Abstract
Objective
Gynecologists occasionally encounter synchronous endometrial and ovarian endometrioid carcinoma (SEO-EC) patients who show favorable prognosis than locally advanced or metastatic disease patients. This study aimed to elucidate prognostic factors of SEO-EC and identify patients who have a sufficiently low risk of recurrence without receiving adjuvant chemotherapy.
Methods
We retrospectively reviewed 46 patients with pathologically confirmed SEO-EC who underwent surgery at the National Cancer Center Hospital between 1997 and 2016. Immunohistochemical evaluation of DNA mismatch repair (MMR) protein expression were performed for both endometrial and ovarian tumors. Patient outcomes were analyzed according to clinicopathologic factors.
Results
From the multivariate analysis, cervical stromal invasion indicated a worse prognosis for progression-free survival (hazard ratio [HR]=6.85; 95% confidence interval [CI]=1.50–31.1) and overall survival (HR=6.95; 95% CI=1.15–41.8). Lymph node metastasis and peritoneal dissemination did not significantly affect survival. MMR deficiency was observed in 13 patients (28.3%), with both endometrial and ovarian tumors showing the same MMR expression status. MMR deficiency was not significantly associated with survival. Of 23 patients with lesions confined to only the uterine body and adnexa, only 2 had recurrence in the group receiving adjuvant therapy, while none of the 10 patients who did not receive adjuvant therapy had recurrence.
Conclusion
SEO-EC patients with tumors localized to the uterine body and adnexa lesions had a low risk for recurrence and may not require adjuvant therapy. SEO-EC may have prognostic factors different from those of endometrial and ovarian cancer.
Keywords: Synchronous Neoplasms, Prognostic Factors, Mismatch Repair Deficiency, Immunohistochemistry, Adjuvant Chemotherapy
INTRODUCTION
Synchronous endometrial and ovarian endometrioid cancer (SEO-EC) is an intractable clinical concern for gynecologists. SEO-EC occurs in 3.1%–10.0% of patients with endometrial cancer and 10% of those with ovarian cancer [1,2,3]. The possible origins of SEO-EC include 3 different scenarios, including dual primary cancer, endometrial cancer with ovarian metastasis, and ovarian cancer with endometrial metastasis. Distinguishing dual primary cancer from metastatic disease is essential for choosing the optimal adjuvant treatment and predicting patient prognosis. Previously reported clinicopathological features of dual primary cancer include younger age, earlier stage, histologically lower grade, and a more favorable prognosis than metastatic disease [2,4,5,6,7,8]. Therefore, some gynecologists believe that patients with dual primary cancer may not always require adjuvant chemotherapy. The traditional approach for selecting the treatment of distinct dual primary cancer from metastatic disease is performed based on the histopathological features, such as histological similarity, tumor volume, depth of myometrial invasion, presence of vascular space invasion, ovarian tumor laterality, presence of atypical endometrial hyperplasia, and/or endometriosis, which were reported by Herrington [9]. However, clinicians occasionally encounter an SEO-EC patient in whom distinguishing between dual primary and metastatic disease using the Scully criteria is difficult (the full description of the criteria provided in Supplementary Table 1).
Recently, the clonality of SEO-EC has been discerned from molecular analyses using next-generation sequencing (NGS) technology [10,11,12]. These studies revealed the clonal relationship between endometrial cancer and ovarian cancer in SEO-EC patients with massively parallel sequencing. Moreover, these studies consistently reported that approximately 95% of the SEO-ECs clinically diagnosed as dual primary cancers were clonal tumors; that is, most SEO-ECs are metastatic disease from either endometrial or ovarian primary tumors.
The clinical and biological behavior of SEO-EC remains to be clarified. Considering recent reports on the clonality of SEO-EC, most of these malignancies are either endometrial cancer with ovarian metastases (The International Federation of Gynecology and Obstetrics [FIGO] stage III) or ovarian cancer with uterine metastases (FIGO Stage II). However, there have been numerous reports of SEO-EC patients with favorable prognosis, despite having metastatic disease [2,4,5,6,7,8,13,14]. The European Society of Medical Oncology (ESMO) and the National Cancer Center Network (NCCN) guidelines recommend adjuvant therapy for patients that have endometrial cancer with ovarian metastasis or ovarian cancer with uterine metastasis [15,16,17,18]. However, adjuvant therapy might not provide a survival benefit for SEO-EC patients with good prognoses. Therefore, there is concern that unnecessary adjuvant therapy will be routinely performed for SEO-EC patients with metastatic cancers disease, as defined by NGS technology in the near future.
This study aimed to determine prognostic factors of SEO-EC, including those of patients diagnosed with primary uterine cancer, primary ovarian cancer, and dual primary cancer, and identify SEO-EC patients who have a sufficiently low risk of recurrence without adjuvant chemotherapy.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board of the National Cancer Center Hospital (approval No. 2016-260). The analyzed cases included patients that were pathologically diagnosed with endometrioid cancer of both the endometrium and ovary and who underwent surgery at the National Cancer Center Hospital between January 1997 and June 2016. First, we identified a total of 1,183 patients with endometrial and/or ovarian cancer who underwent surgery at our hospital during the study period. Only 89 patients with both endometrial and ovarian lesions were included. Of these patients, 22 with histological subtypes other than endometrioid carcinoma were excluded. Therefore, 67 SEO-EC cases were identified. Finally, after exclusion of 19 cases without lymph node dissection and 2 cases with subsequent salvage operation, 46 SEO-EC patients were included in the analysis (Supplementary Fig. 1). Data of clinicopathological characteristics and prognoses were collected from medical records.
Immunohistochemical evaluation of DNA mismatch repair (MMR) protein expression was performed using formalin-fixed, paraffin-embedded blocks of both endometrial and ovarian specimens. Representative whole 4-μm-thick sections were analyzed by immunohistochemistry (IHC). The protein expression of 4 MMR proteins was evaluated using the following antibodies: anti-MLH1 (ES05, 1:200 dilution; Dako, Glostrup, Denmark), anti-MSH2 (FE11, 1:50 dilution; Dako), anti-MSH6 (SP93, 1:200; Spring Bioscience, Pleasanton, CA, USA), and anti-PMS2 (A16-4, 1:200; Biocare Medical, Pacheco, CA, USA). All IHC tests were performed using a Dako autostainer (Dako), according to the manufacturer's instructions. After deparaffinization, tissue sections were stained using antibodies against MLH1, MSH2, MSH6, and PMS2. Slides were counterstained with hematoxylin. MMR-deficient status was defined as the complete loss of nuclear staining for 1 or more MMR proteins. Adjacent normal mucosa, stromal cells, and inflammatory cells with intact nuclear staining served as internal positive controls. When PMS2 or MSH6 loss was observed and to discriminate between MLH1 or MSH2 (concurrent MLH1 and PMS2, or MSH2 and MSH6 loss) and PMS2 or MSH6 (only PMS2 or MSH6 loss) aberrations, an additional IHC test using either an anti-MLH1 or anti-MSH2 antibody was performed.
For the survival analysis, progression-free survival (PFS) was defined as the time from the date of surgery to the date of first recurrence or any cause of death. Overall survival (OS) was defined as the time from the date of surgery to the date of any cause of death. Survival curves were constructed using the Kaplan-Meier method, and a univariate log-rank test was used to assess statistical significance. Multivariate analyses for PFS and OS were performed using Cox proportional hazard modeling. For the analyses, the level of statistical significance was set at p<0.05. All statistical analyses were performed using SPSS version 19 for Mac (IBM Corp., Armonk, NY, USA).
RESULTS
1. Clinicopathological characteristics of SEO-EC patients
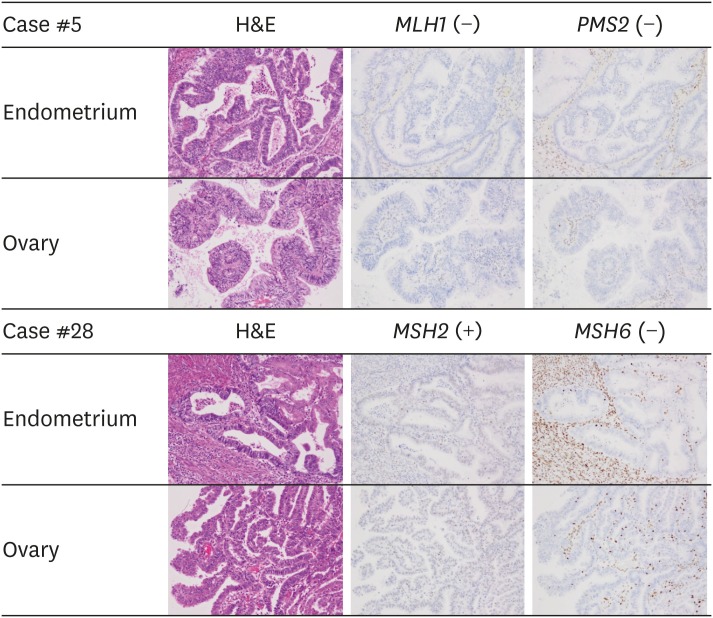
A total of 67 patients with SEO-EC were identified during the study period. Of the 67 patients, 46 met the inclusion criteria and 21 were excluded because they underwent salvage surgery or had insufficient staging procedure (Supplementary Fig. 1). The median follow-up period was 62 months (range, 7–223 months). The patients' characteristics are summarized in Table 1. The median age was 51 years (range, 30–77 years), the median body mass index was 22.0 (range, 16.4–30.7), and 27 patients (58.7%) were nulliparous. All patients achieved a no residual disease status following surgery, and semi-radical hysterectomies were performed for all 4 patients with cervical stromal invasion. Preoperative chemotherapy was not administered in any case. Thirty-two patients (69.5%) received adjuvant therapy after surgery, 29 (63.0%) were treated with chemotherapy, and 3 (6.5%) received radiation therapy. Of them, adriamycin and cisplatin (AP), paclitaxel and carboplatin (TC), cyclophosphamide, doxorubicin and cisplatin (CAP), dose-dense paclitaxel and carboplatin (ddTC), docetaxel and carboplatin (DC) were administered in 11, 8, 5, 4, and 1 patients, respectively. All patients who underwent adjuvant chemotherapy completed 6 cycles of chemotherapy without dose reduction. Most of the patients had pathological grade 1 or 2 endometrial and ovarian cancer and had a unilateral ovarian lesion. Lesions confined to the uterine body and ovary were observed in 23 of the patients (50%), while the other cases showed fallopian tubal involvement (30.4%), cervical stromal invasion (8.7%), peritoneal dissemination (30.4%), lymph node metastasis (32.6%), and positive peritoneal cytology (41.3%). During the follow-up period, 10 patients (21.7%) had recurrence and 9 patients (19.6%) died. Kaplan-Meier estimates for PFS and OS of all the patients are presented in Supplementary Fig. 2. The 5-year PFS of all patients was 80.3% (95% confidence interval [CI]=67.9%–92.6%) and the 5-year OS of all patients was 85.7% (95% CI=75.1%–96.4%). On the immunohistochemical evaluation of MMR protein status, 33 patients (71.7%) were classified as MMR intact, while 13 patients (28.3%) showed MMR deficiency. Of these, loss of MLH1 and PMS2, MSH2 and MSH6, and MSH6-only loss was observed in 7 (15.2%), 3 (6.5%), and 3 (6.5%) patients, respectively (Fig. 1). No tumors were classified as PMS2-only loss. All tumors showed the same MMR protein expression status between endometrial and ovarian tumors.
Table 1. Patients' characteristics (n=46).
| Characteristic | Value | ||
|---|---|---|---|
| Age (yr) | 51 (30–77) | ||
| BMI (kg/m2) | 22.0 (16.4–37.0) | ||
| Parity | |||
| Nulliparous | 27 (58.7) | ||
| Multipara | 19 (41.3) | ||
| Preoperative diagnosis of primary | |||
| Endometrial primary | 27 (58.7) | ||
| Ovarian primary | 4 (8.7) | ||
| Double primary | 15 (32.6) | ||
| Hysterectomy | |||
| Total | 42 (91.3) | ||
| Semi-radical | 4 (8.7) | ||
| Adjuvant therapy | |||
| No | 14 (30.4) | ||
| Chemotherapy | 29 (63.0) | ||
| AP/TC/CAP/ddTC/DC* | 11/8/5/4/1 | ||
| Radiation (whole pelvic 50 Gy) | 3 (6.5) | ||
| Myometrial invasion | |||
| <1/2 | 26 (56.5) | ||
| ≥1/2 | 20 (43.5) | ||
| Endometrial pathological grade | |||
| G1, G2 | 39 (84.8) | ||
| G3 | 7 (15.2) | ||
| LVSI of endometrial lesions | |||
| No | 22 (47.8) | ||
| Yes | 24 (52.2) | ||
| Ovary | |||
| Unilateral | 33 (71.7) | ||
| Bilateral | 13 (28.3) | ||
| Ovarian pathological grade | |||
| G1, G2 | 39 (84.8) | ||
| G3 | 7 (15.2) | ||
| Fallopian tubal involvement | |||
| No | 32 (69.6) | ||
| Yes | 14 (30.4) | ||
| Cervical stromal invasion | |||
| No | 42 (91.3) | ||
| Yes | 4 (8.7) | ||
| Lymph node metastasis | |||
| No | 31 (67.4) | ||
| Yes | 15 (32.6) | ||
| Peritoneal metastasis | |||
| No | 32 (69.6) | ||
| Yes | 14 (30.4) | ||
| Peritoneal cytology | |||
| Negative | 27 (58.7) | ||
| Positive | 19 (41.3) | ||
| MMR protein expression status | |||
| Intact | 33 (71.7) | ||
| MLH1(−), PMS2(−) | 7 (15.2) | ||
| MLH1(+), PMS2(−) | 0 (0.0) | ||
| MSH2(−), MSH6(−) | 3 (6.5) | ||
| MSH2(+), MSH6(−) | 3 (6.5) | ||
Values are presented as number of patients (%) of median (range).
AP, adriamycin and cisplatin; BMI, body mass index; CAP, cyclophosphamide, doxorubicin and cisplatin; ddTC, dose-dense paclitaxel and carboplatin; DC, docetaxel and carboplatin; LVSI, lymphovascular space invasion; MMR, DNA mismatch repair; TC, paclitaxel and carboplatin.
*For all 29 patients completed 6 cycles of adjuvant chemotherapy.
Fig. 1. Mismatch repair protein expression in synchronous endometrial and ovarian endometrioid adenocarcinoma. Patient #5 shows the concurrent loss of MLH1 and PMS2 expression in both endometrial and ovarian tumors, indicating an MLH1 aberration. Patient #28 exhibits retained MSH2 expression and loss of MSH6 in both endometrial and ovarian tumors, suggesting an MSH6 aberration.
2. Prognostic factors for PFS and OS
The univariate analysis of clinicopathological factors and survival outcomes are shown in Table 2. Patients with cervical stromal invasion had significantly lower PFS and OS than patients without cervical stromal invasion (p<0.01 and p=0.03, respectively). Patients with lymph node metastases had significantly lower PFS than patients without lymph node metastases (p=0.04, respectively). Depth of myometrial invasion and lymphovascular space invasion (LVSI) by endometrial lesion were not significantly associated with OS, but patients with myometrial invasion of ≥1/2 and LVSI tended to have worse PFS. MMR protein status was not significantly associated with survival according to the univariate analysis. Multivariate analysis including depth of myometrial invasion, LVSI of endometrial lesion, cervical stromal invasion, and lymph node metastases revealed a significant effect of cervical stromal invasion on PFS (hazard ratio [HR]=6.85; 95% CI=1.50–31.14) and OS (HR=6.95; 95% CI=1.15–41.84) (Table 3).
Table 2. The univariate analysis of clinicopathological factors and survival outcomes.
| Factor | PFS | OS | |||
|---|---|---|---|---|---|
| 5-yr PFS (%) | Univariate p | 5-yr OS (%) | Univariate p | ||
| Age | 0.93 | 0.91 | |||
| <50 | 83.6 | 84.4 | |||
| ≥50 | 78.6 | 87.5 | |||
| Myometrial invasion | 0.08 | 0.28 | |||
| <1/2 | 90.6 | 87.6 | |||
| ≥1/2 | 67.5 | 83.0 | |||
| Endometrial pathological grade | 0.56 | 0.69 | |||
| G1, G2 | 82.2 | 86.0 | |||
| G3 | 68.6 | 83.3 | |||
| LVSI of endometrial lesions | 0.09 | 0.87 | |||
| No | 89.2 | 79.7 | |||
| Yes | 72.8 | 91.3 | |||
| Ovary | 0.96 | 0.14 | |||
| Unilateral | 82.4 | 83.2 | |||
| Bilateral | 73.4 | 92.3 | |||
| Ovarian pathological grade | 0.19 | 0.29 | |||
| G1, G2 | 82.7 | 88.4 | |||
| G3 | 64.3 | 71.4 | |||
| Fallopian tubal involvement | 0.25 | 0.58 | |||
| No | 85.3 | 86.1 | |||
| Yes | 69.3 | 85.1 | |||
| Cervical stromal invasion | <0.01 | 0.03 | |||
| No | 86.5 | 89.9 | |||
| Yes | 25.0 | 50.0 | |||
| Lymph node metastases | 0.04 | 0.19 | |||
| No | 89.3 | 85.1 | |||
| Yes | 61.6 | 86.7 | |||
| Peritoneal metastases | 0.30 | 0.25 | |||
| No | 86.4 | 86.6 | |||
| Yes | 65.0 | 83.3 | |||
| Peritoneal cytology | 0.57 | 0.49 | |||
| Negative | 84.6 | 84.3 | |||
| Positive | 73.1 | 87.5 | |||
| Confined to uterine body and adnexa | 0.04 | 0.01 | |||
| No | 61.0 | 75.8 | |||
| Yes | 90.9 | 95.2 | |||
| MMR protein status | 0.48 | 0.25 | |||
| Intact | 76.3 | 83.2 | |||
| Deficiency | 83.9 | 92.3 | |||
LVSI, lymphovascular space invasion; MMR, DNA mismatch repair; OS, overall survival; PFS, progression-free survival.
Table 3. Multivariate analysis for PFS and OS.
| Factor | 5-year PFS | 5-year OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Myometrial invasion | |||||||
| ≥1/2 | 1.97 | 0.47–8.31 | 0.36 | 2.00 | 0.40–9.44 | 0.40 | |
| LVSI of endometrial lesions | |||||||
| Yes | 1.41 | 0.22–9.15 | 0.72 | 0.20 | 0.03–1.62 | 0.13 | |
| Cervical stromal invasion | |||||||
| Yes | 6.85 | 1.50–31.1 | 0.01 | 6.95 | 1.15–41.8 | 0.03 | |
| Lymph node metastases | |||||||
| Yes | 2.65 | 0.58–12.1 | 0.21 | 5.68 | 0.77–41.8 | 0.09 | |
CI, confidence interval; LVSI, lymphovascular space invasion; OS, overall survival; PFS, progression-free survival.
Although previous studies consistently reported that true primary site of SEO-EC cannot to be strictly determined by clinicopathological and/or genetic features [10,11,12], just for reference, results of survival analyses based on the same FIGO stage were provided in a Supplementary Tables 2 and 3. These survival analyses are based on hypothesis that all the SEO-EC cases were all endometrial primary (FIGO stage III or IV) or all ovarian primary (FIGO stage II or III).
3. Favorable prognosis group among SEO-EC patients
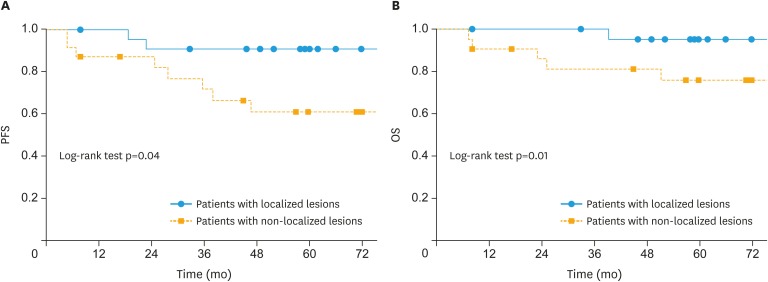
The SEO-EC patients with confined lesions could be considered to achieve complete resection of the tumor by surgery alone; therefore, these patients might not require adjuvant chemotherapy. Considering the results of multivariate analysis described above, we analyzed the prognosis of the patients who had lesions confined to only the uterine body and adnexa (ovary and fallopian tube); the prognosis of these patients is shown in Tables 2 and 4, as well as Fig. 2. The patients with lesions confined uterine body and adnexa (localized lesions) showed significantly longer 5-year PFS and OS (HR=0.29; 95% CI=0.06–0.96; p=0.04 and HR=0.11; 95% CI=0.006–0.61; p=0.009, respectively). Of these 23 patients, 13 received adjuvant therapy, while 10 patients did not. The patients with myometrial invasion ≥1/2 tended to receive adjuvant therapy, as compared to those with superficial myometrial invasion. However, there were no significant differences in the clinicopathological features between the 2 groups. Two patients had recurrence in the group that received adjuvant therapy, while no recurrences were observed in the group that was not administered adjuvant therapy.
Table 4. The clinicopathological features of SEO-EC patients with favorable prognosis.
| Characteristics | Adjuvant therapy | |
|---|---|---|
| No | Yes | |
| Number of patients | 10 | 13 |
| Follow-up period (mon) | 59 (33–138) | 66 (8–202) |
| Recurrence | 0 (0) | 2 (15) |
| Death | 0 (0) | 1 (7.7) |
| Myometrial invasion (≥1/2) | 0 (0) | 4 (31) |
| Endometrial pathological grade (G3) | 0 (0) | 2 (15) |
| LVSI of endometrial lesions (yes) | 2 (20) | 4 (31) |
| Ovary (bilateral) | 3 (30) | 2 (15) |
| Ovarian pathological grade (G3) | 0 (0) | 1 (7.7) |
| Fallopian tubal involvement (yes) | 2 (20) | 3 (23) |
| Peritoneal cytology (positive) | 2 (20) | 5 (39) |
Values are presented as number of patients (%) of median (range).
LVSI, lymphovascular space invasion; SEO-EC, synchronous endometrial and ovarian endometrioid cancer.
Fig. 2. Survival outcomes of patients with localized and non-localized lesions. (A) PFS and (B) OS.
OS, overall survival; PFS, progression-free survival.
DISCUSSION
This study elucidated baseline recurrent risk and prognostic factors of SEO-EC, which have been reported to be biologically clonal lesions. In addition, we identified patients who obtained a benefit from adjuvant therapy. We found that cervical stromal invasion was an independent factor for an unfavorable prognosis and described the clinicopathological features of SEO-EC patients that showed favorable prognosis despite not receiving adjuvant therapy. Furthermore, we clarified the frequency of MMR protein deficiencies in SEO-EC and showed that MMR protein status was the same in both endometrial and ovarian tumors. Recent studies revealed the clonal relationships between endometrial and ovarian tumors in SEO-EC, and all of these results consistently indicated that most SEO-ECs were clonal and metastatic disease [10,11,12]. However, gynecologists occasionally encounter SEO-EC patients that that show a more favorable prognosis than endometrial or ovarian cancers that were diagnosed as locally advanced or metastatic disease. Therefore, this finding raised the clinical question of whether all SEO-ECs should be treated as metastatic disease with a high-risk of recurrence. Although determining if SEO-EC was a uterine or ovarian primary tumor has not been revealed by NGS analysis, we considered that the clinical course and behavior of SEO-EC was quite different from metastatic disease, especially lymphovascular metastasis of endometrial and ovarian cancer.
Univariate and multivariate analyses revealed that cervical stromal invasion had a significant effect on PFS and OS. Notably, lymph node metastasis and peritoneal dissemination did not have a significant impact on survival. If SEO-EC is of endometrial origin, SEO-EC is classified as FIGO stage III endometrial cancer. However, cervical stromal invasion, which is a factor of FIGO stage II, was a significant prognostic factor, while peritoneal dissemination, which is a factor of FIGO stage IV, was not prognostic in the present study. Conversely, if SEO-EC is of ovarian origin, SEO-EC with cervical stromal invasion is classified as FIGO stage II ovarian cancer. However, lymph node metastasis and peritoneal dissemination, which are factors of FIGO stage III, were not significant prognostic factors in this study. According to our knowledge, there have been no previous reports on prognostic factors in SEO-EC patients, including cases that were previously excluded by the classical clinicopathological criteria. Although a limited power to detect differences due to the small sample size should be considered, our results indicate that the prognostic factors of SEO-EC may differ from the established prognostic factors of endometrial and ovarian cancer. More large-scale cohort studies should be performed to validate our findings for identifying SEO-EC patients with a high-risk of recurrence.
Prognosis of SEO-EC patients with lesions confined to only the uterine body and adnexa were analyzed to explore the baseline recurrence risk of these patients. SEO-EC patients with confined lesions could be considered for complete tumor resection by surgery alone; therefore, these patients might not require adjuvant chemotherapy. Considering the significant impact of cervical stromal invasion for a poor prognosis, we assessed patients who had lesions confined to only the uterine body and adnexa. Although there were only 10 patients who did not receive adjuvant therapy, no recurrence was observed in this cohort. SEO-EC localized to the uterine body and adnexa may have low risk of recurrence, although it is defined as metastatic disease by NGS, as well as either stage III endometrial cancer or stage II ovarian cancer. Consequently, for these patients, ESMO and NCCN guidelines recommend adjuvant therapy [15,16,17,18]. However, adjuvant therapy may result in unnecessary treatment for SEO-EC patients whose disease is localized to the uterine body and adnexa. This hypothesis has been supported by some previous studies. Before the NGS-era, some SEO-EC patients were clinically diagnosed as dual primary cancer but showed comparable prognosis to stage I endometrial cancer and stage I ovarian cancer [8,13]. The reason why SEO-EC with lesions confined lesions to the uterine body and adnexa showed relatively favorable prognosis remains unclear. However, SEO-EC caused by implantation through the fallopian tube may not have the same malignant potential as metastatic cancer cells showing lympho-vascular invasion or peritoneal dissemination. The difference in the mode of metastasis may reflect different prognoses.
MMR deficiencies were observed in 13 patients (28.3%), with both endometrial and ovarian tumors showing the same MMR protein expression status in all study patients. Previous studies reported that MMR protein deficiencies were detected in 20%–40% of endometrioid-type endometrial cancers [19,20,21,22,23,24,25] and in 6.4% of ovarian cancers, as well as 10% of endometrioid-type ovarian cancers [26]. The present study showed a comparable frequency of MMR deficiencies, as detected by IHC. However, MMR deficiency in SEO-EC has not been sufficiently described in the literature. Kobayashi et al. reported that MMR protein deficiencies were observed in 62.5% (15/24) of SEO-ECs, with 73% (11/15) showing different MMR protein expression statuses between endometrial and ovarian tumors [27]. However, the results of IHC analysis contained curious combinations of MMR protein loss, such as loss of MLH1 and MSH6. The results included 8 of 32 endometrial cancers and 1 of 32 ovarian cancers with loss of both MLH1 and MSH6. These results implied technical issues in the quality control and assessment by IHC analysis of MMR proteins. Furthermore, the inclusion criteria of the present study were different from those of previous studies, which included only the clinicopathologically diagnosed synchronous primary endometrial and ovarian cancers using the Scully criteria [9]. The differences in inclusion criteria might also explain the discordant results between our study and previous studies.
Previous studies reported that MMR protein expression status was significantly associated with low histological grade, disease stage, and LVSI in endometrioid-type endometrial cancer [19,28] and that MMR deficiency might be correlated to an optimal prognosis [29,30]. However, MMR protein status was not significantly associated with the prognosis of SEO-EC patients in this study. Although the reason for these discordant results remains unclear, this is the first report on the prognostic value of MMR deficiency in SEO-EC patients.
The present study has several limitations. First, we did not examine the clonal relationship between endometrial and ovarian tumors using NGS technology. We assumed that all 46 SEO-EC patients had metastatic disease based on consistent research results from different institutions [10,11,12]; therefore, a very small number of true dual primary SEO-ECs might be included in this study. However, considering that the fact of approximately 95% of SEO-EC patients that were clinically diagnosed as dual primary had metastatic disease, we can expect that few true dual primary SEO-ECs were included and should not affect the main results of this study. Second, the number of the included patients in this study was small due to the rarity of SEO-EC. More large-scale cohort studies should be performed in the future to validate the findings of our study. Nevertheless, the fact that cervical stromal invasion was a significant factor for a poor prognosis in SEO-EC patients was not consistent with conventional FIGO staging for both endometrial and ovarian cancer. Despite the small number of cases, we could point out the possibility that SEO-EC had different prognostic factors from both endometrial and ovarian cancer.
In conclusion, SEO-EC patients with tumors localized to the uterine body and adnexa showed a very low risk for recurrence. Therefore, adjuvant therapy for these patients might not provide a therapeutic benefit. Cervical stromal invasion was a significant factor for a poor prognosis, while MMR protein status was not associated with prognosis for SEO-EC. Recently, most SEO-ECs have been regarded as clonal and metastatic disease by NGS technology; however, prognostic factors of SEO-EC may be different from metastatic endometrial cancer and ovarian cancer. Further large-scale cohort studies are necessary to validate the findings of the present study for identifying SEO-EC patients who may actually obtain benefit with adjuvant therapy.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Y.Y., Y.H., I.M.
- Data curation: Y.Y.
- Formal analysis: Y.Y., Y.H., I.M.
- Funding acquisition: Y.H.
- Investigation: Y.Y., Y.H., I.M.
- Methodology: Y.Y., Y.H.
- Project administration: M.T., K.T.
- Resources: Y.Y., Y.H., I.M., S.H., U.T., K.T.
- Software: Y.Y.
- Supervision: M.T., K.T.
- Validation: Y.Y., Y.H., I.M.
- Visualization: Y.Y.
- Writing - original draft: Y.Y., Y.H.
- Writing - review & editing: Y.Y., Y.H., I.M., S.H., U.T., M.T., K.T.
SUPPLEMENTARY MATERIALS
The Scully's criteria
Univariate and multivariate analysis for PFS and OS in case of the all the SEO-EC cases were endometrial cancer with ovarian metastasis
Univariate and multivariate analysis for PFS and OS in case of the all the SEO-EC cases were ovarian cancer with endometrial metastasis
Number of patients included for analysis.
Kaplan-Meier estimates for PFS and OS of all patients (n=46).
References
- 1.AlHilli MM, Dowdy SC, Weaver AL, St Sauver JL, Keeney GL, Mariani A, et al. Incidence and factors associated with synchronous ovarian and endometrial cancer: a population-based case-control study. Gynecol Oncol. 2012;125:109–113. doi: 10.1016/j.ygyno.2011.12.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaino R, Whitney C, Brady MF, DeGeest K, Burger RA, Buller RE. Simultaneously detected endometrial and ovarian carcinomas--a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83:355–362. doi: 10.1006/gyno.2001.6400. [DOI] [PubMed] [Google Scholar]
- 3.Song T, Seong SJ, Bae DS, Suh DH, Kim DY, Lee KH, et al. Synchronous primary cancers of the endometrium and ovary in young women: a Korean Gynecologic Oncology Group Study. Gynecol Oncol. 2013;131:624–628. doi: 10.1016/j.ygyno.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Soliman PT, Slomovitz BM, Broaddus RR, Sun CC, Oh JC, Eifel PJ, et al. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol. 2004;94:456–462. doi: 10.1016/j.ygyno.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Chiang YC, Chen CA, Huang CY, Hsieh CY, Cheng WF. Synchronous primary cancers of the endometrium and ovary. Int J Gynecol Cancer. 2008;18:159–164. doi: 10.1111/j.1525-1438.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim YK, Padma R, Foo L, Chia YN, Yam P, Chia J, et al. Survival outcome of women with synchronous cancers of endometrium and ovary: a 10 year retrospective cohort study. J Gynecol Oncol. 2011;22:239–243. doi: 10.3802/jgo.2011.22.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MG, Bandera EV, Demissie K, Rodríguez-Rodríguez L. Synchronous primary ovarian and endometrial cancers: a population-based assessment of survival. Obstet Gynecol. 2009;113:783–789. doi: 10.1097/AOG.0b013e31819c7bdf. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, Machida H, Frimer M, Marcus JZ, Pejovic T, Roman LD, et al. Prognosis of women with stage I endometrioid endometrial cancer and synchronous stage I endometrioid ovarian cancer. Gynecol Oncol. 2017;147:558–564. doi: 10.1016/j.ygyno.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrington CS. Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament. Atlas of tumor pathology. Third series, Fascicle 23. Robert E. Scully, Robert H. Young and Philip B. Clement. Armed Forces Institute of Pathology, Washington, DC, 1998. No. of pages: 527. Price: $95.00. ISBN: 1 881041 43 3. J Pathol. 1999;189:145. [Google Scholar]
- 10.Schultheis AM, Ng CK, De Filippo MR, Piscuoglio S, Macedo GS, Gatius S, et al. Massively parallel sequencing-based clonality analysis of synchronous endometrioid endometrial and ovarian carcinomas. J Natl Cancer Inst. 2016;108:djv427. doi: 10.1093/jnci/djv427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anglesio MS, Wang YK, Maassen M, Horlings HM, Bashashati A, Senz J, et al. Synchronous endometrial and ovarian carcinomas: evidence of clonality. J Natl Cancer Inst. 2016;108:djv428. doi: 10.1093/jnci/djv428. [DOI] [PubMed] [Google Scholar]
- 12.Chao A, Wu RC, Jung SM, Lee YS, Chen SJ, Lu YL, et al. Implication of genomic characterization in synchronous endometrial and ovarian cancers of endometrioid histology. Gynecol Oncol. 2016;143:60–67. doi: 10.1016/j.ygyno.2016.07.114. [DOI] [PubMed] [Google Scholar]
- 13.Narin MA, Karalok A, Basaran D, Ureyen I, Turkmen O, Turan T, et al. Does synchronous endometrioid endometrial cancer have any prognostic effect on Stage I endometrioid ovarian cancer? Eur J Obstet Gynecol Reprod Biol. 2016;200:113–116. doi: 10.1016/j.ejogrb.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Rodolakis A, Thomakos N, Akrivos N, Sotiropoulou M, Ioannidis I, Haidopoulos D, et al. Clinicopathologic insight of simultaneously detected primary endometrial and ovarian carcinomas. Arch Gynecol Obstet. 2012;285:817–821. doi: 10.1007/s00404-011-2046-z. [DOI] [PubMed] [Google Scholar]
- 15.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 16.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 17.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 18.Morgan RJ, Jr, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, Chen LM, et al. Ovarian cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1134–1163. doi: 10.6004/jnccn.2016.0122. [DOI] [PubMed] [Google Scholar]
- 19.McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2016;34:3062–3068. doi: 10.1200/JCO.2016.67.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993;53:5100–5103. [PubMed] [Google Scholar]
- 22.MacDonald ND, Salvesen HB, Ryan A, Iversen OE, Akslen LA, Jacobs IJ. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000;60:1750–1752. [PubMed] [Google Scholar]
- 23.Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042–2048. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 24.Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group study. J Clin Oncol. 2015;33:4301–4308. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114:486–490. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Murphy MA, Wentzensen N. Frequency of mismatch repair deficiency in ovarian cancer: a systematic review this article is a US government work and, as such, is in the public domain of the United States of America. Int J Cancer. 2011;129:1914–1922. doi: 10.1002/ijc.25835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Nakamura K, Nomura H, Banno K, Irie H, Adachi M, et al. Clinicopathologic analysis with immunohistochemistry for DNA mismatch repair protein expression in synchronous primary endometrial and ovarian cancers. Int J Gynecol Cancer. 2015;25:440–446. doi: 10.1097/IGC.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 28.Cosgrove CM, Tritchler DL, Cohn DE, Mutch DG, Rush CM, Lankes HA, et al. An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol Oncol. 2018;148:174–180. doi: 10.1016/j.ygyno.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aysal A, Karnezis A, Medhi I, Grenert JP, Zaloudek CJ, Rabban JT. Ovarian endometrioid adenocarcinoma: incidence and clinical significance of the morphologic and immunohistochemical markers of mismatch repair protein defects and tumor microsatellite instability. Am J Surg Pathol. 2012;36:163–172. doi: 10.1097/PAS.0b013e31823bc434. [DOI] [PubMed] [Google Scholar]
- 30.Parra-Herran C, Lerner-Ellis J, Xu B, Khalouei S, Bassiouny D, Cesari M, et al. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod Pathol. 2017;30:1748–1759. doi: 10.1038/modpathol.2017.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Scully's criteria
Univariate and multivariate analysis for PFS and OS in case of the all the SEO-EC cases were endometrial cancer with ovarian metastasis
Univariate and multivariate analysis for PFS and OS in case of the all the SEO-EC cases were ovarian cancer with endometrial metastasis
Number of patients included for analysis.
Kaplan-Meier estimates for PFS and OS of all patients (n=46).