Abstract
Objective
To examine outcomes of patients having treatments for newly diagnosed advanced stage low-grade serous ovarian cancer (LGSC).
Methods
We conducted a retrospective case series of women affected by advanced stage (stage IIIB or more) LGSC undergoing surgery in a single oncologic center between January 2000 and December 2017. Survival outcomes were assessed using Kaplan-Meier and Cox models.
Results
Data of 72 patients were retrieved. Primary cytoreductive surgery was attempted in 68 (94.4%) patients: 19 (27.9%) had residual disease (RD) >1 cm after primary surgery. Interval debulking surgery (IDS) was attempted in 15 of these 19 (78.9%) patients and the remaining 4 patients having not primary debulking surgery. Twelve out of 19 (63.1%) patients having IDS had RD. After a mean (±standard deviation) follow-up was 61.6 (±37.2) months, 50 (69.4%) and 22 (30.5%) patients recurred and died of disease, respectively. Via multivariate analysis, non-optimal cytoreduction (hazard ratio [HR]=2.79; 95% confidence interval [CI]=1.16–6.70; p=0.021) and International Federation of Obstetrics and Gynecologists (FIGO) stage IV (HR=3.15; 95% CI=1.29–7.66; p=0.011) were associated with worse disease-free survival. Via multivariate analysis, absence of significant comorbidities (HR=0.56; 95% CI=0.29–1.10; p=0.093) and primary instead of IDS (HR=2.95; 95% CI=1.12–7.74; p=0.027) were independently associated with an improved overall survival.
Conclusion
LGSC is at high risk of early recurrence. However, owing to the indolent nature of the disease, the majority of patients are long-term survivors. Further prospective studies and innovative treatment modalities are warranted to improve patients care.
Keywords: Ovarian Neoplasms, Drug Therapy, Neoplasm Metastasis, Gynecologic Surgical Procedures, Cytoreduction Surgical Procedures
INTRODUCTION
Ovarian cancer is considered one of the most lethal malignancies in developed countries, due to its high death to incidence ratio. More than 22,000 newly diagnosed cases and 14,000 cancer-related deaths are estimated, in the United States every year [1].
Epithelial ovarian cancer represents the most common type of malignant ovarian neoplasm, account for 90% of all ovarian cancers. They should not be considered a single entity but as a group of heterogeneous disease. Epithelial ovarian cancer encompasses a heterogeneous group of malignancies that vary in etiology, histology, molecular biology, and other characteristics. It can be classified into five main types: high-grade serous, endometrioid, clear-cell, mucinous, and low-grade serous ovarian cancer (LGSC) accounting for 70%, 10%, 10%, 3%, and <5%, respectively [2].
LGSC are rare ovarian cancer type that seems to originate from low-malignant potential serous ovarian tumors or de novo from ovarian or peritoneal surfaces [3,4,5,6]. Accumulating data underlined that LGSC are characterized by partial chemoresistance [3-6]. Few studies reported outcomes of patients affected by LGSC and limited data are still available on prognosis of patients affected by advance stage LGSC (stage IIIB–IV) [6,7,8,9,10,11,12,13].
In the present paper we aimed to audit our large experience of patients affected by advance stage LGSC, thus describing outcomes related to LGSC. Moreover, we sought to identify prognostic factors that might influence survival outcomes of women with LGSC.
MATERIALS AND METHODS
This retrospective study was approved by Institutional Review Board (IRB) of IRCCS National Cancer Institute (IRB approval number: INT/MI/006812). Retrospectively, we collected data of consecutive patients affected by advanced stage LGSC, treated at National Cancer Institute (Milan, Italy), from January 1, 2000 to December 31, 2017. Patients who did not consent to use their clinical information for research purposes were excluded.
Inclusion criteria were: 1) histologically-proven invasive LGSC; 2) presence of gross intra-abdominal disease (stage IIIB–IV). Exclusion criteria were: 1) consent withdrawn; 2) final diagnosis of borderline ovarian tumor; 3) recurrent disease; 4) apparently early stage ovarian cancer upstaged to stage III disease due to nodal involvement; 5) presence of synchronous solid malignancy; and 6) performance status not fit for surgical treatment.
Data have been obtained from the computerized surgical database of our Institution. This database is meticulously updated by trained residents, according to the American College of Surgeons' National Quality Improvement Program platform [11]. Individual patients' records were reviewed in order to identify baseline patients' and diseases' characteristics. Data regarding preoperative cancer antigen 125 (CA125) level and the presence of ascites (more than 500 mL) were recorded. Diagnosis of LGSC was performed by 2 dedicated gynecologic pathologists (MLC and BP) who performed a pathology review of all specimens.
Stage and architectural grade were reported in accord to the 2009 International Federation of Obstetrics and Gynecologists (FIGO) [12]. The World Health Organization taxonomy was used in order to classify histological subtypes [12]. Primary endpoint measure was to investigate the role of neoadjuvant chemotherapy followed by interval debulking surgery (IDS) and adjuvant therapy instead of primary surgery and adjuvant chemotherapy. Secondary endpoint measure was to identify predictors of survival in advanced stage LGSC. During the study period there were no significant differences in the facilities available for patient care and in the referral patterns of our service. Basically, other aspects of patient management unrelated to surgical approach remained consistent over time. Preoperative medical evaluations were performed according to the American Society of Anesthesiologists (ASA) score and the Eastern Cooperative Oncology Group (ECOG) scale of performance status [14]. Medical comorbities were recorded and graded per the Charlson comorbidity index [15]. The aim of surgery was to remove all macroscopic disease. Generally, primary surgery was aborted in case of extensive involvement of the bowel (leading to multiple, more than three resections necessary to reach complete cytoreductive surgery), extensive involvement/retraction of the mesentery and extensive involvement of porta hepatis nodes. Resection of bulky nodes was systematically part of cytoreduction procedures; in case of complete intra-abdominal cytoreduction, systemic lymphadenectomy or lymph nodes sampling were performed [16]. Complete and optimal cytoreduction was defined in case of no macroscopic residual disease (RD) and RD <1.0 cm [17]. Details of our surgical protocols are reported elsewhere [13,16]. All patients were scheduled to have platinum-based adjuvant chemotherapy after surgery. For statistical purpose patients having primary surgical attempt (resulted to be unsatisfactory) followed by neoadjuvant chemotherapy and IDS were included in the interval debulking group.
1. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 6.0 for Mac (GraphPad Software, San Diego CA, USA) and IBM-Microsoft SPSS version 20.0 for Mac (IBM Corp., Armonk, NY, USA). Data are summarized using basic descriptive statistics. Survival outcomes were evaluated with both Kaplan-Meier and Cox models. Hazard ratio (HR) and 95% confidence intervals (CIs) were calculated for each comparison. Univariable and multivariable analysis were performed when appropriate, using Cox proportional hazard model. All covariates with a p-value less than 0.10, based on univariable analysis were included in the multivariable model. All p-values were 2-sided. The p-values <0.05 were statistically significant.
RESULTS
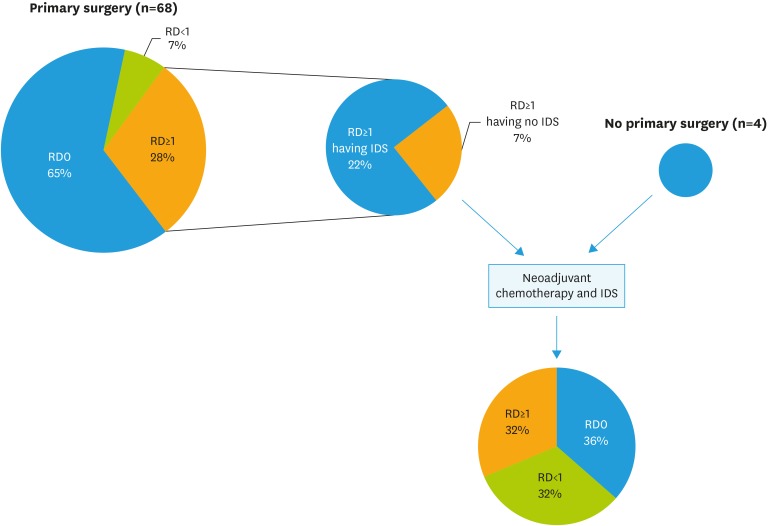
Data of 72 patients were collected. Mean (±standard deviation) patients age was 62.5 (±13.0) years. Patients' characteristics and stage distribution is reported in Table 1. Primary cytoreductive surgery was attempted in 68 (94.4%) patients: complete resection and optimal resection was achieved in 65% (n=44) and 72% (n=49) of patients, respectively; while 19 (27.9%) had RD >1 cm after primary surgery. IDS was attempted in 15 (20.8%) women of these latter group of patients and in 4 (5.5%) patients having not a primary surgical attempt. All these 4 patients were referred to our center for the execution of IDS after having diagnosis of advanced disease and neoadjuvant chemotherapy in other centers. Twelve out of 19 (63.1%) patients having interval debulking had RD at surgery. Fig. 1 shows the flow of patients through the study design.
Table 1. Baseline characteristics.
| Characteristics | Study population (n=72) | |
|---|---|---|
| Age (yr) | 62.5±13.0 | |
| Menopausal status | ||
| No | 16 (22.2) | |
| Yes | 51 (70.9) | |
| NA | 5 (0.7) | |
| FIGO stage | ||
| IIIB | 9 (12.5) | |
| IIIC | 55 (76.4) | |
| IVA | 1 (1.4) | |
| IVB | 7 (9.7) | |
| ECOG performance status | ||
| 0 | 26 (36.1) | |
| 1 | 29 (40.3) | |
| 2 | 2 (2.8) | |
| NA | 15 (20.8) | |
| Type of surgical approach | ||
| Primary cytoreduction | 53 (73.6) | |
| IDS after failure of primary attempt | 15 (20.8) | |
| IDS | 4 (5.5) | |
| RD at primary surgical attempt (n=68) | ||
| Complete | 44 (64.7) | |
| Optimal | 49 (7.2) | |
| Non-optimal | 19 (27.9) | |
| RD at IDS (n=19) | ||
| Complete | 7 (36.8) | |
| Optimal | 6 (31.6) | |
| Non-optimal | 6 (31.6) | |
| Recurrence | ||
| No | 50 (69.4) | |
| Yes | 18 (25.0) | |
| NA | 4 (5.6) | |
Data are reported as mean±standard deviation or as numbers and percentage.
FIGO, International Federation of Gynecology and Obstetrics; ECOG, Eastern Cooperative Oncology Group; IDS, interval debulking surgery; NA, not available; RD, residual disease.
Fig. 1. Flow chart.
IDS, interval debulking surgery; RD, residual disease.
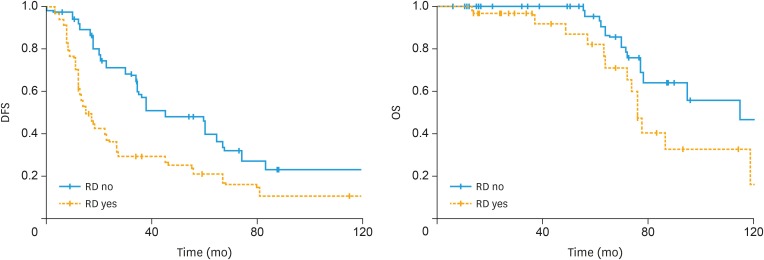
After a mean (±standard deviation) follow-up was 61.6 (±37.2) months, 50 (69.4%) and 22 (30.5%) patients recurred and died of disease, respectively. Table 1 reports baseline characteristics regarding the study population. Median (range) time to recurrence and death was 22.5 (1.0–160.0) and 59.3 (6.1–160.0) months, respectively. Median (range) overall survival (OS) for patients who died after they developed recurrent disease was 72.0 (13.5–119) months. RD at surgery was associated with disease-free survival (DFS) (median DFS: 45 months for patients with no RD vs. 15 months for patients with RD; p=0.005, log-rank test) and to a slightly improvement of OS (median DFS: 115 months for patients with no RD vs. 76 months for patients with RD; p=0.055, log-rank test). Fig. 2 displays the association between RD and survival outcomes of patients affected by LGSC.
Fig. 2. Survival outcomes of advanced stage low-grade serous ovarian cancer according to RD.
DFS, disease-free survival; OS, overall survival; RD, residual disease.
Considering factors associated with survival outcomes, we observed that via univariate analysis, presence of RD (HR=2.16; 95% CI=1.23–3.79; p=0.007), non-optimal cytoreduction (HR=2.99; 95% CI=1.68–5.33; p<0.001) and FIGO stage IV (HR=4.11; 95% CI=1.80–9.34; p=0.001) were associated with an increased risk of recurrence. Via multivariate analysis, non-optimal cytoreduction (HR=2.79; 95% CI=1.16–6.70; p=0.021) and FIGO stage IV (HR=3.15; 95% CI: 1.29–7.66; p=0.011) remained associated with DFS (Table 2).
Table 2. Factors predicting disease-free survival.
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr) | 1.11 (0.93–1.33) | 0.220 | - | - | |
| CCI | 0.903 | - | |||
| >1 | Reference | - | |||
| <1 | 1.01 (0.77–1.34) | - | |||
| ASA score | 0.18 (0.38–1.19) | 0.184 | - | - | |
| ECOG performance status | 0.94 (0.54–1.63) | 0.838 | - | - | |
| CA125 levels | 0.99 (0.97–1.01) | 0.287 | - | - | |
| Type of surgical approach | 0.103 | - | |||
| Primary cytoreductive surgery | Reference | - | |||
| IDS | 1.64 (0.90–2.99) | - | |||
| RD | 0.007 | 0.903 | |||
| No | Reference | Reference | |||
| Yes | 2.16 (1.23–3.79) | 0.94 (0.39–2.29) | |||
| Optimal cytoreduction | <0.001 | 0.021 | |||
| RD <1 cm | Reference | Reference | |||
| RD >1 cm | 2.99 (1.68–5.33) | 2.79 (1.16–6.70) | |||
| FIGO stage of disease | 0.001 | 0.011 | |||
| Stage III | Reference | Reference | |||
| Stage IV | 4.11 (1.80–9.34) | 3.15 (1.29–7.66) | |||
| Lymph node status | 0.620 | - | |||
| Negative | Reference | - | |||
| Positive | 0.79 (0.31–1.99) | - | |||
ASA, American Society of Anesthesiologists; CA125, cancer antigen 125; CCI, Charlson comorbidity index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IDS, interval debulking surgery; RD, residual disease.
OS was influenced by patients' comorbidity (HR=0.42; 95% CI=0.25–0.94; p=0.033), type of surgical approach (HR=3.00; 95% CI=1.25–7.20) for patients who had interval debulking instead of primary surgery (p=0.014), presence of RD (HR=2.25; 95% CI=0.95–5.30; p=0.064) and FIGO stage IV (HR=3.46; 95% CI=1.23–9.70; p=0.018). Via multivariate analysis, the execution of interval debulking instead primary surgery (HR=2.95; 95% CI=1.12–7.74; p=0.027) correlated with an increased risk of death over the time (Table 3).
Table 3. Factors predicting overall survival.
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr) | 1.23 (0.91–1.67) | 0.162 | - | - | |
| CCI | 0.033 | 0.093 | |||
| >1 | Reference | Reference | |||
| <1 | 0.42 (0.25–0.94) | 0.56 (0.29–1.10) | |||
| ASA score | 0.73 (0.31–1.72) | 0.485 | - | - | |
| ECOG performance status | 0.78 (0.33–1.82) | 0.571 | - | - | |
| CA125 levels | 0.99 (0.98–1.01) | 0.881 | - | - | |
| Type of surgical approach | 0.014 | 0.027 | |||
| Primary cytoreductive surgery | Reference | Reference | |||
| IDS | 3.00 (1.25–7.20) | 2.95 (1.12–7.74) | |||
| RD | 0.064 | 0.296 | |||
| No | Reference | Reference | |||
| Yes | 2.25 (0.95–5.30) | 1.78 (0.60–5.26) | |||
| Optimal cytoreduction | 0.130 | - | |||
| RD <1 cm | Reference | - | |||
| RD >1 cm | 1.97 (0.81–4.75) | - | |||
| FIGO stage of disease | 0.018 | 0.172 | |||
| Stage III | Reference | Reference | |||
| Stage IV | 3.46 (1.23–9.70) | 2.24 (0.70–7.13) | |||
| Lymph node status | 0.226 | - | |||
| Negative | Reference | - | |||
| Positive | 0.28 (0.03–2.15) | - | |||
ASA, American Society of Anesthesiologists; CA125, cancer antigen 125; CI, confidence interval; CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IDS, interval debulking surgery; RD, residual disease.
DISCUSSION
The present paper investigated the role and timing of surgery in patients affected by advanced stage LGSC, thus reporting a number of noteworthy findings. First, patients affected by advanced stage LGSC are at high risk of developing recurrent disease within the first 2 years after diagnosis. However, despite the occurrence of recurrent disease death of disease occurred at a median of 6.0 years after first diagnosis. Second, surgery plays an important role in the outcomes of patients affected by LGSC. In fact, the amount of RD impacted on survival outcomes. Third, less than 4 out of 10 patients having neoadjuvant chemotherapy had complete cytoreduction at the time of IDS, thus underlined the relative low efficacy of this strategy in advanced stage LGSC.
LGSC constitutes a smaller group of epithelial ovarian cancer, and are generally treated as the high-grade serous counterpart [7-10,13,17,18]. Gockley et al. [19], identified 16,854 (95.7%) patients with high-grade serous ovarian cancer (HGSC) and 755 (4.3%) patients with LGSC from the National Cancer Database [19]. They observed that compared with HGSC, LGSC is associated with improved survival. In patients with advanced-stage LGSC, lymphadenectomy but not adjuvant chemotherapy was associated with improved survival [19]. Other investigations highlighted that LGSC is not responsive to chemotherapy as HGSC [19-23]. In particular, the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) group investigated the response rate to chemotherapy in LGSC (n=39) and HGSC (n=80) having sub-optimal debulking (RD >1.0 cm). They observed that response rate to chemotherapy was 23.1% and 90.1% in LGSC and HGSC [23]. Similarly, the MD Anderson Cancer Center study group, underlined that chemotherapy alone is not effective in LGSC. Gershenson et al. [22], evaluating a large cohort of patients affected by stage II–IV LGSC, observed that the addition of hormonal maintenance therapy to conventional chemotherapy improved DFS of those patients (median DFS: 26.4 months for chemotherapy vs. 64.9 months for chemotherapy plus hormonal maintenance therapy; p<0.001) [22].
As aforementioned LGSC are characterized by chemoresistence that might explain the limited value of neoadjuvant chemotherapy in this group of patients. In fact, we observed that complete cytoreduction was achieved in a limited number of patients. Interestingly, more 20% of patients having interval debulking had had not a previous surgical exploration, and were referred to our center for the execution of IDS. Owing to the relative limited value of chemotherapy in LGSC, surgery represent the mainstay of treatment in this cluster of patients. Accumulating data support that optimal and complete cytoreduction are associated with improved prognosis. This finding is also confirmed by our study. Crane et al. [24], suggested that cytoreduction is effective even in case of recurrent disease. Analyzing 41 patients with recurrent LGSC, the authors reported that median progression-free survival was about 60.3 and 10.7 months for patients with no RD and with RD, respectively [24]. This data was confirmed by another investigation of our study group [25]. The inherent biases of the retrospective study design represents the main weakness of our investigation. However, the relative large sample size and central pathology review are the main strengths of the research.
In our series patients having IDS experienced worse OS than patients having primary cytoreductive surgery. Obviously, we have to underline that patients having interval debulking might have a higher burden of disease than patients having primary surgery. Unfortunately, we have no data regarding the amount of peritoneal involvement in those patients. Therefore, our data needs to be corroborated by other prospective experiences.
In conclusion the present paper analyzed prognostic factors associated with survival outcomes of patients diagnosed by advanced stage LGSC. Patients with advanced stage LGSC are at high risk of developing recurrence disease, but are characterized by a prolonged survival. We observed that non-optimal cytoreduction and FIGO stage IV correlate with worse DFS; while the execution of neoadjuvant chemotherapy and IDS might have detrimental effects on OS. Further prospective studies are warranted to identify new therapies for LGSC.
ACKNOWLEDGMENTS
We thank Maria Luisa Carcangiu for the help in pathology review of all specimens.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: B.G., D.A., M.F., L.D., R.F.
- Data curation: B.G., L.R.M.U., P.B., D.A., M.F., L.D., R.F.
- Formal analysis: B.G., P.B., L.D., R.F.
- Investigation: B.G., L.R.M.U., P.B.
- Methodology: B.G., P.B., R.F.
- Project administration: L.R.M.U., P.B., R.F.
- Resources: P.B.
- Software: B.G., L.R.M.U.
- Supervision: P.B., R.F.
- Validation: B.G., P.B., M.F., L.D., R.F.
- Visualization: B.G., L.R.M.U., P.B., M.F., L.D., R.F.
- Writing - original draft: B.G., L.R.M.U., L.D., R.F.
- Writing - review & editing: B.G., P.B., D.A., M.F., L.D., R.F.
References
- 1.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(Suppl 10):x111–x117. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 3.Gershenson DM. Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol. 2016;27(Suppl 1):i45–i49. doi: 10.1093/annonc/mdw085. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo K, Machida H, Grubbs BH, Sood AK, Gershenson DM. Trends of low-grade serous ovarian carcinoma in the United States. J Gynecol Oncol. 2018;29:e15. doi: 10.3802/jgo.2018.29.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Wong KK, Fotopoulou C, Blake EA, Robertson SE, Pejovic T, et al. Impact of lympho-vascular space invasion on tumor characteristics and survival outcome of women with low-grade serous ovarian carcinoma. J Surg Oncol. 2018;117:236–244. doi: 10.1002/jso.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA. Low-grade serous ovarian cancer: a review. Gynecol Oncol. 2016;143:433–438. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 7.Sallum LF, Andrade L, Bastos Eloy da Costa L, Ramalho S, Ferracini AC, Natal RA, et al. BRCA1, Ki67, and β-Catenin immunoexpression is not related to differentiation, platinum response, or prognosis in women with low- and high-grade serous ovarian carcinoma. Int J Gynecol Cancer. 2018;28:437–447. doi: 10.1097/IGC.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 8.Okoye E, Euscher ED, Malpica A. Ovarian low-grade serous carcinoma: a clinicopathologic study of 33 cases with primary surgery performed at a single institution. Am J Surg Pathol. 2016;40:627–635. doi: 10.1097/PAS.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 9.Fader AN, Java J, Ueda S, Bristow RE, Armstrong DK, Bookman MA, et al. Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol. 2013;122:225–232. doi: 10.1097/AOG.0b013e31829ce7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segev Y, Kaldawy A, Auslender R, Lavie O. Low grade ovarian cancer. Harefuah. 2017;156:507–511. [PubMed] [Google Scholar]
- 11.Hutter MM, Rowell KS, Devaney LA, Sokal SM, Warshaw AL, Abbott WM, et al. Identification of surgical complications and deaths: an assessment of the traditional surgical morbidity and mortality conference compared with the American College of Surgeons-National Surgical Quality Improvement Program. J Am Coll Surg. 2006;203:618–624. doi: 10.1016/j.jamcollsurg.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Ditto A, Martinelli F, Bogani G, Lorusso D, Carcangiu M, Chiappa V, et al. Long-term safety of fertility sparing surgery in early stage ovarian cancer: comparison to standard radical surgical procedures. Gynecol Oncol. 2015;138:78–82. doi: 10.1016/j.ygyno.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Raspagliesi F, Bogani G, Ditto A, Martinelli F, Chiappa V, Borghi C, et al. Implementation of extensive cytoreduction resulted in improved survival outcomes for patients with newly diagnosed advanced-stage ovarian, tubal, and peritoneal cancers. Ann Surg Oncol. 2017;24:3396–3405. doi: 10.1245/s10434-017-6030-0. [DOI] [PubMed] [Google Scholar]
- 14.Eastern Cooperative Oncology Group. ECOG performance status [Internet] Philadelphia, PA: Eastern Cooperative Oncology Group; [cited 2016 Jul 2]. Available from: http://ecog-acrin.org/resources/ecog-performance-status. [Google Scholar]
- 15.Bogani G, Uccella S, Ghezzi F. Impact of comorbidity index on survival in endometrial cancer. Am J Clin Oncol. 2014;37:642–643. doi: 10.1097/COC.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 16.Wagner U. Lymphadenectomy In Ovarian Neoplasms (LION) protocol (AGO-OVAR OP.3) [Internet] Bethesda, MD: U.S. National Library of Medicine; cited 2018 Jun 2. Available from: https://clinicaltrials.gov/ct2/show/NCT00712218. [Google Scholar]
- 17.Bogani G, Matteucci L, Tamberi S, Arcangeli V, Ditto A, Maltese G, et al. The impact of number of cycles of neoadjuvant chemotherapy on survival of patients undergoing interval debulking surgery for stage IIIC–IV unresectable ovarian cancer: results from a multi-institutional study. Int J Gynecol Cancer. 2017;27:1856–1862. doi: 10.1097/IGC.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 18.Torres D, Kumar A, Wallace SK, Bakkum-Gamez JN, Konecny GE, Weaver AL, et al. Intraperitoneal disease dissemination patterns are associated with residual disease, extent of surgery, and molecular subtypes in advanced ovarian cancer. Gynecol Oncol. 2017;147:503–508. doi: 10.1016/j.ygyno.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gockley A, Melamed A, Bregar AJ, Clemmer JT, Birrer M, Schorge JO, et al. Outcomes of women with high-grade and low-grade advanced-stage serous epithelial ovarian cancer. Obstet Gynecol. 2017;129:439–447. doi: 10.1097/AOG.0000000000001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fader AN, Bergstrom J, Jernigan A, Tanner EJ, 3rd, Roche KL, Stone RL, et al. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: Reducing overtreatment without compromising survival? Gynecol Oncol. 2017;147:85–91. doi: 10.1016/j.ygyno.2017.07.127. [DOI] [PubMed] [Google Scholar]
- 21.Buttarelli M, Mascilini F, Zannoni GF, Ciucci A, Martinelli E, Filippetti F, et al. Hormone receptor expression profile of low-grade serous ovarian cancers. Gynecol Oncol. 2017;145:352–360. doi: 10.1016/j.ygyno.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC. Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum. J Clin Oncol. 2017;35:1103–1111. doi: 10.1200/JCO.2016.71.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabowski JP, Harter P, Heitz F, Pujade-Lauraine E, Reuss A, Kristensen G, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO study group metadatabase. Gynecol Oncol. 2016;140:457–462. doi: 10.1016/j.ygyno.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Crane EK, Sun CC, Ramirez PT, Schmeler KM, Malpica A, Gershenson DM. The role of secondary cytoreduction in low-grade serous ovarian cancer or peritoneal cancer. Gynecol Oncol. 2015;136:25–29. doi: 10.1016/j.ygyno.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogani G, Tagliabue E, Signorelli M, Ditto A, Martinelli F, Chiappa V, et al. A score system for complete cytoreduction in selected recurrent ovarian cancer patients undergoing secondary cytoreductive surgery: predictors- and nomogram-based analyses. J Gynecol Oncol. 2018;29:e40. doi: 10.3802/jgo.2018.29.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]