar cancer: Evaluation of feasibility and morbidity of different surgical techniques. Surg Oncol. 2017;26:511–521. doi: 10.1016/j.suronc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Benedetti Panici P, Di Donato V, Bracchi C, Marchetti C, Tomao F, Palaia I, et al. Modified gluteal fold advancement V-Y flap for vulvar reconstruction after surgery for vulvar malignancies. Gynecol Oncol. 2014;132:125–129. doi: 10.1016/j.ygyno.2013.10.037. [DOI] [PubMed] [Google Scholar]